Abstract
Objective
Cardiac valvular endothelium is unique in its ability to undergo endothelial-to-mesenchymal transformation, a differentiation process that is essential for valve development and has been proposed as mechanism for replenishing the interstitial cells of mature valves. We hypothesized that the valvular endothelium contains endothelial cells that are direct precursors to osteoblastic valvular interstitial cells (VICs).
Methods and Results
Clonal cell populations from ovine mitral valve leaflets were isolated by single cell plating. Mitral valvular endothelial and mesenchymal clones were tested for osteogenic, adipogenic, and chondrogenic differentiation, determined by the expression of lineage-specific markers. Mitral valvular endothelial clones showed a propensity for osteogenic, as well as chondrogenic differentiation that was comparable to a mitral valvular VIC clone and to bone marrow–derived mesenchymal stem cells. Osteogenic differentiation was not detected in nonvalvular endothelial cells. Regions of osteocalcin expression, a marker of osteoblastic differentiation, were detected along the endothelium of mitral valves that had been subjected in vivo to mechanical stretch.
Conclusion
Mitral valve leaflets contain endothelial cells with multilineage mesenchymal differentiation potential, including osteogenic differentiation. This unique feature suggests that postnatal mitral valvular endothelium harbors a reserve of progenitor cells that can contribute to osteogenic and chondrogenic VICs.
Keywords: endothelium, heart valves, vascular biology, EMT, endothelial cells, mitral valve, osteogenic differentiation
Endothelial-to-mesenchymal transformation (EMT) occurs in endocardial cushions in the developing heart: a subset of endothelial cells (ECs) lining the endocardial surface of the cushions downregulate cellular adhesion molecules, retract from the endothelium, transiently upregulate the contractile protein α-smooth muscle actin (α-SMA), and migrate into the interstitium of the nascent valve to become valvular interstitial cells (VICs) (reviewed elsewhere1). Lineage-tracing studies revealed that essentially all of the cells in mature valves, including the mitral valve leaflets, are derived from the endothelial cells lining the endocardial cushion (for review, see2). Hence, during embryonic development, the mitral valvular endothelial cells and interstitial cells are derived from a common cellular source.
EMT is rarely observed or induced in cultured nonvalvular ECs.3–5 In studies where EMT has been reported in nonvalvular ECs, a confounding factor is the potential for a small number of mesenchymal cells within an endothelial primary culture to grow more rapidly than the ECs when culture conditions are manipulated to induce EMT. To avoid this possibility, we used clonally derived populations of ECs from ovine and human valves to demonstrate EMT, induced by adding transforming growth factor (TGF)-β.6 – 8 In vivo, cells coexpressing endothelial markers and α-SMA have been detected along the valve endothelium and in subendothelial locations, which indicates EMT in mature aortic and pulmonary valves. These observations prompted us to speculate that EMT may contribute to replenishment of the VICs on an as-needed basis throughout postnatal life.7
In ischemic mitral regurgitation (MR), an increase in mitral leaflet area has been detected by 3D echocardiography and proposed as an adaptive mechanism to minimize functional MR.9 To explore the cellular mechanisms that might contribute this adaptation, Dal-Bianco et al8 showed, using an ovine model, that imposing an altered leaflet geometry that mimicked the tethering that occurs in ischemic MR caused an increase in leaflet area and thickness. A 4-fold increase in mitral valve ECs (MVECs) undergoing EMT was shown to coincide with the increase in leaflet size.8 These results suggest that EMT played an important role in the adaptive response of the mitral valve to leaflet tethering and furthermore, EMT might be an important mechanism in the adaptive processes to circumvent functional MR.
VICs are the prominent cell type in valve cusps and leaflets, residing throughout the fibrosa, spongiosa, and atrialis/ventricularis layers, and are viewed as essential for the production of layer-specific extracellular matrix (ECM). The fibrosa layer is rich in collagen 1 fibrils and thought to provide mechanical strength and resilience to the valve,10 but it is also the primary site of calcification in diseased aortic valves. The spongiosa layer is enriched in glycosaminoglycans and proteoglycans that provide compressibility to the valve. Interestingly, the spongiosa layer is characterized by expression of cartilage-related proteins11–13 such as Sox9, aggrecan, and collagen 2a1, indicating a cartilage-like feature to the VICs in this region. The atrialis (mitral valve) and ventricularis (aortic valve) layers are rich in elastin.14 For orientation, the atrialis and ventricularis layers are the surfaces of the atrioventricular and semilunar valves, respectively, that are exposed directly to blood flow. The distinct ECM of each layer and the emerging patterns of layer-specific gene expression14–16 strongly suggest that there are unique cellular phenotypes within the VIC population.
A recent review article has provided a framework for delineating VICs into distinct phenotypes.17 Quiescent VICs are the predominant cell type in healthy valves but become activated VICs, expressing abundant α-SMA, in response to injury or abnormal mechanical forces. Furthermore, osteogenic VICs have been described that can differentiate into osteoblasts in vitro and are thought to be the source of calcification in vivo in diseased aortic valves.18–22 A recent study of porcine aortic valves from healthy 8-month-old animals has shown that the VIC population is highly enriched in osteogenic progenitors.23 The cells were identified by their distinctive morphology in vitro, expression of α-SMA, and lack of CD31 expression, indicating the absence of contaminating ECs. Consistent with this study, the gene expression profiles of a murine osteoblastic cell line and embryonic day 17.5 valves were found to be similar,24 as well as protein expression pattern between human bone marrow–derived mesenchymal stem cells (BM-MSCs) and human VICs.25
Progenitor VICs are postulated to have 2 sources. Resident progenitor cells have been shown to give rise to α-SMA–positive activated VICs17 and, as described above, to undergo multilineage differentiation. Hematopoietic cell recruitment and differentiation into VIC-like cells has been demonstrated in a murine model.26 What has not been reported previously is the potential for valvular ECs to differentiate into multipotent VICs or into osteogenic cells. Here, we show that clonally derived ECs isolated from ovine mitral valve leaflets are able to differentiate into multiple mesenchymal lineages, with a propensity for osteogenic differentiation.
Methods
Materials
The following materials were used: endothelial basal medium (EBM) (Lonza Inc), FBS (Hyclone), glutamine-penicillin-streptomycin sulfate (GPS), trypsin-EDTA, and other cell culture materials, Gibco-BRL; human TGF-β1 and vascular endothelial growth factor (VEGF)-A165 (R&D Systems); basic fibroblast growth factor (Roche Applied Biosciences); and NuPAGE Novex Bis-Tris gels (4 to 12%) (Invitrogen).
