Abstract
We reported a novel interaction between Beclin 1, a key regulator of autophagy, and survivin, a member of the IAP family. We found that knock-down of Beclin 1 down-regulated survivin protein, and the turnover rate of survivin was increased when Beclin 1 expression was silenced. Knock-down of Beclin 1 sensitized glioma cells to TRAIL-induced apoptosis, and introduction of survivin antagonized the sensitizing effect, suggesting that down-regulation of survivin mediates the enhanced sensitivity to TRAIL-induced apoptosis. These results demonstrate a novel interaction between Beclin 1 and survivin, and may provide a potential mechanism underlying the cross-talk between autophagy and apoptosis.
Keywords: apoptosis, autophagy, Beclin 1, survivin, TRAIL
1. Introduction
IAPs are known to play a key role in apoptosis suppression, signal transduction and cell proliferation in tumor cells [1]. Survivin, a structurally unique IAP protein, is the smallest member of the IAP family in mammalian cells and is able to antagonize apoptosis, promote tumor-associated angiogenesis and act as a resistance determinant to various anticancer therapies [2]. Survivin has also been reported to play a pivotal role in oncogenesis and progression of malignant brain tumors [3]. Beclin 1, the mammalian homologue of the yeast Atg6, is a key autophagy-promoting protein that regulates the formation of autophagosome. The function of Beclin 1 has been shown to contribute to inhibition of tumorigenesis in human malignancies [4]. Notably, it has been demonstrated recently that Beclin 1 can interact with the anti-apoptotic protein Bcl-2 and this interaction impacts on the activity of both apoptosis and autophagy [5]. Beclin 1 may exert its pro-survival effects via interactions with either Bcl-2 or Bcl-xL, and Bcl-2 can function as an anti-autophagic protein through its inhibitory interaction with Beclin 1 [6, 7]. These studies indicate that the apoptotic and autophagic machineries might be functionally related and cross-talked. In the current study, we observed a novel interaction between Beclin 1 and survivin. We demonstrated that Beclin 1 was able to physically bind to survivin, and that knockdown of Beclin 1 resulted in a down-regulation of survivin protein. Furthermore, we showed that decrease of Beclin 1 sensitized the human glioma cells, T98G and LN229, to TRAIL-induced apoptosis, and this sensitizing effect appeared to be a consequence of the down-regulation of survivin caused by depletion of Beclin 1. Our finding of the interaction between survivin and Beclin 1 may aid in the elucidation of the mechanisms underlying the cross-talk between autophagy and apoptosis, and provide evidence for develop novel strategies to sensitizing tumor cells to apoptosis inducers such as TRAIL via suppressing Beclin 1 expression.
2. Materials and Methods
2.1. Cell culture
The human glioblastoma cell lines, T98G and LN229, and the human kidney epithelial cell line 293T, were purchased from American Type Culture Collection. The human glioblastoma cell line U251 was a gift from Dr. Jong Yun (Penn State College of Medicine). T98G cells were cultured in Ham's F-10/DMEM 10:1; LN229, U251, and 293T cells were cultured in DMEM. All cell culture media were supplemented with 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin. Cells were maintained at 37°C in a humidified atmosphere containing 5% CO2/95% air.
2.2. Reagents and antibodies
MG132 and cyclohexamide were obtained from Calbiochem. 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma. Recombinant human TRAIL was obtained from PeproTech. All cell culture media and supplements were purchased from Invitrogen. The following antibodies were used in this study: rabbit anti-survivin, anti-Beclin 1, anti-cleaved PARP, anti-cleaved caspase 7, and anti-cleaved caspase 9 (Cell Signaling); mouse anti-flag and anti-tubulin (Sigma); mouse anti-LC3 (Nanotools); mouse anti-caspase 8 (Alexis Biochemicals).
2.3. siRNA transfection
Cells were cultured in 6-well plates at 30% to 40% confluence on the day of transfection. Control or Beclin 1 (Dharmacon, J-010552-05) siRNA at final concentration of 17 nM was mixed with 2.5 μl RNAi MAX (Invitrogen) for each well, according to the manufacturer's protocol. After 72 h of transfection, cells were collected for further experiments.
