Abstract
Elongation factor-2 kinase (eEF-2 kinase, also known as calmodulin-dependent protein kinase III), is a unique calcium/calmodulin-dependent enzyme that inhibits protein synthesis by phosphorylating and inactivating elongation factor-2 (eEF-2). We previously reported that expression/activity of eEF-2 kinase was up-regulated in several types of malignancies including Gliomas, and was associated with response of tumor cells to certain therapeutic stress. In the current study, we sought to determine whether eEF-2 kinase expression affected sensitivity of glioma cells to treatment with tumor the necrosis factor-related apoptosis-inducing ligand (TRAIL), a targeted therapy able to induce apoptosis in cancer cells but causes no toxicity in most normal cells. We found that inhibition of eEF-2 kinase by RNA interference (RNAi) or by a pharmacological inhibitor (NH125) enhanced TRAIL-induced apoptosis in the human glioma cells, as evidenced by an increase in apoptosis in the tumor cells treated with eEF-2 kinase siRNA or the eEF-2 kinase inhibitor. We further demonstrated that sensitization of tumor cells to TRAIL was accompanied by a down-regulation of the anti-apoptotic protein, Bcl-xL, and that overexpression of Bcl-xL could abrogate the sensitizing effect of inhibiting eEF-2 kinase on TRAIL. The results of this study may help devise a new therapeutic strategy for enhancing the efficacy of TRAIL against malignant glioma by targeting eEF-2 kinase.
Keywords: eEF-2 kinase, TRAIL, Bcl-xl, apoptosis, glioblastoma
1. Introduction
Glioblastoma multiforme (GBM), one of the common childhood malignancies and a cancer of increasing significance in adults, is a fatal disease characterized by survival of glioma cells following initial treatment, invasion through the brain, and ultimately resistance to treatments [1]. To improve the treatment outcome of this devastating disease, new therapies are urgently needed. The tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is considered a promising candidate as an anticancer agent based on its ability to trigger rapid apoptosis and its specific cytotoxicity in malignant cells [2]. TRAIL belongs to the TNF family, and is able to induce caspase-8-dependent apoptosis through its binding to the death receptors DR4 (TRAIL-RI) and DR5 (TRAIL-RII) to form the death-inducing signaling complex (DISC) and to trigger the recruitment of Fas-Associated protein with Death Domain (FADD) [3]. Nevertheless, despite expression of TRAIL receptor in tumor cells, cellular insensitivity to TRAIL-induced apoptosis is still often encountered [4]. How tumor cells become insensitive to TRAIL and how to sensitize tumor cells to this therapy remain poorly understood.
Elongation factor-2 (eEF-2) kinase is an essential enzyme that participates in regulation of protein synthesis under stressful conditions. This kinase phosphorylates eEF-2, a 100 kDa protein that promotes ribosomal translocation from the A to the P-site, which is the reaction that induces movement of mRNA along the ribosome during translation[5]. Phosphorylation of eEF-2 at Thr56 by eEF-2 kinase terminates peptide elongation by decreasing the affinity of eEF-2 for the ribosome. Previous studies found that the activity and expression of eEF-2 kinase is up-regulated in several types of cancers including glioma and breast cancer [6, 7], and that inhibiting this kinase results in a decreased viability of tumor cells [8]. We have recently reported that inactivation of eEF-2 kinase suppresses autophagy but promotes apoptosis under various stresses such as nutrient deprivation, growth factor inhibition [9], Akt inhibition [10] and energy stress caused by the glycolytic inhibitor, 2-deoxy-D-glucose [11]. These studies suggest that over-expression of eEF-2 kinase in tumor cells may contribute to apoptosis resistance, which is often associated with failure of cancer therapy. Therefore, in this study we intended to determine the effects of eEF-2 kinase activity on sensitivity of glioma cells to TRAIL. We found that inhibiting eEF-2 kinase by genetic or pharmacologic approaches can sensitize glioma cells to TRAIL treatment, and this sensitizing effect is associated with the down-regulation of the anti-apoptotic protein Bcl-xL.