Mitral Valve Cells
Primary cultures from mitral valve leaflets from sheep were prepared6,7 and plated as single cells in 96-well plates.7 Briefly, the primary culture was trypsinized to yield a single cell suspension, diluted to 3 cells/mL, and plated at 100 μL/well. Visual inspection was performed to assess that single colonies appeared in a subset of wells. Clones designated MVEC-4, MVEC-5, and mitral VIC-(MVIC)-7 were expanded on 1% gelatin-coated dishes in EBM-B medium (10% heat-inactivated FBS, 1% GPS, and 2 ng/mL basic fibroblast growth factor). Experiments were performed using MVEC-4 at passages 6 and 7, MVEC-5 at passages 9 to 11, and MVIC-7 at passages 7 and 8. Six additional MVEC clones were characterized and analyzed in osteogenesis assays (Figure I in the online Data Supplement, available at http://atvb.ahajournals.org). Ovine aortic valve endothelial clone-1 (wav-1) was isolated and cultured as described.27,28
Nonvalvular ECs
ECs from ovine carotid arteries (CAECs) were isolated by collagenase (0.2% vol/vol final) and dispase (2.5 U/mL final) digestion for 1 hour at 37°C. The tissue digest was passed through a 100-μm strainer and plated on gelatin-coated plates in EGM-2 medium (Lonza Inc). Endothelial-like colonies were collected and plated by limiting dilution as described above. Clones CAEC-3 and CAEC-13 were analyzed by flow cytometry (supplemental Figure II). Sheep endothelial progenitor cells (SPECs) were isolated from peripheral blood samples as described.29 Human cord blood endothelial progenitor cells (cbEPCs) and human dermal microvascular ECs (HDMECs) were isolated and characterized as described.27,30 BM-MSCs were isolated as described.31 Nonvalvular ECs are not transformed cell lines, and additionally they were grown in the same medium and under identical conditions as the MVECs for all experiments presented here.
Immunofluorescence, Flow Cytometry, and Western Blotting
Immunofluorescence was performed on methanol-fixed cells using anti-human VE-cadherin (Santa Cruz Biotechnology), murine anti-human α-SMA (clone 1A4, Sigma-Aldrich), and murine anti-bovine osteocalcin (QED Biosciences). Secondary antibodies conjugated with either fluorescein isothiocyanate or Texas Red were used after the primary antibody incubation step. Flow cytometry for CD31 and α-SMA expressing cells was performed as described.8 CD90 was detected using a murine anti-human CD90-PE (BD Pharmingen). For Western blotting, cells were lysed as described.28 Murine anti-human α-SMA (1:2000), goat anti-human CD31 (1:1000) (M-20, Santa Cruz Biotechnology), and goat anti-human VE-cadherin (1:300) (Santa Cruz Biotechnology) were used to detect α-SMA, CD31, and VE-cadherin, respectively. All antibodies were shown to cross-react with their ovine homologs.
Cellular Proliferation Assay
Mitral valve clones were plated at 2000 cells/cm2 in EBM-B. One row of cells was counted on day 1 to determine plating efficiency. Cells were trypsinized at days 2, 3, 5, and 7 and counted. Each condition was performed in triplicate.
Osteogenic, Adipogenic, and Chondrogenic Differentiation
Control medium consisted of DMEM low-glucose medium with 10% FBS, 1× GPS.
Osteogenesis
Mitral valve clones were cultured for 2 or 3 weeks in DMEM low-glucose medium with 10% FBS, 1× GPS, and osteogenic supplements (1 μmol/L dexamethasone, 10 mmol/L β-glycerophosphate, 60 μmol/L ascorbic acid-2-phosphate). Differentiation was assessed by von Kossa and alkaline phosphatase staining,32 osteocalcin staining, and detection of mRNA transcripts encoding osteogenic markers.
Adipogenesis
Mitral valve cells were cultured for 2 or 3 weeks in DMEM low-glucose medium with 10% FBS, 1× GPS, and adipogenic supplements (5 μg/mL insulin, 1 μmol/L dexamethasone, 0.5 mmol/L isobutylmethylxanthine, 60 μmol/L indomethacin). Differentiation into adipocytes was assessed by oil red O staining and expression of peroxisome proliferator-activated receptor (PPAR)γ2.
Chondrogenesis
Mitral valve cell suspensions were transferred into 15 mL of polypropylene centrifuge tubes (500 000 cells/tube) and gently centrifuged. The resulting pellets were statically cultured in DMEM high-glucose medium with 1× GPS and chondrogenic supplements (1× insulin-transferrin-selenium, 1 μmol/L dexamethasone, 100 μmol/L ascorbic acid-2-phosphate, and 10 ng/mL TGF-β1). After 2 or 3 weeks, pellets were fixed in 10% buffered formalin overnight, embedded in paraffin, and sectioned (7 μm thick). Differentiation into chondrocytes was assessed by Alcian Blue staining for the presence of glycosaminoglycans and mRNA transcripts for Sox9 and collagen 2a1. Sections of mouse articular cartilage served as control.
RNA Extraction and RT-PCR
Total RNA was isolated using an RNeasy Mini Kit (Qiagen), and 2 μg was treated with DNAse I (Invitrogen). Reverse transcription was performed with iScript cDNA Synthesis Kit (Bio-Rad). Oligonucleotide primer sequences are in the Table. Polymerase chain reaction (PCR) was performed with iQ Supermix (Bio-Rad) on a PT-100 machine (MJ Research). PCR conditions were as follows: Human osteopontin: initial denaturation at 94°C for 2 minutes, followed by 35 cycles of denaturation at 94°C for 30 seconds, annealing at 55°C for 2 minutes, and extension at 72°C for 2 minutes, followed by an extension at 72°C for 10 minutes; Human PPARγ2: initial denaturation at 94°C for 5 minutes, followed by 35 cycles of denaturation at 94°C for 45 seconds, annealing at 55°C for 45 seconds, and extension at 72°C for 90 seconds, followed by an extension at 72°C for 10 minutes; All other primer sets: initial denaturation at 94°C for 5 minutes, followed by 35 cycles of denaturation at 94°C for 30 seconds, annealing at 55°C for 30 seconds, and extension at 72°C for 1 minute, followed by an extension at 72°C for 10 minutes. All PCR products were sequenced using ABI DNA sequencer (Children's Hospital Boston core facility) to verify the sequence corresponded to the gene of interest.
Table.