2.4. Western blotting
Cells were lysed in Complete-M buffer (Roche) supplemented with protease inhibitor cocktail (Roche) at room temperature for 5 minutes followed by centrifugation at 14,000 × g for 10 minutes. Concentrations of protein of the cell lysates were measured by the Bio-Rad DC assay (Bio-Rad), according to the manufacturer's instruction. Protein (20-40 μg) were resolved on SDS-PAGE and transferred to PVDF membrane (Bio-Rad). The blots were incubated with indicated antibodies in 3% BSA/TBST at 4°C for overnight followed by incubation with secondary antibodies at room temperature for 1 h. The signal was detected by ECL (Thermo Scientific).
2.5. Real-time reverse transcription-PCR
Total RNAs were isolated from the treated cells using the TriPure isolation reagent (Roche) according to the manufacturer's instruction. The concentrations of total RNAs were measured using a NanoDrop (Thermo Scientific). First-strand cDNA was synthesized using Omniscript reverse transcription kit (Qiagen) with random primers, according to the manufacturer's instruction. Real-time PCR was performed on a Stratagene Mx3005P using Brilliant II SYBR Green QPCR master mix (Stratagene) and following primer sets: survivin, 5’-CCCGATGACAACCCGATA-3’ (forward) and 5’-CATCTGCTTCTTGACAGTGAG-3’ (reverse); glyceraldehydes-3-phosphate dehydrogenase, 5’-TGCCTCTTTAGTTGTCATGCAG-3’ (forward) and 5’-CCCGTTCAGCTCAGGGATGA-3’ (reverse). After 40 cycles, data were collected and analyzed by MxPro software (Stratagene).
2.6. Pulse – chase experiments
Cells were transfected with a control siRNA or Beclin 1 siRNA for 72 h. Following the transfection, the cells were treated with cyclohexamide (10μg/ml) for 16h, followed by release into cyclohexamide-free media for the indicated times. Cell extracts were prepared and run on 7.5% SDS-PAGE, followed by Western blot using anti-survivin, anti-Beclin 1 or and anti-β-actin antibodies.
2.7. Immunoprecipitation
293T cells grown on 10-cm tissue culture dishes were transfected with a flag-beclin and survivin expression vector using Lipofactamine 2000 (Invitrogen), according to the manufacturer's protocol. After 24 h of transfection, cells were lysed in 1% Triton X-100, 50 mM Tris pH 7.5, 300 mM NaCl, and 5 mM EDTA with protease inhibitor cocktail (Roche), and centrifuged at 14,000 × g for 10 minutes. Supernatant was collected and pre-cleared with Protein A/G agarose beads (Santa Cruz) at 4°C for 30 minutes. Control IgG (rabbit) and specific antibodies were added to lysates and incubated at 4°C for overnight. The mixture was further incubated with 30 μl of Protein A/G agarose beads at 4°C for 2 h. Beads were washed 3 times with 0.1% Triton X-100, 50 mM Tris pH 7.5, 300 mM NaCl, and 5 mM EDTA. Following a final wash with PBS, the samples were mixed with 1x SDS-PAGE loading buffer, heated at 70°C for 10 minutes, and subjected to SDS-PAGE and Western blot analysis.
2.8. Caspase Activity Assay
For assaying the pan - caspase activity, the Guava multi-caspase detection protocol was followed. Briefly, 10 μl of caspase detection reagent was added to 1 × 104 cells (in 100 μl) and the cells were incubated for 1 h at 37 °C with 5% CO2. Following the incubation, the cells were washed twice with washing buffer and then re-suspended in 200 μl of the caspase 7-AAD working solution. The samples were further incubated for 10 min at room temperature, followed by analysis of caspase activity on a Guava flow cytometry.
2.8. Cell viability assay
Cell viability was determined using MTT assay. Briefly, cells were seeded in 96-well tissue culture plates and subjected to different treatments. At the end of treatments, cells were incubated with 20 μl of 5 mg/ml MTT reagent. After 4 h of incubation at 37°C in a humidified atmosphere containing 5% CO2, formazan crystals were dissolved in 200 μl DMSO. Absorbance at 570 nm was measured using a Bio-Tek plate reader.
