Abstract
A novel technique using the incorporation of 5-ethynyl-2'-deoxyuridine (EdU) into replicating DNA is described for the analysis of replicating banding patterns of human metaphase chromosomes. Human lymphocytes were synchronized with excess thymidine and treated with EdU during the late S phase of the cell cycle. The incorporated EdU was then detected in metaphase chromosomes using Alexa Fluor® 488 azides, through the 1,3-dipolar cycloaddition reaction of organic azides with the terminal acetylene group of EdU. Chromosomes with incorporated EdU showed a banding pattern similar to G-banding of normal human chromosomes. Imaging by atomic force microscopy (AFM) in liquid conditions showed that the structure of the chromosomes was well preserved even after EdU treatment. Comparison between fluorescence microscopy and AFM images of the same chromosome 1 indicated the presence of ridges and grooves in the chromatid arm, features that have been previously reported in relation to G-banding. These results suggest an intimate relationship between EdU-induced replication bands and G- or R-bands in human chromosomes. This technique is thus useful for analyzing the structure of chromosomes in relation to their banding patterns following DNA replication in the S phase.
Keywords: G-banding, 5-ethynyl-2'-deoxyuridine, atomic force microscopy, human, metaphase chromosome
I. Introduction
Incorporation of the thymidine analog, 5-bromo-2'-deoxyuridine (BrdU), into DNA during the synthesis phase (S phase) of the cell cycle was introduced in 1973 by Latt [12]. BrdU is incorporated during DNA replication in this phase instead of thymidine and is widely used in studies of cellular proliferation [16]. BrdU is crudely detected by differential staining using Hoechst fluorescence. This enables the sites of replication on chromosomes to be determined in relation to chromosome bands. Subsequent immunohistochemical studies also visualized R- or G-banding patterns in chromosomes when BrdU was introduced into cells during early or late S phase, respectively [7, 19]. However, the immunohistochemical detection of BrdU requires strong denaturation of DNA [1, 6] followed by immunoreaction of the incorporated BrdU with an anti-BrdU antibody, resulting in unstable detection of the incorporated BrdU and the disorganization of the higher order structure of the chromosomes.
Recently, a thymidine analog, 5-ethynyl-2'-deoxyuridine (EdU), has been introduced as an alternative to BrdU. In EdU, the terminal methyl group is replaced with an alkyne group, which allows detection using a fluorescent azide that covalently binds to the alkyne group in a reaction known as “click chemistry” [2, 13, 15]. This reaction is thus more specific and rapid than BrdU immunocytochemistry because the molecular size of the reagent is almost 500 times smaller than that of an antibody molecule. In addition, DNA denaturation is not required for the reaction between the terminal acetylene group and the fluorescent azide; therefore, the chromosome structure is not seriously damaged by this method. Thus, EdU has recently been used instead of BrdU to detect DNA synthesis in proliferating cells [3, 14, 20].
In the present study, we used EdU to analyze the replication banding that is produced by the incorporation of EdU into DNA in the late S phase of the cell cycle. We will show the reliability of this method and also demonstrate its applicability for analyzing the structure of chromosomes in relation to replication banding patterns using atomic force microscopy (AFM).
II. Materials and Methods
Preparation of EdU-incorporated chromosomes
EdU-incorporated chromosomes were prepared based on a method described for BrdU incorporation into replicating DNA [7, 19], in which cells were synchronized with excess thymidine before EdU incorporation.
Briefly, human lymphocytes were isolated by Ficoll-Paque gradient density centrifugation (GE Healthcare, Uppsala, Sweden) from heparinized peripheral blood, obtained from normal healthy volunteers (aged between 25 and 44 years) from whom informed consent had been obtained. These lymphocytes were cultivated in a karyotyping medium (PB-Max; Gibco, CA, USA) under 5% CO2 at 37°C for 48 hr. To synchronize the cell cycles of the lymphocytes, thymidine (300 µg/ml, Wako, Osaka, Japan) was added to the medium and the cells incubated for a further 15.5 hr. Next, the cells were rinsed twice with Dulbecco’s modified Eagle’s medium (Gibco), treated with 25 µg/ml EdU (Invitrogen, Carlsbad, CA, USA), and incubated again in the karyotyping medium for an additional 6.5 hr under 5% CO2 at 37°C. Colcemid (Demecolcine, Wako, Osaka, Japan) was then added to the culture medium at a final concentration of 0.05 µg/ml and cells incubated for 30 min. The cell suspension was treated with 75 mM KCl for 30 min at room temperature and fixed with a mixture of methanol and acetic acid (3:1). Spreads of chromosomes were made by dropping the cell suspension onto glass slides followed by air drying under humid conditions for 10 min [4].