Oligonucleotide Primer Sequences Used for PCR
| Gene | Accession No. | Oligonucleotides | Product Size (bp) |
|---|---|---|---|
| Osteopontin (human) | NM_001040060.1 | 5’-CCAAGTAAGTCCAACGAAAG-’3 | 347 |
| 5’-GGTGATGTCCTCGTCTGTA-’3 | |||
| Osteopontin (sheep) | AF152416.1 | 5’-CTGATTTTCCCACTGACATT-’3 | 354 |
| 5’-CTATGGAATTCTTGGCTGAG-’3 | |||
| Osteonectin (human and sheep) | NM_001166202.1 | 5’-CGACTCTTCCTGCCACTTCT-’3 | 63 |
| 5’-TTGTGGCCCTTCTTGGT-’3 | |||
| Osteocalcin (human) | X51699 | 5’-ATGAGAGCCCTCACACTCCTC-’3 | 293 |
| 3’- GCCGTAGAAGCGCCGATAG-’3 | |||
| Osteocalcin (sheep) | NM_001040009.1 | 5’-AGCTCATCACAGTCAGGGTTG-’3 | 121 |
| 3’- AGCGAGGTGGTGAAGAGA-’3 | |||
| Peroxisome proliferator-activated receptorγ 2 (human) | NM_015869.4 | 5’-GCTGTTATGGGTGAAACTCT-’3 | 116 |
| 5’-TCGCAGGCTCTTTAGAAACTC-’3 | |||
| Peroxisome proliferator-activated receptorγ 2 (sheep) | AY137204.1 | 5’-ATGTCTCATAATGCCATCAGGTT-’3 | 224 |
| 5’-GATAACAAACGGTGATTTGTCTGTC-’3 | |||
| Collagen type 2 (human) | NM_001844 | 5’-CTCCTGGAGCATCTGGAGAC-’3 | 142 |
| 5’-ACCACGATCACCCTTGACTC –’3 | |||
| Collagen type 2 (sheep) | FJ378650 | 5’-CGTCACCTACCACTGCAAGA-’3 | 134 |
| 5’-CGGTGTATGTGAACCTGCTG-’3 | |||
| SRY-box containing gene 9 (human and sheep) | NM_000346.3 | 5’-TCTGAACGAGAGCGAGAAGC-’3 | 81 |
| 5’-GTAATCCGGGTGGTCCTTCT-’3 | |||
| PECAM-1 (human) | NM_000442 | 5’-CAGTCAGAGTCATTCTTGCC-’3 | 176 |
| 5’-GTTGTTGGAGTTCAGAAGTGG-’3 | |||
| PECAM-1 (sheep) | GQ268014.1 | 5’-GTTCAGCGAAGTTCTGCGAG –’3 | 228 |
| 5’-CTTGCTGGCTGTGGTCTTGT-’3 | |||
| Glyceraldehyde-3-phosphate Dehydrogenase (human and sheep) | NM_002046 | 5’-TGCACCACCAACTGCTTAG-’3 | 172 |
| 5’-GATGCAGGGATGATGTTC-’3 |
Immunohistochemistry on Mitral Valve Leaflets
Mitral valve specimens were obtained from sham-operated sheep and sheep in which the mitral valve was subjected to mechanical stretch over 2 months.8 Anterior and posterior leaflets were excised, frozen in OCT compound (Sakura Finetek), sectioned in 6-μm slices and stained by the avidin–biotin–peroxidase method.33 ECs were identified with polyclonal anti-CD31 antibody (BD Biosciences). Adjacent sections were stained with murine monoclonal antibody against bovine osteocalcin (QED Bioscience). Double-label immunofluorescence was carried out by incubating sections with anti-α-SMA antibody, followed by biotinylated secondary antibody, and Texas Red-conjugated streptavidin (Amersham). Next, sections were treated with an avidin-biotin blocking kit (Vector Laboratories) and stained with anti-CD31 or anti-osteocalcin, then appropriate secondary antibodies, and finally fluorescent-conjugated streptavidin (Amersham). Images were captured with a Nikon DXM1200-F (Nikon Inc) using imaging software Elements (version 3.10).
Results
Endothelial and Mesenchymal Phenotypes of Mitral Valve Clones
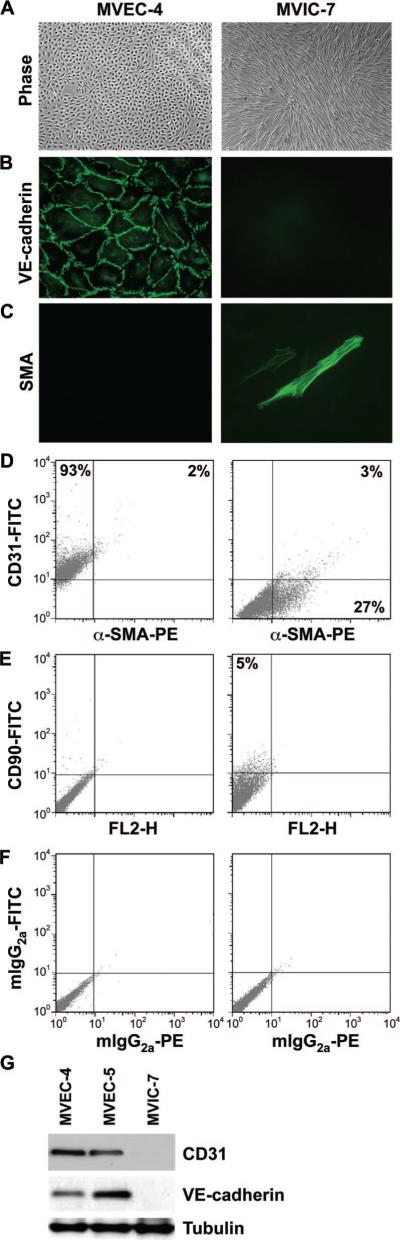
Clonal populations from ovine mitral valves were isolated from anterior and posterior leaflets using a brief collagenase-A procedure used previously for semilunar valves.6,7 Clones with endothelial morphology and one clone with mesenchymal morphology were expanded and characterized (Figure 1 and supplemental Figure I). MVEC clone 4 (MVEC-4) showed a cobblestone endothelial morphology (Figure 1A), VE-cadherin at the cell-cell borders (Figure 1B), and no α-SMA (Figure 1C). Mitral VIC clone 7 (MVIC-7) showed a spindle-shaped morphology, no VE-cadherin (Figure 1B) and some strongly α-SMA–positive cells. Flow cytometry (Figure 1D through 1F) showed MVEC-4 cells were positive for CD31, with only 2% of the cells double-positive for α-SMA, and negative for the mesenchymal marker CD90 (also known as Thy1 antigen). In contrast, MVIC-7 cells were negative for CD31, but 27% of the cells were positive for α-SMA. Only 5% of MVIC-7 cells were positive for CD90, indicating VICs differ in this regard from mesenchymal stem or progenitor cells.31 Negative staining with IgG isotype–matched controls is shown in Figure 1F. A second endothelial clone, MVEC-5, showed the same endothelial phenotype in these assays (data not shown). Western blot for the 2 endothelial markers CD31 and VE-cadherin (Figure 1G) further confirmed the endothelial phenotype of MVEC-4 and MVEC-5 and the nonendothelial character of MVIC-7. In summary, we show by several assays that MVEC-4 and MVEC-5 are bona fide ECs.
Figure 1.
Phenotype of MV clones. A, Phase-contrast images of MVEC-4 and MVIC-7. B and C, Immunofluorescence staining of MVEC-4 and MVIC-7 for VE-cadherin (B) and for α-SMA (C). D, Cells immunostained simultaneously for CD31 and α-SMA and analyzed by flow cytometry. E, Cells stained for CD90 only. F, Cells stained with isotype-matched phycoerythrin- and fluorescein isothiocyanate–conjugated control antibodies. G, Western blot for CD31, VE-cadherin, and tubulin in MVEC-4, MVEC-5, and MVIC-7.