3. Results and discussion
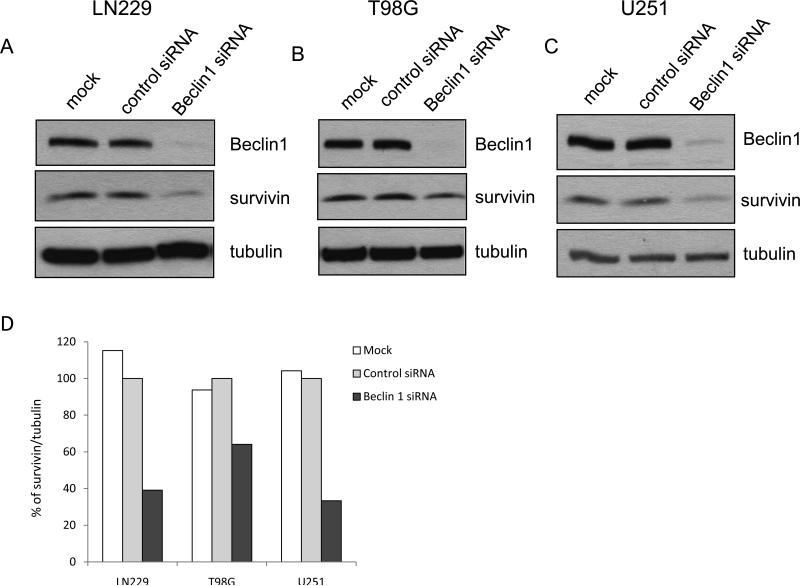
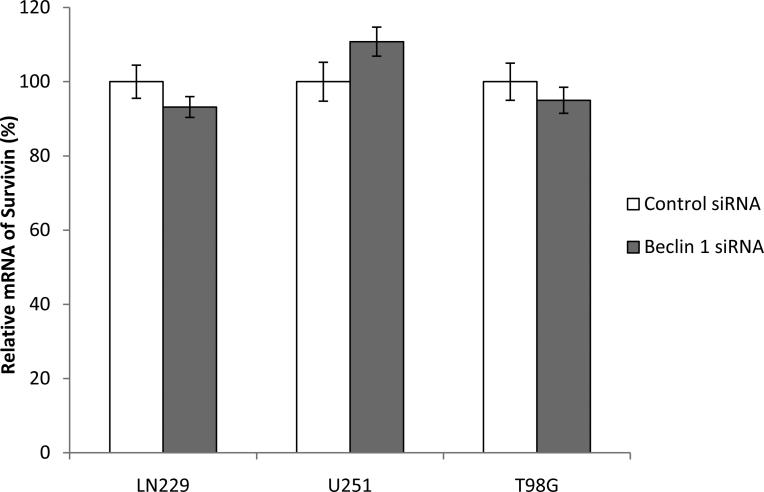
In study of the relationship between regulators of autopahgy and apoptosis, we found in human glioma cell lines LN229, T98G and U251 that when expression of Beclin 1 was silenced using siRNA, the expression of survivin protein was also decreased (50%, 40%, and 70%, respectively), as examined by Western blot (Fig. 1A, B and C). Since transcriptional repression of survivin by tumor suppressors such PTEN and p53 through different mechanisms has been previously reported [8,9], we next asked whether or not the down-regulation of survivin by knock-down of Beclin 1 resulted from transcriptional repression of this anti-apoptotic protein. We performed the real-time RT-PCR analysis of survivin mRNA in cells transfected with a beclin 1-targeted siRNA or a non-targeted siRNA. We found that the mRNA levels of survivin were not decreased in glioma cells with silencing of Beclin 1, as compared with that in non-targeting siRNA-transfected cells (Fig. 2), indicating that the down-regulation of survivin induced by suppression of Beclin 1 expression was transcription-independent.
Figure 1. Knock-down of Beclin 1 down-regulates survivin in human glioma cells.
Human glioma cell lines LN229 (A), T98G (B), and U251 (C) were transfected with null siRNA (mock), a control, non-targeting RNA, or a Beclin 1 siRNA. After 72 h of transfection, cells were collected and subjected to Western blot analysis with the specific antibodies as indicated. Tubulin was used as a loading control. (D) Quantification of panel A, B and C. Results shown are the representative of three identical experiments.
Figure 2. Survivin transcription is not affected by knock-down of Beclin 1.
LN229, U251 and T98G cells were transfected with a control or Beclin 1 siRNA for 72 h; total RNAs from the treated cells were isolated and subjected to quantitative real-time RT-PCR, as described in “Material and Methods”. GAPDH was used as an internal control, and survivin mRNA was normalized to GAPDH. Results shown are the mean ± SD of triplicate determinations from one of three identical experiments.