Detection of EdU in chromosomes
EdU was detected by the “click reaction,” the reaction of the alkyne group of EdU with the azide group of Alexa Fluor® 488 (Click-iT EdU Alexa Fluor Cell Proliferation Assay kit, Invitrogen) through the 1,3-dipolar cycloaddition reaction of the organic azide with the terminal acetylene of EdU. After the chromosome spreads were reacted with the fluorescent probe, they were further stained with YOYO-3 (dilution 1:10,000, Molecular Probes, Eugene, OR, USA) to detect the shape of the chromosomes. They were gently covered with a coverslip with an excess of phosphate-buffered saline (PBS) sandwiched between sample and coverslip, and observed under a fluorescence microscope (Eclipse TE2000-U, Nikon, Tokyo, Japan). The chromosomes to be studied were photographed using a digital image acquisition system (Olympus DP71, Tokyo, Japan).
Atomic force microscopy
Before and after EdU treatment, AFM was used to observe chromosomes in PBS to study any changes in chromosome structure due to EdU staining. Chromosome 1 was observed in PBS by AFM to analyze the structure of the chromosome in relation to its banding induced by EdU incorporation. AFM imaging was performed using a commercial atomic force microscope (SPI 4000 probe station, SII NanoTechnology, Chiba, Japan). Since an optical microscope is placed over the AFM unit, the target chromosomes can be accurately identified for observation by the AFM. AFM was operated in a dynamic force mode (i.e., intermittent contact mode) in PBS solution. The maximum scan range of the piezo scanner was about 20 µm in width (x–y) and 1.2 µm in height (z). Reduction in oscillation amplitude was used as a feedback parameter by the slope detection method. Commercially available V-shaped silicon nitride cantilevers with a nominal spring constant of 0.32 N/m (DNP-S; Veeco, Santa Barbara, CA, USA) were used for imaging. The resonance frequency was about 12.5 kHz in the liquid medium. The size of the obtained images was 512×512 pixels, and both height mode and error mode images were obtained simultaneously. The height images were displayed by a computer in the “gradation mode” for showing the height of specimens as color gradations.
III. Results and Discussion
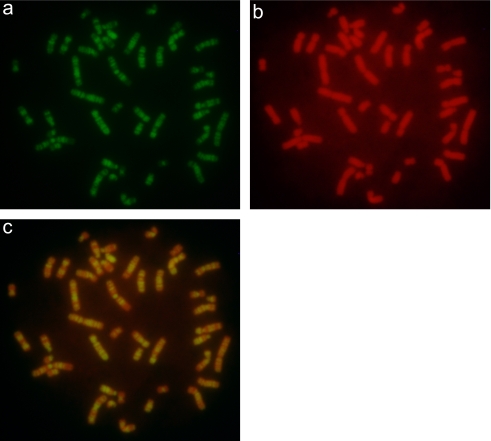
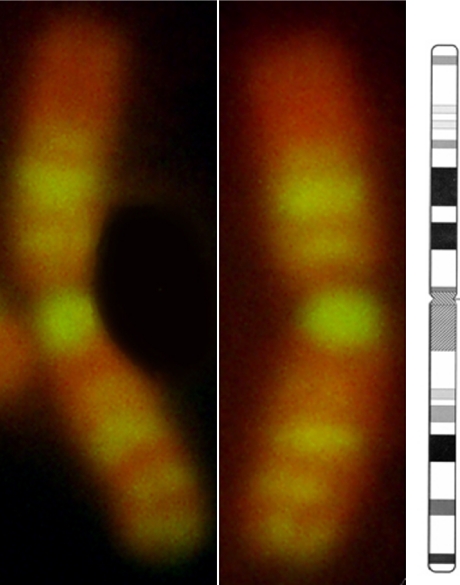
Figure 1 shows images of a set of chromosomes from mitotic lymphocytes that were synchronized with excess thymidine and then treated with EdU in the late S phase of the cell cycle. EdU-incorporated DNA was clearly detected as green fluorescent spots as a result of labeling with Alexa Fluor 488 (Fig. 1a). The shape of chromosomes was observed by YOYO-3 staining (Fig. 1b). Superimposition of the two images provided a clear image of the position of the replication bands on the chromosomes (Fig. 1c). A closer view of chromosome 1 in Figure 1 is shown in Figure 2, where the chromosome is compared with a G-banded ideogram of normal human chromosome 1 [11]. This indicates that the replication banding pattern induced by EdU incorporation in the late S phase showed a good similarity with the G-banding pattern of the chromosome.
Fig. 1.
(a) Fluorescence micrograph of replicated G-banded human chromosomes treated with EdU and detected by the reaction of the EdU ethynyl group with Alexa Fluor 488® azides. (b) The same sample counterstained with YOYO-3. (c) Superimposition of images (a) and (b). Bands are clear and consistent in all chromosomes.
Fig. 2.