2. Materials and Methods
2.1. Cell lines and culture
The human glioma cell lines U251 and T98G were purchased from American Type Culture Collection (Manassas, VA, USA); the normal human astrocyte cell line, SVGp12, was a kind gift from Dr. James Connor (Penn State College of Medicine). T98G cells were cultured in Ham’s F-10: DMEM (10:1) medium; U251 and SVGp12 cells were cultured in DMEM medium. All of the cell culture media were supplemented with 10% fetal bovine serum, 100 units/mL penicillin, and 100 µg/mL streptomycin. Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2/95% air. All cultures were monitored routinely and found to be free of contamination by mycoplasma or fungi. All cell lines were discarded after three months and new lines propagated from frozen stocks.
2.2. Reagents and antibodies
Recombinant human TRAIL was purchased from PeproTech (Rocky Hill, NJ); 1-Hexadecyl-2-methyl-3-(phenyl methyl)-1H-imidazolium iodide (NH125) and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were purchased from Sigma (St Louis, MO, USA) ; antibodies to PARP, Caspase 8, phospho-eEF-2 (Thr56), eEF-2 kinase were purchased from Cell Signaling Technology (Danvers, MA, USA); antibodies to Mcl-1, XIAP, and Bcl-xL were purchased from BD Biosciences (San Diego, CA, USA). α-Tubulin antibody was obtained from Santa Cruz (Santa Cruz, CA, USA); Antibody Microarray Assay Kit was obtained from Full Moon Biosystems (Sunnyvale, CA, USA). All of the cell culture media and other reagents were from Invitrogen (Carlsbad, CA, USA).
2.3. siRNA transfection
siRNA duplexes targeting eEF-2 kinase and control siRNA were prepared by Dharmacon RNAi Technologies (Lafayette, CO, USA). Transfection of siRNA was performed according to the manufacturer’s protocol. Briefly, cells in exponential phase of growth were plated in six-well cell culture plates at 1×105 cells per well, grown for 24 h, and then transfected with siRNA using Oligofectamine and OPTI MEMI-reduced serum medium (Invitrogen, Carlsbad, CA). The concentrations of siRNA were chosen based on dose-response studies.
2.4. Bcl-xL plasmid and transfection
U251 and T98G cells subjected to inhibition of eEF-2 kinase were transiently transfected with an empty vector or a Bcl-xL-expressing plasmid (a gift from Dr Wang HG, Penn State College of Medicine) using FuGENE 6 transfection reagent (Roche, Indianapolis, IN, USA) according to the manufacturer’s instructions. Twenty - four hours after transfection, the transfected tumor cells were treated with TRAIL and used for further experiments. The overexpression of Bcl-xL was confirmed by Western blotting.
2.5. Western blot
Cells were lysed in M-PER mammalian protein extraction reagent (Pierce Biotechnology, Inc., Rockford, IL) supplemented with a protease inhibitor cocktail (Roche, Indianapolis, IN, USA), followed by centrifugation at 14,000× g for 10 min. At the end of centrifugation, cell lysates were collected and protein concentrations of cell lysates were measured. Protein (10 – 20µg) were resolved by SDS-PAGE, and then transferred to PVDF membrane (Bio-Rad Hercules, CA, USA). The blots were incubated with primary antibodies in 3% BSA/TBST at 4°C overnight, followed by incubation with secondary antibodies at room temperature for 1 h. The protein signals were detected by ECL method.
2.6. Apoptosis assays
Apoptosis was assayed by: 1) flow cytometric analysis of Annexin V and 7-AAD staining. Briefly, 100 µl of Guava Nexin reagent (Millipore, Bedford, MA, USA) was added to 1×105 cells (in 100 µl) and the cells were incubated with the reagent for 20 min at room temperature in the dark. At the end of incubation, the cells were analyzed by a Guava 16 EasyCyte™ Plus FlowCytometry System (Millipore); 2) Western blot analysis of the cleaved PARP and caspase-8.
2.7. Cell viability assay
Cell viability was measured by MTT assay. Briefly, cells were plated at a density of 5×103 cells per well on 96-well plates and subjected to different treatment. Following 48 h incubation at 37°C in a humidified atmosphere containing 5% CO2/95% air, the cells were incubated for another 4 h with MTT reagent. The formazan product was dissolved in DMSO and read at 570 nm on a Victor3 Multi Label plate reader (PerkinElmer, Boston, MA, USA).
2.8. Statistical analysis
All data are expressed as means ± standard deviation (SD), and the data were compared using the Student’s t-test. The results considered significant at P < 0.05 (*) or P < 0.01 (**).