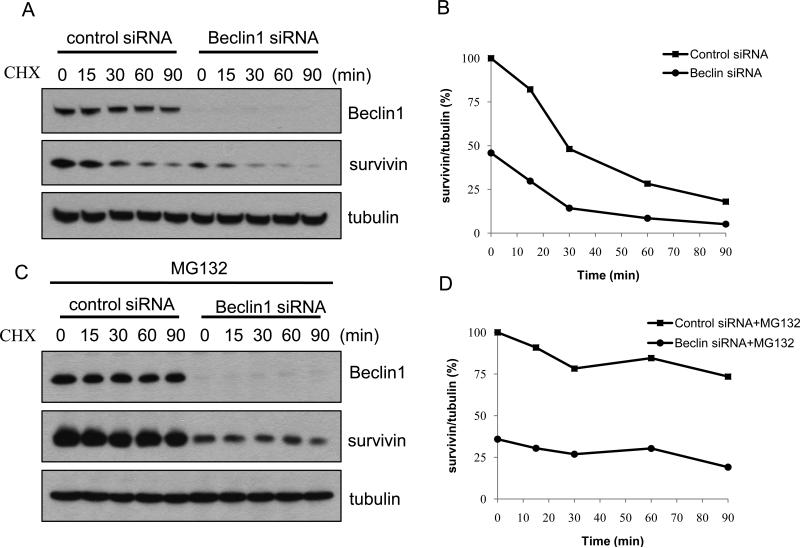
Survivin is a short-lived protein that is degraded by the ubiquitin-proteasome pathway [10]. Therefore, we wanted to know whether knockdown of Beclin 1 reduced survivin through facilitating the degradation of survivin by the ubiquitin-proteasome system. To compare the turnover rate of survivin in cells with or without silencing of Beclin 1 expression, we pulse-chased the survivin protein in LN229 cells transfected with a beclin 1-targeted siRNA or a non-targeting siRNA in the presence of cyclohexamide (CHX), an inhibitor of protein synthesis. We observed a slight increase in the turnover rate of survivin in the cells whose expression of Beclin 1 was silenced, as compared with that in control cells (t1/2 of survivin protein was 23 min and 30 min, respectively) (Fig. 3A and B). Treatment with the proteasome inhibitor, MG132, caused an accumulation of survivin in the cells either transfected with a beclin 1-targeted siRNA or a non-targeting siRNA (Fig. 3C and D). These results presented an evidence for a role of Beclin 1 in regulating the cellular content of survivin. Nevertheless, whether or not the interaction between survivin and Beclin 1 affects the turnover of Beclin 1 remains to be explored. Additionally, in the presence of MG132, survivin level in the cells transfected with a Beclin 1 siRNA could not be fully rescued to the control level (Fig. 3C), suggesting that the effects of Beclin 1 on the turnover of survivin could be mediated through other mechanism in addition to the proteasomal pathway.
Figure 3. Effect of silencing Beclin 1 expression on degradation rate of survivin.
(A) LN229 cells were transfected with a control siRNA or Beclin 1 siRNA for 72 h, and then chased for survivin in the presence of 50 μg/ml CHX at indicated time points, as described in “Materials and Methods”. Survivin was detected using Western blot. (B) Quantification of panel A. (C) LN229 cells were transfected with a control or Beclin 1 siRNA for 72 h, followed by the treatment with 10 μg/ml of MG132 for 1 h. Survivin protein was chased in the presence of 50 μg/ml CHX at indicated time points. (D) Quantification of panel C. Results shown are the representative of three identical experiments.