Comparison between replication banding of chromosome 1 obtained after EdU treatment (detailed view of the chromosome in Fig. 1) and a G-banded ideogram for normal human chromosome 1 (representative of a haploid karyotype of approximately 400 bands). The ideogram was obtained from ISCN 2009. The EdU-induced banding pattern of chromosome 1 showed good similarity with the G-banded ideogram.
Replication banding of chromosomes is usually performed by introducing BrdU into cells during the S phase followed by Hoechst differentiation staining or immunohistochemistry of chromosomes during subsequent mitosis [12]. A previous study also showed that BrdU incorporated into synchronized cells during the late S phase was restricted in metaphase chromosomes to a replicated banding pattern similar to G-banding [5]. In the present study, we introduced EdU into cells instead of BrdU and showed clear evidence that G-banding is closely related to the position of DNA that was replicated in the late S phase of the cell cycle.
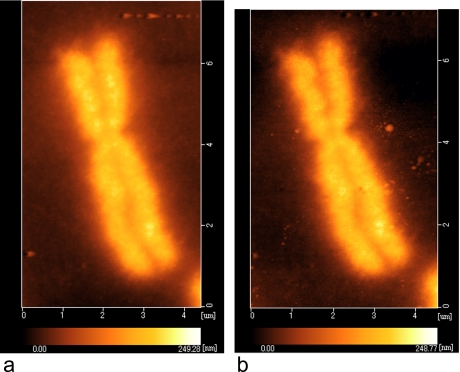
To assess possible structural damage of chromosomes due to EdU staining, we observed chromosomes using AFM in a liquid medium before and after EdU staining with the fluorescent probe. AFM is useful for analyzing the structure of chromosomes under liquid conditions because severe damage to their structure is avoided [8, 10]. These images showed that the three-dimensional structures of chromosomes were well preserved even after EdU staining (Fig. 3), which indicates that the chromosome structure is not seriously damaged by this staining method. By AFM imaging, we have also confirmed that chromosomes treated by BrdU instead of EdU and immunostained with anti-BrdU antibody had structural damages (Hoshi, O., unpublished data, 2010).
Fig. 3.
AFM images of a chromosome before (a) and after (b) EdU treatment using the click chemistry reaction. The fine structure of the chromosome was well preserved irrespective of EdU click chemistry.
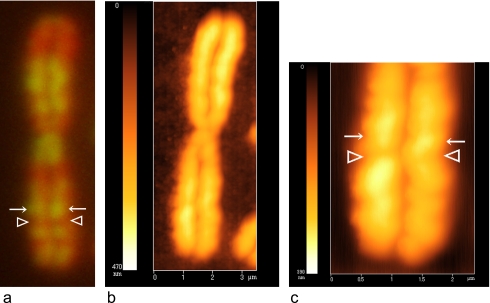
In our previous study using AFM, we showed the presence of alternating ridges and grooves on the chromatid arm of G/Q-banded human metaphase chromosomes that corresponded to G/Q-positive and -negative bands, respectively [9, 17, 18]. In the present study, the comparison between fluorescence microscopy and AFM images of the same chromosome 1 treated with EdU indicates that the ridges corresponded to the replication bands produced by EdU incorporation into DNA in the late S phase (Fig. 4). This finding indicates that the ridges and grooves on the chromatid arm are intrinsic structures, which are related not only to chromosome banding but also to the replication timing in the S phase.
Fig. 4.
(a) Fluorescence micrograph of the banding pattern of human chromosome 1 obtained after EdU treatment. (b) AFM image of the same chromosome 1. (c) Magnified view of part of (a). In the fluorescence micrograph, green (G-positive, arrows) and not green (G-negative, arrowheads) bands correspond to ridges (arrows) and grooves (arrowheads), respectively, on the surface of the chromosome in the AFM image.
In conclusion, we have described a reliable method for studying the replication banding pattern of human chromosomes by using EdU treatment in the S phase followed by EdU click chemistry. This method combined with AFM is useful for analyzing the structure of chromosomes in relation to their replication banding pattern. The high-resolution analysis of the EdU-incorporated chromosomes by AFM is expected to provide new insights into the functional significance of higher-order structures of chromosomes.
IV. Acknowledgments
This study was supported in part by a Grant for Promotion of Niigata University Research Projects, Japan (to O.H.).