3. Results
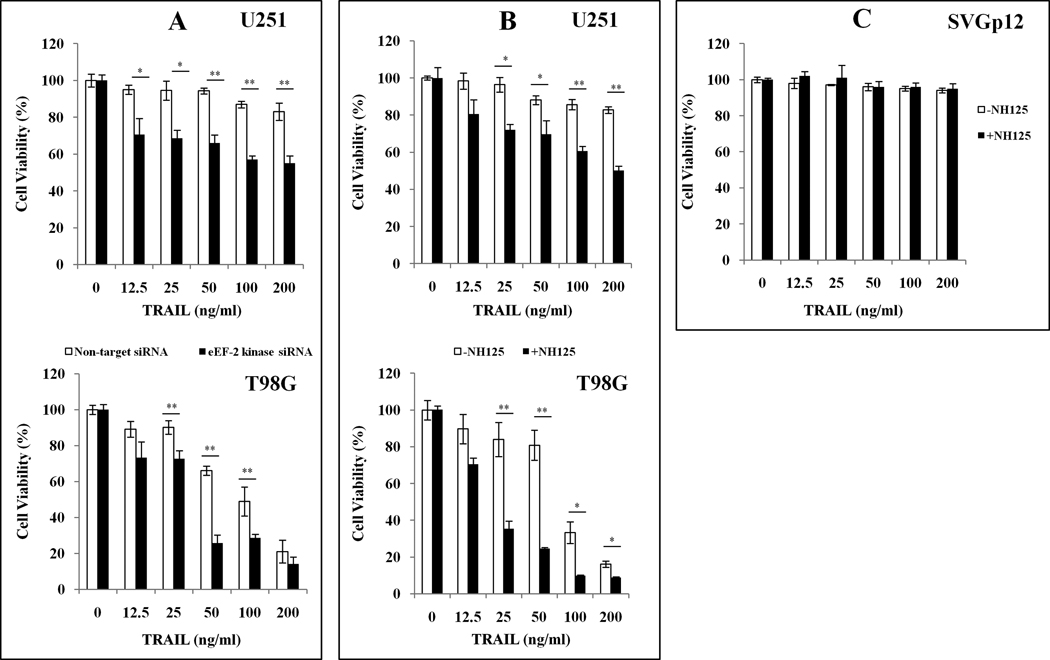
To determine whether or not the increased expression and activity of eEF-2 kinase in tumor cells affected efficacy of TRAIL, we first compared the cellular viability of glioma cells with or without silencing of eEF-2 kinase following treatment with a series of concentrations of TRAIL. Figure 1A shows that silencing of eEF-2 kinase expression by siRNA significantly enhanced the cytotoxicity of TRAIL in the human glioma cell lines, U251 and T98G. Combinatorial treatment with TRAIL and the small molecule inhibitor of eEF-2 kinase, NH125, also sensitized tumor cells to TRAIL, as compared to the treatment with TRAIL alone (Fig. 1B). Neither NH125 and TRAIL alone, nor the combination of NH125 and TRAIL showed cytotoxicity in normal human astrocytes, SVGp12 (Fig. 1C).
Figure.1. Inhibition of eEF-2 kinase enhances sensitivity of glioma cells to TRAIL.
(A) Human glioma cell lines U251 and T98G with or without silencing of eEF-2 kinase expression were treated with the indicated concentrations of TRAIL for 48 h. At the end of treatment, cell viability was measured by MTT assay. (B) U251 and T98G cells were treated with the indicated concentrations of TRAIL for 48 h in the presence or absence of 0.5 µM of NH125. At the end of treatment, cell viability was measured by MTT assay. (C) Normal human astrocytes were treated with the indicated concentrations of TRAIL for 48 h in the presence or absence of NH125 (0.5 µM). At the end of treatment, cell viability was measured by MTT assay. Each point represents mean ± S.D. of triplicate determinations; results shown are the representative of three identical experiments. *p<0.05; **p<0.01, t-test.