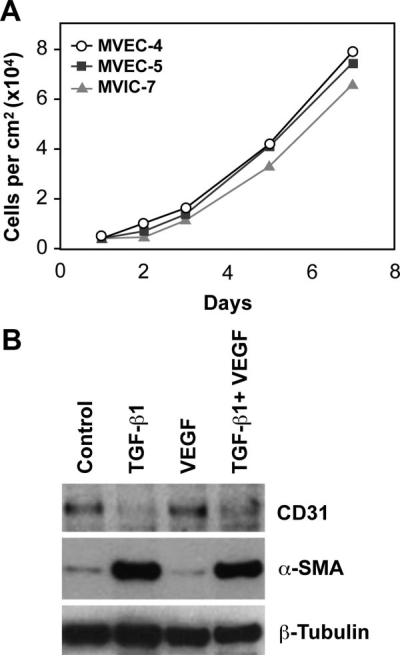
Growth and Differentiation of Mitral Valve ECs
The growth rates of the 3 clones were similar over 7 days, undergoing up to 4 population doublings within 8 days after plating (Figure 2A). In Figure 2B, the ability of MVEC-5 to undergo EMT in response to TGF-β1, in the presence or absence of VEGF-A, was tested to determine whether the cells were similar to pulmonic and aortic valvular ECs.6,7,28 TGF-β1 treatment for 5 days caused a dramatic upregulation of α-SMA and downregulation of CD31, consistent with EMT. VEGF-A did not block TGF-β1-induced EMT, as we had seen previously in some human pulmonary valve clones.6 Similar EMT was observed in MVEC-4 (data not shown).
Figure 2.
Growth and EMT. A, Increase in MVEC-4 (○), MVEC-5 (■), and MVIC-7 (▲) cell number over 7 days. B, MVEC-5 treated with TGF-β1 (2 ng/mL), VEGF-A (10 ng/mL), or both for 5 days and analyzed by Western blot for α-SMA and CD31. β-Tubulin served as a loading control.
Osteogenic, Adipogenic and Chondrogenic Differentiation Potential
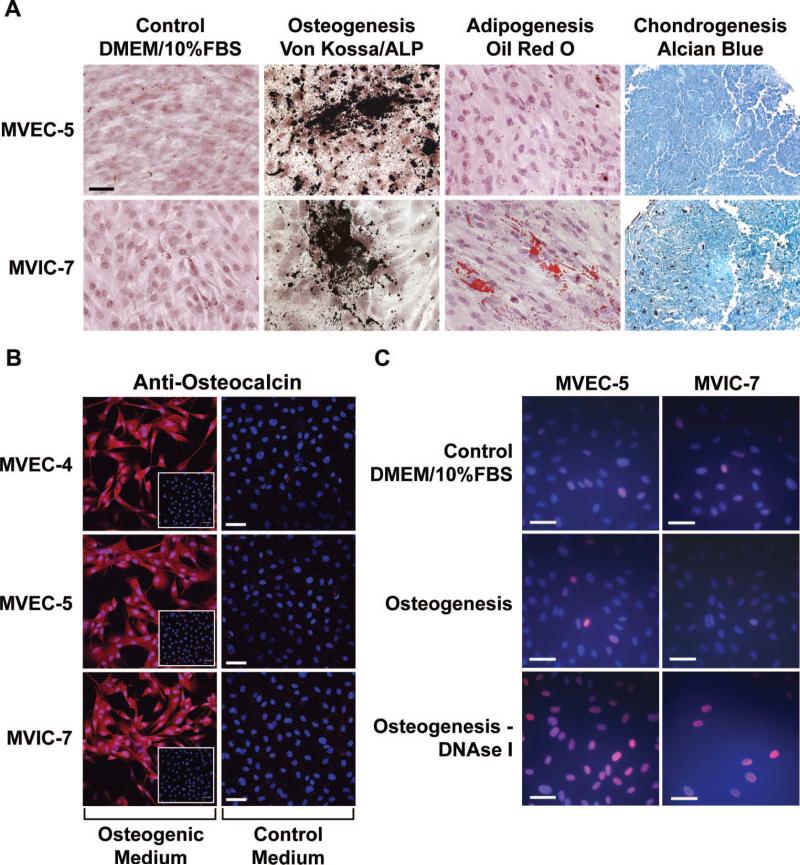
The MVEC clones, in parallel with MVIC-7, were tested for ability to differentiate toward osteogenic, adipogenic and chondrogenic lineages following well-established in vitro protocols34 we used previously.31 After 2 weeks, cultures were stained for cellular markers of differentiation (Figure 3A through 3C). MVEC-5 cells were induced to differentiate toward the osteogenic and chondrogenic lineages. Lipid accumulation indicative of adipogenesis was not detected at the 2 week time point (Figure 3A). MVEC-4 displayed similar osteogenic and chondrogenic differentiation (data not shown). MVIC-7 showed differentiation toward all 3 lineages at the 2 week time point. Expression of osteocalcin protein was confirmed by staining cells incubated in osteogenic versus control medium with anti-osteocalcin (Figure 3B). Insets in Figure 3B show staining with isotype-matched control IgG. To determine whether the mineralization visualized by the von Kossa staining procedure was attributable to apoptotic cells, cultures were subjected to a direct TUNEL-labeling assay (Figure 3C) using an In Situ Cell Death Detection Kit (Roche Inc). None of the cultures, differentiated or control, endothelial or interstitial, showed appreciable numbers of TUNEL-positive cells.
Figure 3.
Mesenchymal differentiation potential. A, MVEC-5 and MVIC-7 in control, osteogenic, adipogenic, and chondrogenic differentiation media for 2 weeks and stained for mineralization (von Kossa/alkaline phosphatase [ALP], lipid accumulation [oil red O], and glycosaminoglycans [Alcian blue)]). Scale bar=50 μm. B, MVEC-4, MVEC-4, and MVIC-7 cells were incubated in osteogenic or control medium for 3 weeks and stained with anti-osteocalcin (10 μg/mL) or isotype-matched IgG1 (insets). Scale bar = 20 μm. C, Apoptosis was measured by TUNEL. Prior treatment with DNAse I served as a positive control. Scale bar=50 μm.
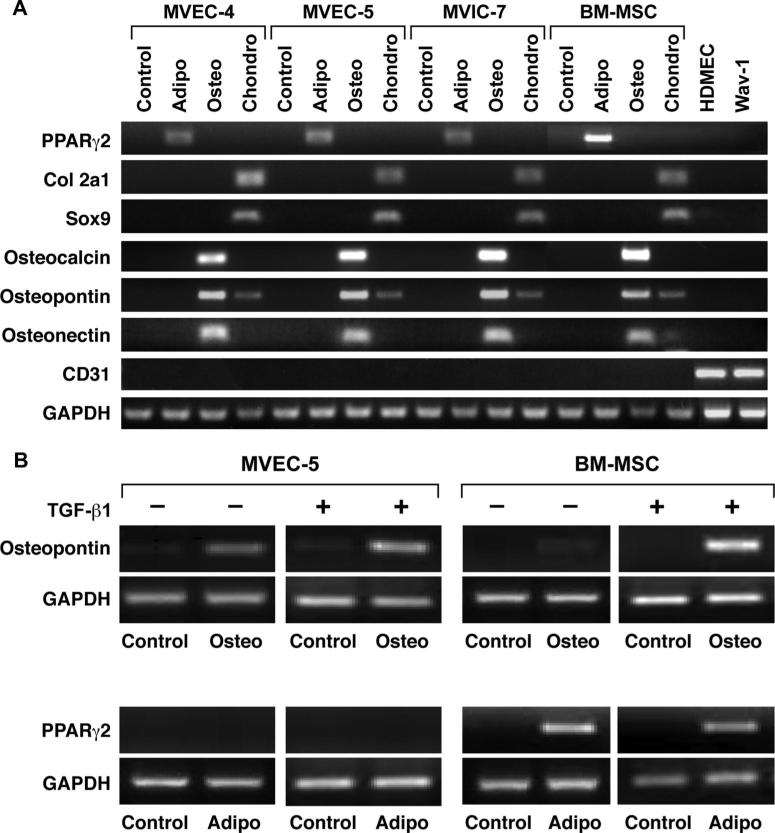
To confirm the mesenchymal differentiation potential, MVEC-4 and MVEC-5, in parallel with MVIC-7 and BM-MSCs, were subjected to differentiation protocols as in Figure 3. Total RNA was isolated after 3 weeks in control or differentiation media, and analyzed by RT-PCR (Figure 4A). Molecular markers for the specific lineages included PPARγ2 for adipocytes, collagen type 2a1 (col2a1) and Sox 9 for chondrocytes and osteocalcin, osteopontin, and osteonectin for osteoblasts. CD31 was amplified to determine whether the MVEC clones had downregulated the endothelial marker. Two controls for human and ovine ECs, HDMECs, and Wav-1 respectively, are shown. Primers used for RT-PCR are provided in the Table. The expression of lineage specific genes confirmed that MVEC-4 and MVEC-5 were induced to undergo chondrogenic and osteogenic differentiation. The high degree of sensitivity of RT-PCR revealed weak PPARγ2 expression after 3 weeks in differentiation medium. MVIC-7 also displayed multilineage differentiation, consistent with previous studies on VICs from aortic valves.23
Figure 4.