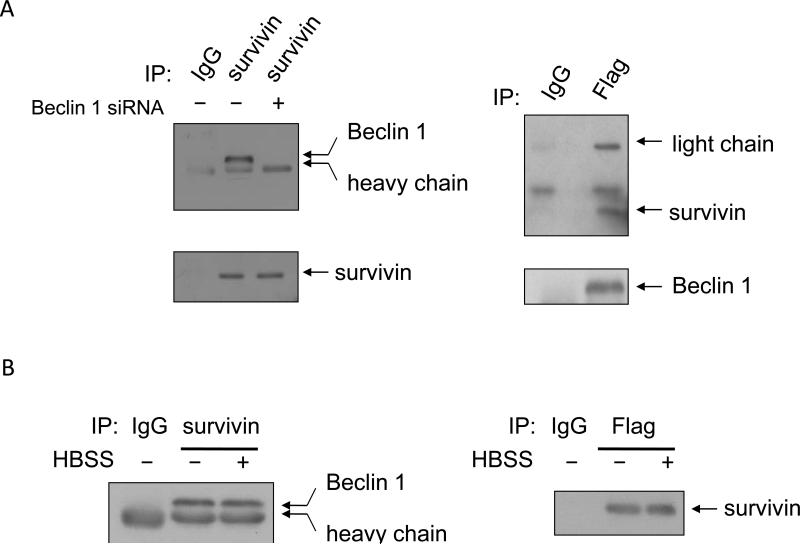
To explore how Beclin 1 regulates the content of survivin, we determined whether Beclin 1 and survivin physically interacted using a co-immunoprecipitation approach. In these experiments, 293T cells with or without silencing of Beclin 1 expression were transfected with a Flag-Beclin 1 and a survivin expression vector, and cell lysates from the transfected cells were then subjected to immunoprecipitation with an antibody to survivin and immunoblottign with an antidody to Beclin 1, or subjected to immunoprecipitation with an antibody to Flag and immunoblottign with an antibody to survivin. Figure 4A demonstrated that Beclin 1 was co-immunoprecipitated with the anti-survivin antibodies, and vice versa. However, Beclin 1 was not detected in the survivin immunoprecipitates when Beclin 1 was silenced (Fig. 4A, left panel). Furthermore, the interaction between Beclin 1 and survivin was also identified in the cells subjected to nutrient starvation (cultured in HBSS) (Fig. 4B). These results suggest that there is indeed a physical association between Beclin 1 and survivin, and this interaction might account, at least in part, for the effect of Beclin 1 on survivin protein levels as shown in Figure 1. However, We did not observed changes in the physical association between survivin and beclin 1 following nutrient starvation (Fig. 4B), probably due to that, this level of interaction is adequate for inducing autophagy and for modulating cell survival, or there exist other factors (e.g., Bcl-2, Bcl-xL, etc.) controlling the interaction between these two proteins._ In addition, the exact binding sites on both of the proteins, and whether this interaction is spatially – restricted or tissue – selective, are currently unknown, and remain to be investigated.
Figure 4. Detection of association of survivin with Beclin 1.
(A) 293T cells were co-transfected with a flag-Beclin 1 and a survivin expression vector in the presence or absence of a Beclin 1 - targeted siRNA. Forty-eight h after transfection, cells were collected for immunoprecipitation with either flag (right panel) or survivin (left panel) antibodies. IgGs were used as a negative control. Immunoprecipitates were subjected to Western blot analysis using an anti-Beclin 1 or anti-survivin antibody. (B) Cells transfected with a survivin expression vector were treated with or without HBSS for 4 h, followed by immunoprecipitation with a survivin antibody and immunoblotting with a Beclin 1 antibody. Results shown are the representative of three similar experiments.