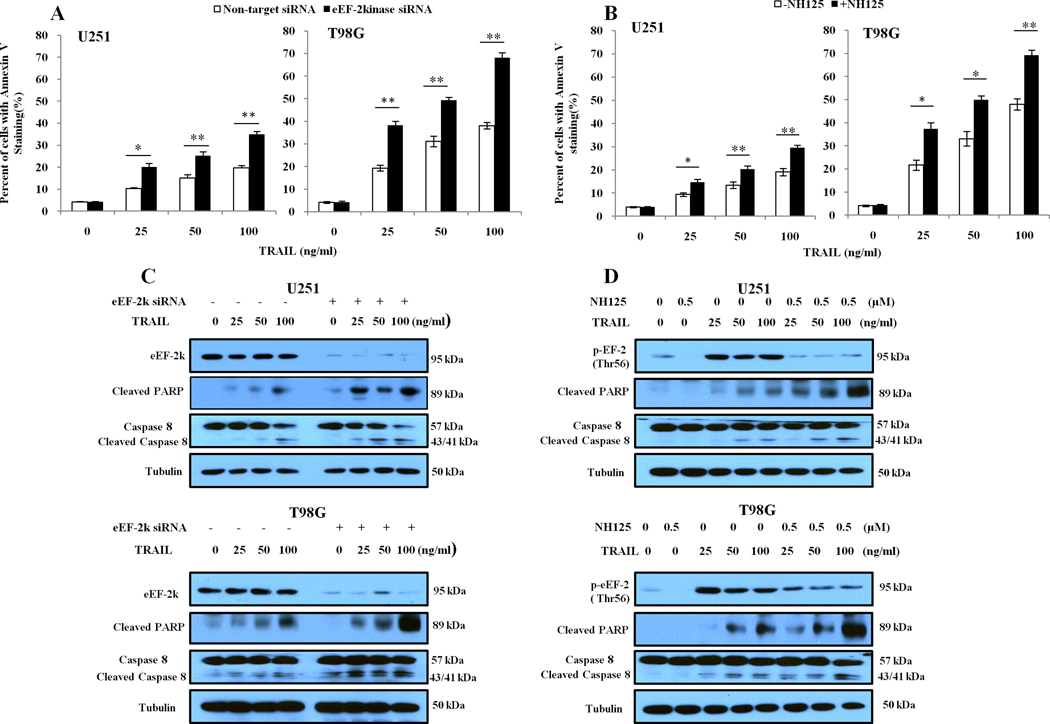
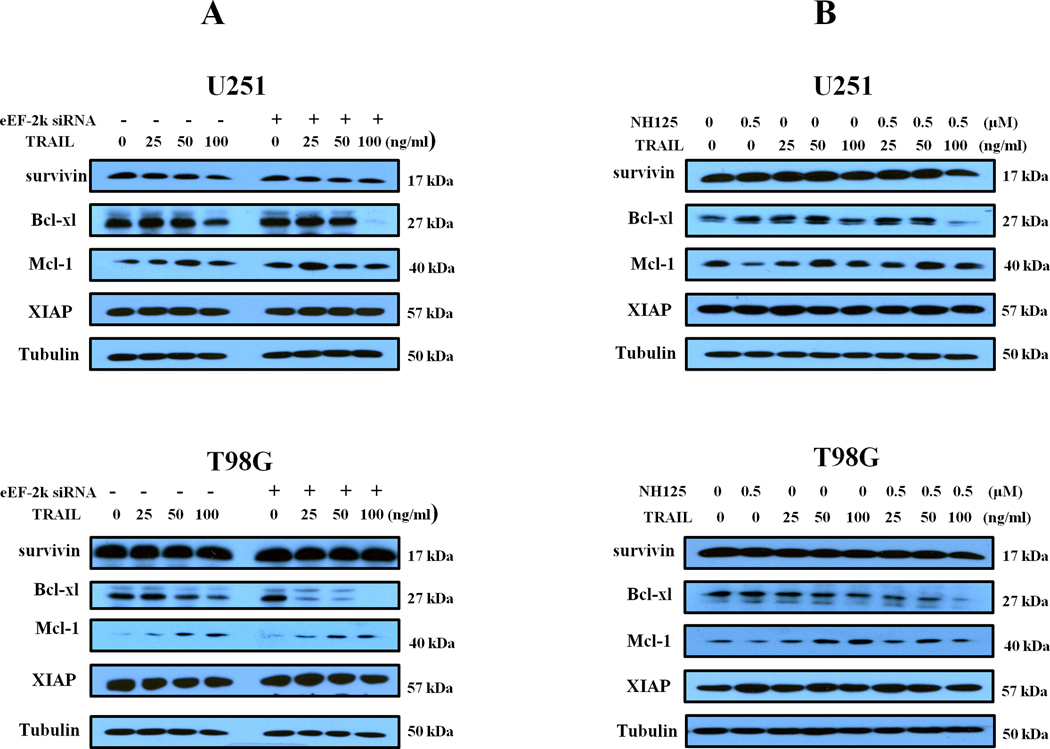
We next examined the effects of inhibiting eEF-2 kinase on TRAIL-induced apoptosis in those glioma cells. We found that both eEF-2 kinase-targeted siRNA and the inhibitor of the kinase, NH125, could significantly augment the apoptosis induced by TRAIL, as indicated by the increases in Annexin V staining (Fig. 2A and 2B). The augmenting effect of eEF-2 kinase inhibition on TRAIL-induced apoptosis was also demonstrated by increases in the levels of cleaved caspase-8 and cleaved PARP (Fig. 2C and Fig. 2D), two biochemical indicators of apoptosis. To further explore pathways possibly involved in the augmentation of TRAIL-induced apoptosis by inactivating eEF-2 kinase, based on our comparisons of the apoptosis-related proteins in glioma cells with or without silencing of eEF-2 kinase expression, which showed differential levels of the proteins known to be involved in resistance to TRAIL, including XIAP, survivin, Bcl-xL, Mcl-1 (Supplemental data), we examined the expression of these anti-apoptotic proteins in the treated cells. Fig. 3 shows that as compared to TRAIL treatment alone, the combination of TRAIL with either eEF-2 kinase-targeted siRNA (Fig. 3A) or NH125 (Fig. 3B) led to a reduction of Bcl-xL protein in U251 and T98G cells, as determined by Western blot. The decrease of Bcl-xL was most remarkable in the glioma cells treated with 100 ng/ml of TRAIL. The expression of survivin, XIAP and Mcl-1 did not appear to be altered in the cells subjected to both TRAIL and inhibition of eEF-2 kinase, as compared to TRAIL treatment alone (Fig. 3).
Figure.2. Inhibition of eEF-2 kinase augments TRAIL-induced apoptosis in glioma cells.