Induction of adipogenic, osteogenic, and chondrogenic Markers. A, MVEC-4, MVEC-5, MVIC-7, and BM-MSCs in control, osteogenic, adipogenic, and chondrogenic differentiation media for 3 weeks. RNA was analyzed by RT-PCR; glyceraldehyde phosphate dehydrogenase (GAPDH) served as a control. HDMECs and ovine aortic valve wav-1 served as positive controls for CD31 and negative controls for the lineage-specific markers. B, MVEC-5 and BM-MSCs treated with TGF-β1 for 5 days and then plated in control, osteogenic, or adipogenic differentiation medium for 2 weeks. RT-PCR for osteopontin, PPARγ2, and GAPDH.
To determine whether prior TGF-β-induced EMT would alter or accelerate the mesenchymal lineage differentiation, MVEC-5 cells were treated with TGF-β1 for 5 days before adding osteogenic or adipogenic differentiation media for 2 weeks (Figure 4B). The shorter time point of 2 weeks instead of 3 weeks was chosen to detect accelerated differentiation. TGF-β1 treated MVEC-5 cells were induced to express osteopontin at 2 weeks as were untreated MVEC-5. PPARγ2 was not detected at 2 weeks in TGF-β-treated or untreated MVEC-5 cells. This analysis shows that prior TGF-β1 treatment does not substantially alter differentiation toward the osteogenic lineage.
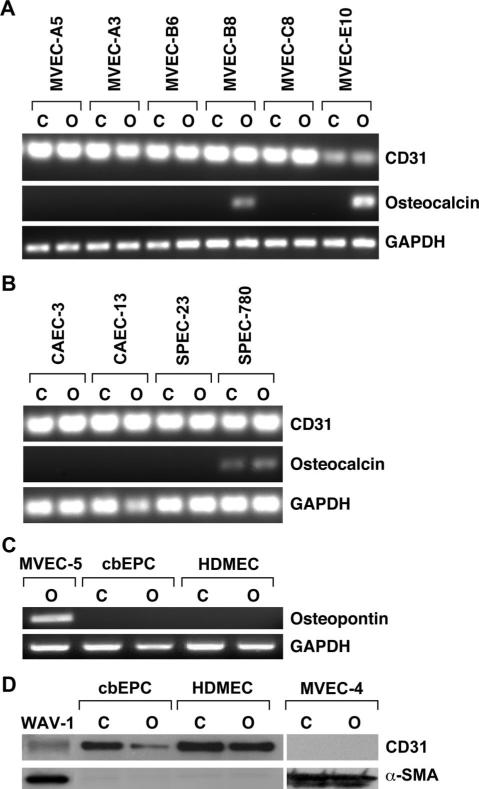
To determine the prevalence of the osteogenic differentiation potential, we screened an additional 6 MVEC clones for osteocalcin expression (Figure 5A). Two of the six, MVEC-B8 and MVEC-E10, were found to express osteocalcin after 3 weeks in differentiation medium. (Phenotypic characterization of these 6 additional clones is provided in supplemental Figure I.) In summary, of 8 MVECs clones analyzed, 4 displayed osteogenic differentiation potential. TGF-β1 induced EMT in 6 of the 8 MVECs clones (data not shown), indicating that 2 clones were able to undergo EMT but not osteogenesis. To address the specificity of the osteogenic differentiation, we analyzed 2 sources of ovine ECs (carotid artery ECs [CAECs] and progenitor ECs [SPECs] isolated from peripheral blood.29 (Phenotypic characterization of CAECs is shown in supplemental Figure II.) These nonvalvular ECs were handled and treated identically to the MVECs. Neither the CAECs nor SPECs showed inducible osteocalcin expression when cultured in osteogenic differentiation medium for 3 weeks (Figure 5B). In addition, we analyzed 2 sources of human ECs (cbEPCs), which we have extensively characterized in terms of endothelial function and vasculogenic potential in vivo27,31 and HDMECs from neonatal foreskin: neither showed osteocalcin expression (Figure 5C). In the same differentiation protocol, we verified by Western blot that cbEPCs and HDMECs retained expression of CD31 and did not begin to express α-SMA. In contrast, MVEC-4 no longer expressed CD31 and had strong expression of α-SMA (Figure 5D). These analyses indicate that osteogenic differentiation is restricted to valvular ECs.
Figure 5.
Additional MVEC clones and nonvalvular ECs. A, Six additional MVEC clonal populations were subjected to control (designated as “C”) or osteogenic (designated as “O”) differentiation. RNA was analyzed by RT-PCR for CD31, osteocalcin, and GAPDH. B, Ovine carotid artery ECs (CAECs) and ovine progenitor ECs (SPECs) were analyzed as in A. C, cbEPCs and HDMECs in control or osteogenic differentiation medium for 3 weeks analyzed for osteopontin and GAPDH. MVEC-5 served as a positive control. D, cbEPCs, HDMECs, and MVEC-4 in control or osteogenic medium for 21 days and analyzed for CD31 and α-SMA by Western blot. Wav-1 cells treated with TGF-β1 served as a positive control for detection of CD31 and α-SMA.