Abbreviations
- AFM
atomic force microscopy
- BrdU
5-bromo-2'-deoxyuridine
- EdU
5-ethynyl-2'-deoxyuridine
- PBS
phosphate-buffered saline
V. References
- 1.Bérubé D., Gagné R. Exonuclease digestion of human chromosomes for in situ hybridization and R-banding. Nucleic Acids Res. 1990;18:2831. doi: 10.1093/nar/18.9.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Buck S. B., Bradford J., Gee K. R., Agnew B. J., Clarke S. T., Salic A. Detection of S-phase cell cycle progression using 5-ethynyl-2'-deoxyuridine incorporation with click chemistry, an alternative to using 5-bromo-2'-deoxyuridine antibodies. BioTechniques. 2008;44:927–929. doi: 10.2144/000112812. [DOI] [PubMed] [Google Scholar]
- 3.Chehrehasa F., Meedeniya A. C., Dwyer P., Abrahamsen G., Mackay-Sim A. EdU, a new thymidine analogue for labeling proliferating cells in the nervous system. J. Neurosci. Methods. 2009;177:122–130. doi: 10.1016/j.jneumeth.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Czepulkowski B. BIOS Scientific Publishers Ltd; Oxford: 2001. Analyzing Chromosomes; pp. 47–48. [Google Scholar]
- 5.Drouin R., Lemieux N., Richer C. L. High-resolution R-banding at the 1250-band level 1. Technical considerations on cell synchronization and R-banding (RHG and RBG) Cytobios. 1988;56:107–125. [PubMed] [Google Scholar]
- 6.Drouin R., Messier P. E., Richer C. L. DNA denaturation for ultrastructural banding and the mechanism underlying the fluorochrome-photolysis-Giemsa technique studied with anti-5-bromodeoxyuridine antibodies. Chromosoma. 1989;98:174–180. doi: 10.1007/BF00329681. [DOI] [PubMed] [Google Scholar]
- 7.Fenti R., Drouin R., Richer C. L., Lemieux N. Complementary replication R- and G-band patterns induced by cell blocking at the R-band/G-band transition, a possible regulatory checkpoint within the S phase of the cell cycle. Cytogenet. Cell Genet. 1996;75:172–179. doi: 10.1159/000134472. [DOI] [PubMed] [Google Scholar]
- 8.Hoshi O., Owen R., Miles M., Ushiki T. Imaging of human metaphase chromosomes by atomic force microscopy in liquid. Cytogenet. Genome Res. 2004;107:28–31. doi: 10.1159/000079568. [DOI] [PubMed] [Google Scholar]
- 9.Hoshi O., Ushiki T. Three-dimensional structure of G-banded human metaphase chromosomes observed by atomic force microscopy. Arch. Histol. Cytol. 2001;64:475–482. doi: 10.1679/aohc.64.475. [DOI] [PubMed] [Google Scholar]
- 10.Hoshi O., Ushiki T. Atomic force microscopy imaging of human metaphase chromosomes in liquid. Methods Mol. Biol. 2011;736:109–115. doi: 10.1007/978-1-61779-105-5_8. [DOI] [PubMed] [Google Scholar]
- 11.Shaffer L. G., Slovak M. L., Campbell L. J., (eds). Karger; Basel: 2009. ISCN 2009: An International System for Human Cytogenetic Nomenclature; p. 16. [Google Scholar]
- 12.Latt S. A. Microfluorometric detection of deoxyribonucleic acid replication in human metaphase chromosomes. Proc. Natl. Acad. Sci. U S A. 1973;70:3395–3399. doi: 10.1073/pnas.70.12.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rostovtsev V. V., Green V. V., Fokin V. V., Sharpless K. B. A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. Engl. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Salic A., Mitchison T. J. A chemical method for fast and sensitive detection of DNA synthesis in vivo. Proc. Natl. Acad. Sci. U S A. 2008;105:2415–2420. doi: 10.1073/pnas.0712168105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tornøe C. W., Christensen C., Meldal M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 16.Tsutsumi Y., Fushiki S. Comparison of cell kinetics between the boundary and the interboundary areas during hindbrain segmentation in the chick embryo. Acta Histochem. Cytochem. 2000;33:141–147. [Google Scholar]
- 17.Ushiki T., Hoshi O. Atomic force microscopy for imaging human metaphase chromosomes. Chromosome Res. 2008;16:383–396. doi: 10.1007/s10577-008-1241-7. [DOI] [PubMed] [Google Scholar]
- 18.Ushiki T., Hoshi O., Iwai K., Kimura E., Shigeno M. The structure of human metaphase chromosomes: its histological perspective and new horizons by atomic force microscopy. Arch. Histol. Cytol. 2002;65:377–390. doi: 10.1679/aohc.65.377. [DOI] [PubMed] [Google Scholar]
- 19.Viegas-Péquignot E., Dutrillaux B. Une méthod simple pour obtenir des prophases et des prométaphases. Ann. Génét. 1978;21:122–125. [Google Scholar]
- 20.Zeng C., Pan F., Jones L. A., Lim M. M., Griffin E. A., Sheline Y. I., Mintun M. A., Holtzman D. M., Mach R. H. Evaluation of 5-ethynyl-2'-deoxyuridine staining and reliable method for studying cell proliferation in the adult nervous system. Brain Res. 2010;1319:21–32. doi: 10.1016/j.brainres.2009.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]