(A) U251 and T98G cells with or without silencing of eEF-2 kinase expression were treated with the indicated concentrations of TRAIL for 24 h. (B) U251 and T98G cells were treated with the indicated concentrations of TRAIL for 24 h in the presence or absence of 0.5 µM of NH125. At the end of treatment, apoptosis was determined by flow cytometric analysis of Annexin V staining (A and B) and Western blot analysis of PARP and cleaved caspase-8 (C and D). α-Tubulin was used as a loading control. Each bar represents mean ± S.D. of triplicate determinations; results shown are the representative of three identical experiments. *p <0.05; **p < 0.01, t-test.
Figure.3. Inhibition of eEF-2 kinase cooperates with TRAIL modulated Bcl-xL expression in glioma cells.
(A) U251 and T98G cells with or without silencing of eEF-2 kinase expression were treated with the indicated concentrations of TRAIL for 24 h. (B) U251 and T98G cells were treated with the indicated concentrations of TRAIL for 24 h in the presence or absence of 0.5 µM of NH125. At the end of treatment, survivin, XIAP, Bcl-xL and Mcl-1 were examined by Western blot. Tubulin was used as a loading control.
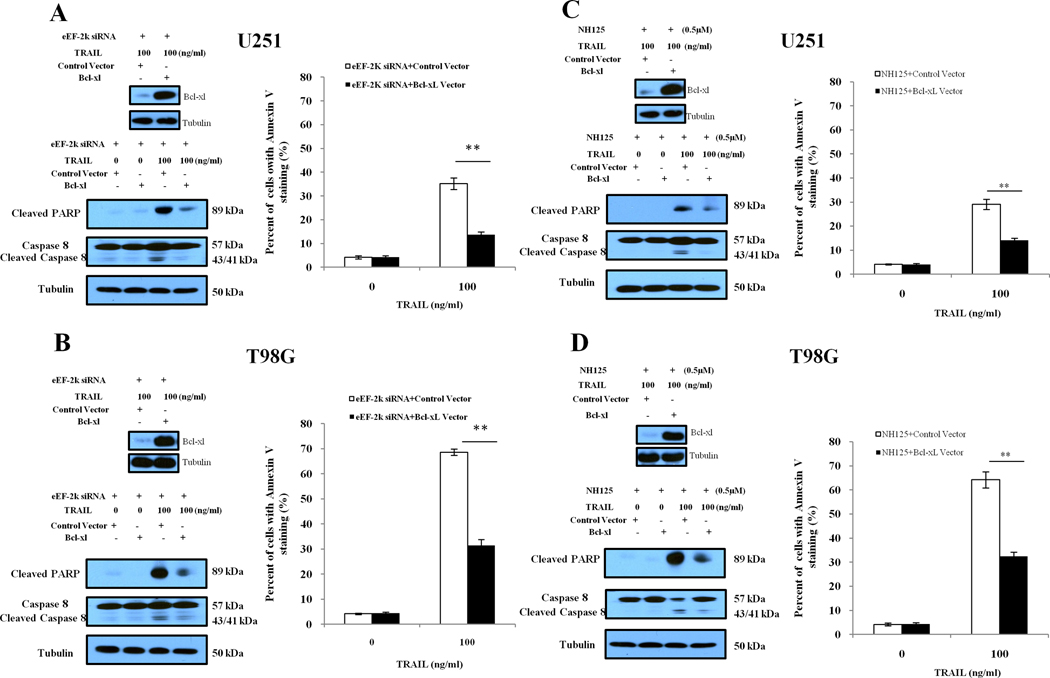
To verify the role of Bcl-xL down-regulation in conferring sensitivity of glioma cells to TRAIL, we transfected U251 and T98G cells with a Bcl-xL-expressing plasmid, and then analyzed apoptosis in the cells co-treated with the inhibitors of eEF-2 kinase and TRAIL or vehicle. Fig. 4 demonstrates that forced expression of Bcl-xL partially blocked the augmentation of TRAIL-induced apoptosis by eEF-2 kinase inhibition (Fig. 4A and 4B: siRNA; Fig. 4C and 4D: NH125), as indicated by the decreases in Annexin V staining and in the amounts of cleaved caspase-8 and PARP. These results suggest that the down-regulation of Bcl-xL is likely to be responsible for the sensitizing effect of inhibiting eEF-2 kinase on TRAIL efficacy.