Endothelial-Osteogenic Phenotype in Mitral Valve Leaflets In Vivo
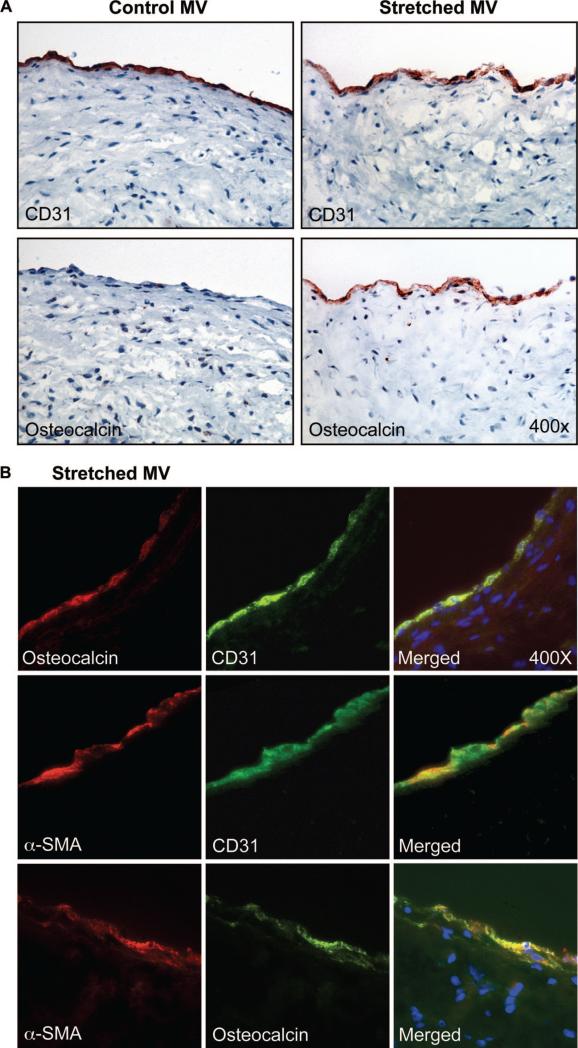
To determine whether the osteogenic differentiation potential of MVECs in culture could be displayed in vivo, mitral valve leaflets from healthy juvenile sheep in which a surgical procedure was used to model the mechanical stretch that occurs in ischemic MR8 were immunostained for CD31 and osteocalcin (Figure 6). In this model, under cardiopulmonary bypass, the papillary muscle tips were retracted apically, short of producing regurgitation. The tethering applied a stretch to the mitral valve leaflets over a 2-month time period; this isolated the effects of mechanical stretch from effects of ischemia or turbulent flow that occur in ischemic MR.8 After 2 months, mitral valve leaflets were sectioned and immunostained with anti-CD31, anti-α-SMA and anti-osteocalcin. Specimens from sham-operated age-matched animals were obtained as well. In stretched valves, sporadic regions along the endothelium along the atrialis stained positive for osteocalcin. Immunostaining for CD31 in adjacent sections confirmed the endothelial phenotype. The endothelium in sham-operated controls was positive for CD31, as expected, but osteocalcin staining was not observed. To gain further insight into the phenotype of the stretched mitral valve endothelium, sections were double-labeled with anti-osteocalcin (red) and anti-CD31 (green) (Figure 6B, top images), anti–α-SMA (red), and anti-CD31 (green) (middle images) or anti–α-SMA (red) and anti-osteocalcin (green) (Figure 6B, bottom images). Merged fluorescent images show colocalization of CD31/osteocalcin, CD31/α-SMA, and α-SMA/osteocalcin. These focal regions of endothelium positive for osteocalcin correlate with the atrial changes, increased α-SMA and collagen deposition, seen in the stretched mitral valve specimens.8 In summary, the expression of endothelial-localized osteocalcin supports the in vitro findings and suggests that the stretch imposed on the mitral valve leaflets over 2 months induced some ECs to express osteogenic markers, which suggests a competency for further differentiation.
Figure 6.
Osteogenic endothelium in vivo. A, Ovine mitral valve leaflets from sham-operated animals and animals in which mechanical stretch was applied to the mitral valve for 2 months were analyzed for CD31 and osteocalcin (n=2) by immunohistochemistry. B, “Stretched” ovine mitral valve leaflets were double-labeled with anti-osteocalcin and anti-CD31 (top images), anti–α-SMA and anti-CD31 (middle images), or with anti–α-SMA and anti-osteocalcin (bottom images). Magnification=×400.
Discussion
We identify an entirely novel capability of MVECs by demonstrating that clonal populations of MVECs readily differentiate toward osteogenic and chondrogenic phenotypes under well-defined protocols.34 Adipogenic differentiation, at the level of cytoplasmic lipid droplets, was not observed although low levels of PPARγ2 transcripts were detected. Treating MVECs first with TGF-β1 to induce EMT did not affect subsequent differentiation capability. We detected no osteogenic differentiation in 4 different normal and robust EC cultures: 2 were ovine, carotid artery ECs (CAECs) and peripheral blood endothelial progenitor cells (SPECs); and 2 were human, cord blood-derived EPCs and neonatal foreskin-derived HDMECs. The detection of osteocalcin-positive endothelium in vivo, in mitral valves subjected to mechanical stretch, supports the relevance of the osteogenic differentiation shown here in mitral valve ECs in vitro. In summary, our results show for the first time the ability of normal valvular ECs to differentiate into specific mesenchymal lineages, and, furthermore, we suggest that this ability is unique to valvular endothelium.
A limitation of our study is that we do not know which region of the MVEC clones were derived from because anterior and posterior leaflets were combined, minced, and digested with collagenase to obtain a single cell population that would be representative of mitral valve. A second limitation is that experiments were conducted with mitral valvular cells; however, similar osteogenic differentiation potential was observed in vitro with an ovine aortic valvular EC clone designated wav-1 (data not shown). A third limitation of the study is the potential for cells to become altered by the in vitro conditions. However, the expression of osteocalcin, a definitive marker for osteoblasts, along the endothelium of mechanically stretched mitral valve leaflets supports our in vitro findings.
In previous studies, we showed that postnatal valvular ECs undergo EMT.6–8,28 We speculated this might reflect a reuse of the embryonic program to replenish VICs. Based on the results shown here, we further speculate that the osteogenic and chondrogenic differentiation potential of MVECs reflects an ability to differentiate into VICs with phenotypes suited for residence in a specific region of the leaflet. Lincoln and Yutzey have described the tendon and bone-like character of cells within the fibrosa, a region of dense collagen fibers, predominantly type I collagen in postnatal valves,35 which is consistent with the bone-forming potential of VICs in aortic valve fibrosa.20,21,23,36 The VICs in the spongiosa layer are thought to exhibit cartilage-like features: abundant production of glycosaminoglycans and cartilage proteoglycans such as aggrecan.14 The transcription factor Sox 9 is needed for expression of cartilage-specific genes in cartilage and in heart valves, and its deletion appears to disrupt the balance of VIC phenotypes toward the osteogenic, calcifying phenotype.37 In the atrialis/ventricularis layers, elastin fibers intermixed with short collagen fibers are thought to confer elasticity and extensibility to these surfaces that face the flow of blood.35 The unique ECM environments of the fibrosa, spongiosa, and atrialis/ventricularis are likely to influence the phenotype of the VICs that reside within the layer, and, in turn, VICs resident in each layer are likely to produce ECM components that are needed to maintain the integrity and structure of the layer. Our data suggest that MVECs can differentiate into VICs with phenotypes that match the fibrosa and spongiosa layers with the valve.