Figure.4. Forced expression of Bcl-xL diminishes the sensitizing effect of eEF-2 kinase inhibition on TRAIL-induced apoptosis in glioma cells.
(A) U251 and (B) T98G cells with silencing of eEF-2 kinase by siRNA were transfected with an empty control vector or Bcl-xL-expressing plasmids for 24 h, followed by treatment with the indicated concentrations of TRAIL for another 24h. At the end of treatment, cell lysates were prepared and analyzed for protein levels of Bcl-xL, PARP, and cleaved caspase-8 by Western blot analysis. Apoptosis was determined by flow cytometric analysis of Annexin V staining. (C) U251 and (D) T98G cells were transfected with an empty control vector or a Bcl-xL-expressing plasmids for 24 h, followed by treatment with the indicated concentrations of TRAIL and 0.5 µM of NH125 for another 24 h. At the end of treatment, cell lysates were prepared and analyzed for protein levels of Bcl-xL, PARP, and cleaved caspase-8 by Western blot analysis. Apoptosis was determined by flow cytometric analysis of Annexin V staining. Each point represents mean ± S.D. of triplicate determinations; results shown are the representative of three identical experiments. **p < 0.01, t-test.
4. Discussion
Although TRAIL can cause variable cytotoxicity in human cancer cells both in vitro and in vivo [12], approximately one-third of human malignancies are resistant to TRAIL treatment, and an additional one-third only have a moderate response [13]. TRAIL resistance can result from a variety of mechanisms, which can occur at various points in the apoptotic pathway or in other cellular signaling pathways [14, 15]. Here, we report that eEF-2 kinase, a critical regulator of protein synthesis, plays an important role in determining sensitivity of glioma cells to TRAIL, and that inhibiting eEF-2 kinase cooperates with TRAIL in killing glioma cells.