Several previous studies show that VICs have significant osteogenic differentiation potential and can become calcifying cells within aortic valves.21,23,36 Pericytes also show multilineage differentiation and may contribute to ectopic calcification.38 Of note, calcification of mitral valve leaflets is typically observed in the annulus but can extend into the bodies of the leaflets, causing moderate regurgitation and stenosis, which correlate with adverse prognosis.39 In addition, VICs from porcine mitral valve were shown to calcify to a similar degree as aortic VICs.22 Clonal populations of calcifying vascular cells (CVCs), isolated from populations of bovine aortic smooth muscle cells, have been shown to have osteogenic and chondrogenic, but not adipogenic differentiative potential40; the morphology and expression of markers strongly supports a smooth muscle/myofibroblast phenotype for these cells, but expression of endothelial markers was not shown. Here, we show that MVECs are potentially a direct source of osteogenic VICs. This is an entirely unique differentiation capability that appears to be specific to valvular endothelium because it was not detected in healthy nonvalvular ECs. However, clonally isolated prostate tumor ECs have been shown to undergo mesenchymal transition and differentiation toward osteogenic and chondrogenic phenotypes.32 von Kossa staining was strongly detected in prostate tumor blood vessel endothelium providing an in vivo correlate to the in vitro differentiation studies.32 Unlike MVECs that have differentiated toward an osteogenic phenotype (Figures 4A and 5D), prostate tumor EC coexpressed the endothelial marker CD31 and the bone marker alkaline phosphatase, which indicates the tumor ECs did not down-regulate the endothelial program before or on osteogenic differentiation. We speculate that the MVEC differentiation toward osteogenic- and chondrogenic-like VICs represents a regulated physiological process by which the valvular endothelium contributes to valvular homeostasis and repair, whereas the osteogenic phenotype of prostate tumor endothelium represents an abnormality caused by the tumor environment.
In summary, our results reveal the multi-mesenchymal lineage differentiation potential of mitral valvular endothelium. This suggests that valvular endothelium contains a reservoir of progenitor cells with robust self-renewal and ability to differentiate into osteogenic-like and chondrogenic-like VICs that have been described.16,17,35,37 We speculate that the osteogenic differentiation capability may become aggravated in disease or with age and contribute to stiffness within the leaflets and affect function.
Supplementary Material
Supplemental Figure I
Flow cytometric analyses of mitral valve EC clones shown in Figure 5A. Clones shown to undergo osteogenesis in Figure 5A are denoted by +.
Supplemental Figure II
Flow cytometric analyses of ovine carotid artery EC clones shown in Figure 5B. Osteogenesis was not detected.
Acknowledgments
We thank Dr Juan Melero-Martin, Department of Cardiac Surgery, Children's Hospital Boston, for providing ovine carotid artery ECs.
Sources of Funding
This work was supported initially by NIH grant HL060490 and then by Leducq Fondation Mitral Network grant 07CVD04.
Footnotes
Disclosures
None.
References
- 1.Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development. Int Rev Cytol. 2005;243:287–335. doi: 10.1016/S0074-7696(05)43005-3. [DOI] [PubMed] [Google Scholar]
- 2.Combs MD, Yutzey KE. Heart valve development: regulatory networks in development and disease. Circ Res. 2009;105:408–421. doi: 10.1161/CIRCRESAHA.109.201566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arciniegas E, Sutton AB, Allen TD, Schor AM. Transforming growth factor beta 1 promotes the differentiation of endothelial cells into smooth muscle-like cells in vitro. J Cell Sci. 1992;103(Pt 2):521–529. doi: 10.1242/jcs.103.2.521. [DOI] [PubMed] [Google Scholar]
- 4.Frid MG, Kale VA, Stenmark KR. Mature vascular endothelium can give rise to smooth muscle cells via endothelial-mesenchymal transdifferentiation: in vitro analysis. Circ Res. 2002;90:1189–1196. doi: 10.1161/01.res.0000021432.70309.28. [DOI] [PubMed] [Google Scholar]
- 5.Ishisaki A, Hayashi H, Li AJ, Imamura T. Human umbilical vein endothelium-derived cells retain potential to differentiate into smooth muscle-like cells. J Biol Chem. 2003;278:1303–1309. doi: 10.1074/jbc.M207329200. [DOI] [PubMed] [Google Scholar]
- 6.Paruchuri S, Yang JH, Aikawa E, Melero-Martin JM, Khan ZA, Loukogeorgakis S, Schoen FJ, Bischoff J. Human pulmonary valve progenitor cells exhibit endothelial/mesenchymal plasticity in response to vascular endothelial growth factor-A and transforming growth factor-beta2. Circ Res. 2006;99:861–869. doi: 10.1161/01.RES.0000245188.41002.2c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paranya G, Vineberg S, Dvorin E, Kaushal S, Roth SJ, Rabkin E, Schoen FJ, Bischoff J. Aortic valve endothelial cells undergo transforming growth factor-beta-mediated and non-transforming growth factor-beta-mediated transdifferentiation in vitro. Am J Pathol. 2001;159:1335–1343. doi: 10.1016/s0002-9440(10)62520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dal-Bianco JP, Aikawa E, Bischoff J, Guerrero JL, Handschumacher MD, Sullivan S, Johnson B, Titus JS, Iwamoto Y, Wylie-Sears J, Levine RA, Carpentier A. Active adaptation of the tethered mitral valve: insights into a compensatory mechanism for functional mitral regurgitation. Circulation. 2009;120:334–342. doi: 10.1161/CIRCULATIONAHA.108.846782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaput M, Handschumacher MD, Tournoux F, Hua L, Guerrero JL, Vlahakes GJ, Levine RA. Mitral leaflet adaptation to ventricular remodeling: occurrence and adequacy in patients with functional mitral regurgitation. Circulation. 2008;118:845–852. doi: 10.1161/CIRCULATIONAHA.107.749440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoen FJ. Evolving concepts of cardiac valve dynamics: the continuum of development, functional structure, pathobiology, and tissue engineering. Circulation. 2008;118:1864–1880. doi: 10.1161/CIRCULATIONAHA.108.805911. [DOI] [PubMed] [Google Scholar]
- 11.Lincoln J, Alfieri CM, Yutzey KE. BMP and FGF regulatory pathways control cell lineage diversification of heart valve precursor cells. Dev Biol. 2006;292:292–302. doi: 10.1016/j.ydbio.2005.12.042. [DOI] [PubMed] [Google Scholar]
- 12.Wirrig EE, Snarr BS, Chintalapudi MR, O'Neal JL, Phelps AL, Barth JL, Fresco VM, Kern CB, Mjaatvedt CH, Toole BP, Hoffman S, Trusk TC, Argraves WS, Wessels A. Cartilage link protein 1 (CRTL1), an extra-cellular matrix component playing an important role in heart development. Dev Biol. 2007;310:291–303. doi: 10.1016/j.ydbio.2007.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao B, Etter L, Hinton RB, Jr, Benson DW. BMP and FGF regulatory pathways in semilunar valve precursor cells. Dev Dyn. 2007;236:971–980. doi: 10.1002/dvdy.21097. [DOI] [PubMed] [Google Scholar]
- 14.Hinton RB, Jr, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res. 2006;98:1431–1438. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- 15.Levay AK, Peacock JD, Lu Y, Koch M, Hinton RB, Jr, Kadler KE, Lincoln J. Scleraxis is required for cell lineage differentiation and extra-cellular matrix remodeling during murine heart valve formation in vivo. Circ Res. 2008;103:948–956. doi: 10.1161/CIRCRESAHA.108.177238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lincoln J, Kist R, Scherer G, Yutzey KE. Sox9 is required for precursor cell expansion and extracellular matrix organization during mouse heart valve development. Dev Biol. 2007;305:120–132. doi: 10.1016/j.ydbio.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu AC, Joag VR, Gotlieb AI. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. Am J Pathol. 2007;171:1407–1418. doi: 10.2353/ajpath.2007.070251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mohler ER., III Mechanisms of aortic valve calcification. Am J Cardiol. 2004;94:1396–1402. doi: 10.1016/j.amjcard.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 19.Mohler ER, III, Chawla MK, Chang AW, Vyavahare N, Levy RJ, Graham L, Gannon FH. Identification and characterization of calcifying valve cells from human and canine aortic valves. J Heart Valve Dis. 1999;8:254–260. [PubMed] [Google Scholar]
- 20.Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–2184. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osman L, Yacoub MH, Latif N, Amrani M, Chester AH. Role of human valve interstitial cells in valve calcification and their response to atorvastatin. Circulation. 2006;114(suppl I):I–547–I-552. doi: 10.1161/CIRCULATIONAHA.105.001115. [DOI] [PubMed] [Google Scholar]
- 22.Bouchard-Martel J, Roussel E, Drolet MC, Arsenault M, Couet J. Inter-stitial cells from left-sided heart valves display more calcification potential than from right-sided valves: an in-vitro study of porcine valves. J Heart Valve Dis. 2009;18:421–428. [PubMed] [Google Scholar]
- 23.Chen JH, Yip CY, Sone ED, Simmons CA. Identification and characterization of aortic valve mesenchymal progenitor cells with robust osteogenic calcification potential. Am J Pathol. 2009;174:1109–1119. doi: 10.2353/ajpath.2009.080750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chakraborty S, Cheek J, Sakthivel B, Aronow BJ, Yutzey KE. Shared gene expression profiles in developing heart valves and osteoblast progenitor cells. Physiol Genomics. 2008;35:75–85. doi: 10.1152/physiolgenomics.90212.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Latif N, Sarathchandra P, Thomas PS, Antoniw J, Batten P, Chester AH, Taylor PM, Yacoub MH. Characterization of structural and signaling molecules by human valve interstitial cells and comparison to human mesenchymal stem cells. J Heart Valve Dis. 2007;16:56–66. [PubMed] [Google Scholar]
- 26.Visconti RP, Ebihara Y, Larue AC, Fleming PA, McQuinn TC, Masuya M, Minamiguchi H, Markwald RR, Ogawa M, Drake CJ. An in vivo analysis of hematopoietic stem cell potential. Hematopoietic origin of cardiac valve interstitial cells. Circ Res. 2006;98:690–696. doi: 10.1161/01.RES.0000207384.81818.d4. [DOI] [PubMed] [Google Scholar]
- 27.Melero-Martin JM, Khan ZA, Picard A, Wu X, Paruchuri S, Bischoff J. In vivo vasculogenic potential of human blood-derived endothelial progenitor cells. Blood. 2007;109:4761–4768. doi: 10.1182/blood-2006-12-062471. [DOI] [PubMed] [Google Scholar]
- 28.Yang JH, Wylie-Sears J, Bischoff J. Opposing actions of notch1 and vegf in post-natal cardiac valve endothelial cells. Biochem Biophys Res Commun. 2008;374:512–516. doi: 10.1016/j.bbrc.2008.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaushal S, Amiel GE, Guleserian KJ, Shapira OM, Perry T, Sutherland FW, Rabkin E, Moran AM, Schoen FJ, Atala A, Soker S, Bischoff J, Mayer JE., Jr Functional small-diameter neovessels created using endothelial progenitor cells expanded ex vivo. Nat Med. 2001;7:1035–1040. doi: 10.1038/nm0901-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kraling BM, Bischoff J. A simplified method for growth of human microvascular endothelial cells results in decreased senescence and continued responsiveness to cytokines and growth factors. In Vitro Cell Dev Biol Anim. 1998;34:308–315. doi: 10.1007/s11626-998-0007-z. [DOI] [PubMed] [Google Scholar]
- 31.Melero-Martin JM, De Obaldia ME, Kang SY, Khan ZA, Yuan L, Oettgen P, Bischoff J. Engineering robust and functional vascular networks in vivo with human adult and cord blood-derived progenitor cells. Circ Res. 2008;103:194–202. doi: 10.1161/CIRCRESAHA.108.178590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dudley AC, Khan ZA, Shih SC, Kang SY, Zwaans BM, Bischoff J, Klagsbrun M. Calcification of multipotent prostate tumor endothelium. Cancer Cell. 2008;14:201–211. doi: 10.1016/j.ccr.2008.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aikawa E, Whittaker P, Farber M, Mendelson K, Padera RF, Aikawa M, Schoen FJ. Human semilunar cardiac valve remodeling by activated cells from fetus to adult: implications for postnatal adaptation, pathology, and tissue engineering. Circulation. 2006;113:1344–1352. doi: 10.1161/CIRCULATIONAHA.105.591768. [DOI] [PubMed] [Google Scholar]
- 34.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 35.Lincoln J, Lange AW, Yutzey KE. Hearts and bones: shared regulatory mechanisms in heart valve, cartilage, tendon, and bone development. Dev Biol. 2006;294:292–302. doi: 10.1016/j.ydbio.2006.03.027. [DOI] [PubMed] [Google Scholar]
- 36.Simmons CA, Grant GR, Manduchi E, Davies PF. Spatial heterogeneity of endothelial phenotypes correlates with side-specific vulnerability to calcification in normal porcine aortic valves. Circ Res. 2005;96:792–799. doi: 10.1161/01.RES.0000161998.92009.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peacock JD, Levay AK, Gillaspie DB, Tao G, Lincoln J. Reduced sox9 function promotes heart valve calcification phenotypes in vivo. Circ Res. 2010;106:712–719. doi: 10.1161/CIRCRESAHA.109.213702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Collett GD, Canfield AE. Angiogenesis and pericytes in the initiation of ectopic calcification. Circ Res. 2005;96:930–938. doi: 10.1161/01.RES.0000163634.51301.0d. [DOI] [PubMed] [Google Scholar]
- 39.Volzke H, Haring R, Lorbeer R, Wallaschofski H, Reffelmann T, Empen K, Rettig R, John U, Felix SB, Dorr M. Heart valve sclerosis predicts all-cause and cardiovascular mortality. Atherosclerosis. 2010;209:606–610. doi: 10.1016/j.atherosclerosis.2009.10.030. [DOI] [PubMed] [Google Scholar]
- 40.Tintut Y, Alfonso Z, Saini T, Radcliff K, Watson K, Bostrom K, Demer LL. Multilineage potential of cells from the artery wall. Circulation. 2003;108:2505–2510. doi: 10.1161/01.CIR.0000096485.64373.C5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure I
Flow cytometric analyses of mitral valve EC clones shown in Figure 5A. Clones shown to undergo osteogenesis in Figure 5A are denoted by +.
Supplemental Figure II
Flow cytometric analyses of ovine carotid artery EC clones shown in Figure 5B. Osteogenesis was not detected.