To explore the pathways underlying the sensitizing effect of eEF-2 kinase inhibition on TRAIL-induced apoptosis, we compared the expression of XIAP, survivin, Bcl-xL, and Mcl-1 (Fig. 3), as the balance between the levels of these apoptosis-regulatory proteins are known to be associated with sensitivity of tumor cells to TRAIL [16]. Among those apoptosis-related proteins examined, we found a significant reduction only in the anti-apoptotic protein Bcl-xL, in the cells co-treated with TRAIL and eEF-2 kinase inhibitors (Figure 3), suggesting that the effect of eEF-2 kinase on TRAIL-induced apoptosis might be mediated through modulating Bcl-xL expression. The role of Bcl-xL in altering the sensitivity of tumor cells to TRAIL-induced apoptosis in tumor cells subjected to eEF-2 kinase inhibition was further verified by the experiments showing that forced expression of Bcl-xL blocked the sensitizing effect of NH125 or eEF-2 kinase - targeted siRNA on TRAIL-induced apoptosis (Fig. 4). Nevertheless, the precise mechanism by which eEF-2 kinase regulates Bcl-xL expression remains unclear, and would need further studies. Bcl-xL resides within the mitochondrial membrane where it acts by inhibiting adaptor molecules needed for activation of the effector caspases [17], and is known to suppress apoptosis induced by TRAIL [18, 19, 20] and some other therapeutic insults [21]. Our results provide additional evidence for the critical role of Bcl-xL in inhibiting TRAIL-induced apoptosis.
In order to improve and reinforce the efficacy of TRAIL in cancer therapy, many strategies and approaches to modulating TRAIL sensitivity have been reported. For instance, it has been shown that TRAIL in combination with irinotecan (CPT-11) increased the expression of the pro-apoptotic protein, Bax, but decreased Bcl-xL expression in prostate cancer cells [22]. PS-341, a proteasome inhibitor, was shown to enhance the TRAIL-induced cytotoxicity through decreasing Bcl-2 and Bcl-xL in malignant glioma cells [23]. We show here that inhibiting eEF-2 kinase can significantly enhance glioma cells sensitivity to TRAIL - induced apoptosis, likely via down-regulating the expression of the anti-apoptotic protein, Bcl-xL. Taken together, the results of the current study reveal eEF-2 kinase as a potential new target that can be exploited to reinforce the efficacy of TRAIL in killing tumor cells, and may thus provide a rationale for combined use of TRAIL and an eEF-2 kinase inhibitor as a new therapeutic strategy for malignant glioma or other types of cancers.
Highlights.
> Inhibiting eEF-2 kinase sensitizes human glioma cells to TRAIL therapy. > The enhanced sensitivity to TRAIL is accompanied by down-regulation of Bcl-xL. > Overexpression of Bcl-xL can abrogate this sensitizing effect on TRAIL. > Targeting eEF-2 kinase may represent a new adjuvant therapy with TRAIL.
Supplementary Material
Acknowledgments
This study was supported by a grant (R01CA135038) from the US Public Health Service and by the National Natural Sciences Foundation of China (K113416510).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prados MD, Levin V. Biology and treatment of malignant glioma. Semin Oncol. 2000;27:1–10. [PubMed] [Google Scholar]
- 2.Bellail AC, Qi L, Mulligan P, Chhabra V, Hao C. TRAIL agonists on clinical trials for cancer therapy: the promises and the challenges. Rev Recent Clin Trials. 2009;4:34–41. doi: 10.2174/157488709787047530. [DOI] [PubMed] [Google Scholar]
- 3.Bellail AC, Tse MC, Song JH, Phuphanich S, Olson JJ, Sun SY, Hao C. DR5-mediated DISC controls caspase-8 cleavage and initiation of apoptosis in human glioblastomas. J Cell Mol Med. 2010;14:1303–1317. doi: 10.1111/j.1582-4934.2009.00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hao C, Beguinot F, Condorelli G, Trencia A, Van Meir EG, Yong VW, Parney IF, Roa WH, Petruk KC. Induction and intracellular regulation of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) mediated apotosis in human malignant glioma cells. Cancer Res. 2001;61:1162–1170. [PubMed] [Google Scholar]
- 5.Ryazanov AG, Rudkin BB, Spirin AS. Regulation of protein synthesis at the elongation stage. New insights into the control of gene expression in eukaryotes. FEBS Lett. 1991;285:170–175. doi: 10.1016/0014-5793(91)80798-8. [DOI] [PubMed] [Google Scholar]
- 6.Parmer TG, Ward MD, Yurkow EJ, Vyas VH, Kearney TJ, Hait WN. Activity and regulation by growth factors of calmodulin-dependent protein kinase III (elongation factor 2-kinase) in human breast cancer. Br J Cancer. 1999;79:59–64. doi: 10.1038/sj.bjc.6690012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu H, Yang JM, Jin S, Zhang H, Hait WN. Elongation factor-2 kinase regulates autophagy in human glioblastoma cells. Cancer Res. 2006;66:3015–3023. doi: 10.1158/0008-5472.CAN-05-1554. [DOI] [PubMed] [Google Scholar]
- 8.Zhang L, Zhang Y, Liu XY, Qin ZH, Yang JM. Expression of elongation factor-2 kinase contributes to anoikis resistance and invasion of human glioma cells. Acta Pharmacol Sin. 2011;32:361–367. doi: 10.1038/aps.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng Y, Li H, Ren X, Niu T, Hait WN, Yang J. Cytoprotective effect of the elongation factor-2 kinase-mediated autophagy in breast cancer cells subjected to growth factor inhibition. PLoS One. 5:e9715. doi: 10.1371/journal.pone.0009715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cheng Y, Ren X, Zhang Y, Patel R, Sharma A, Wu H, Robertson GP, Yan L, Rubin E, Yang JM. eEF-2 Kinase Dictates Cross-Talk between Autophagy and Apoptosis Induced by Akt Inhibition, Thereby Modulating Cytotoxicity of Novel Akt Inhibitor MK-2206. Cancer Res. 71:2654–2663. doi: 10.1158/0008-5472.CAN-10-2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu H, Zhu H, Liu DX, Niu TK, Ren X, Patel R, Hait WN, Yang JM. Silencing of elongation factor-2 kinase potentiates the effect of 2-deoxy-D-glucose against human glioma cells through blunting of autophagy. Cancer Res. 2009;69:2453–2460. doi: 10.1158/0008-5472.CAN-08-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fulda S, Meyer E, Debatin KM. Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression. Oncogene. 2002;21:2283–2294. doi: 10.1038/sj.onc.1205258. [DOI] [PubMed] [Google Scholar]
- 13.Buchsbaum DJ, Zhou T, Lobuglio AF. TRAIL receptor-targeted therapy. Future Oncol. 2006;2:493–508. doi: 10.2217/14796694.2.4.493. [DOI] [PubMed] [Google Scholar]
- 14.Dong Y, Yin S, Li J, Jiang C, Ye M, Hu H. Bufadienolide compounds sensitize human breast cancer cells to TRAIL-induced apoptosis via inhibition of STAT3/Mcl-1 pathway. Apoptosis. 2011;16:394–403. doi: 10.1007/s10495-011-0573-5. [DOI] [PubMed] [Google Scholar]
- 15.Karasic TB, Hei TK, Ivanov VN. Disruption of IGF-1R signaling increases TRAIL-induced apoptosis: a new potential therapy for the treatment of melanoma. Exp Cell Res. 2010;316:1994–2007. doi: 10.1016/j.yexcr.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005;12:228–237. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]
- 17.Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science. 1997;275:1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 18.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 19.Liu F, Hu X, Zimmerman M, Waller JL, Wu P, Hayes-Jordan A, Lev D, Liu K. TNFalpha cooperates with IFN-gamma to repress Bcl-xL expression to sensitize metastatic colon carcinoma cells to TRAIL-mediated apoptosis. PLoS One. 2011;6:e16241. doi: 10.1371/journal.pone.0016241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Walensky LD. BCL-2 in the crosshairs: tipping the balance of life and death. Cell Death Differ. 2006;13:1339–1350. doi: 10.1038/sj.cdd.4401992. [DOI] [PubMed] [Google Scholar]
- 21.Griffiths GJ, Koh MY, Brunton VG, Cawthorne C, Reeves NA, Greaves M, Tilby MJ, Pearson DG, Ottley CJ, Workman P, Frame MC, Dive C. Expression of kinase-defective mutants of c-Src in human metastatic colon cancer cells decreases Bcl-xL and increases oxaliplatin- and Fas-induced apoptosis. J Biol Chem. 2004;279:46113–46121. doi: 10.1074/jbc.M408550200. [DOI] [PubMed] [Google Scholar]
- 22.Ray S, Almasan A. Apoptosis induction in prostate cancer cells and xenografts by combined treatment with Apo2 ligand/tumor necrosis factor-related apoptosis-inducing ligand and CPT-11. Cancer Res. 2003;63:4713–4723. [PubMed] [Google Scholar]
- 23.Yin D, Zhou H, Kumagai T, Liu G, Ong JM, Black KL, Koeffler HP. Proteasome inhibitor PS-341 causes cell growth arrest and apoptosis in human glioblastoma multiforme (GBM) Oncogene. 2005;24:344–354. doi: 10.1038/sj.onc.1208225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.