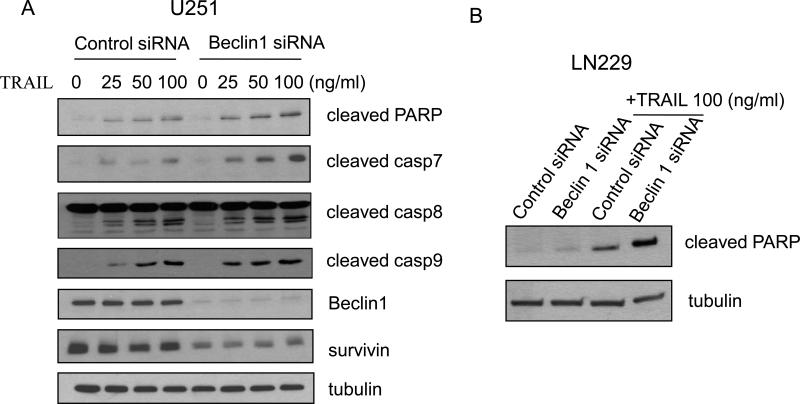
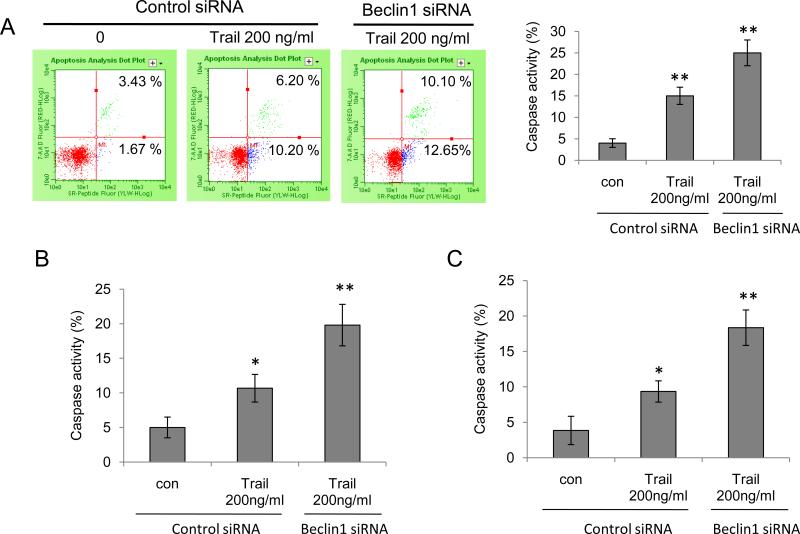
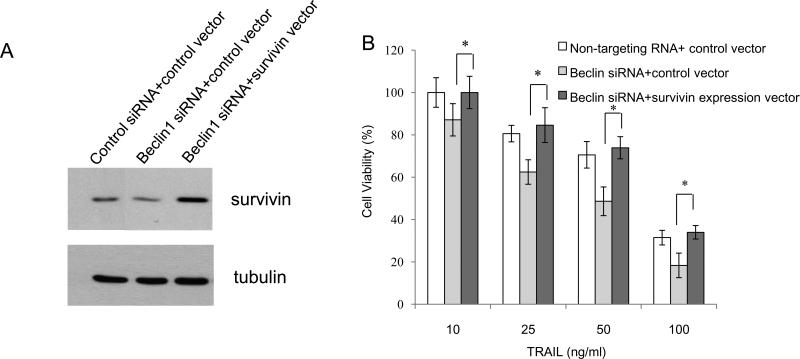
To begin to understand the functional significance of the interaction between Beclin 1 and survivin, we tested the effect of knocking down Beclin 1 on sensitivity of glioma cells to TRAIL, an apoptosis inducer. It has been reported recently that reductions of survivin renders glioma cells sensitivity to TRAIL-induced apoptosis in glioma cells and overexpression of survivin attenuated apoptosis induced by TRAIL [11]. As shown in Figure 5A, while knock down of Beclin 1 reduced survivin levels, it also lead to an increase in apoptosis, as indicated by a dose-dependent increase in the levels of cleaved PARP, cleaved caspase 7, cleaved caspase 8, and cleaved caspase 9 in glioma cells treated with TRAIL. Enhanced sensitivity to TRAIL-induced apoptosis was also observed in LN229 cells with silencing of Beclin 1 expression (Fig. 5B). To verify the effect of knock down of Beclin 1 on TRAIL-induced apoptosis, we measured the pan - caspase activity using flow cytometry. As shown in Figure 6, inhibition of Beclin 1 increased the caspase activities in T98G, LN229 and U251 cells treated with TRAIL. To corroborate that the sensitizing effect of suppressing Beclin 1 expression on TRAIL-induced apoptosis was mediated by down-regulation of survivin, we co-transfected glioma cells with a Beclin 1 siRNA and a survivin expression vector, followed by the treatment with TRAIL for 24 hours. An empty vector was used as a control. As shown in Figure 7, transfection with the survivin expression plasmid significantly lessened the TRAIL-induced cytotoxicity in tumor cells with silencing of Beclin 1, as compared with the transfection with a control plasmid (Fig. 7), suggesting that the reduction of survivin indeed contributes to the enhanced sensitivity to the TRAIL-induced apoptosis in cells with depletion of Beclin 1. Nevertheless, at present the indirect effect of interaction between Beclin 1 and survivin on cell viability can not be excluded.
Figure 5. Knock down of Beclin 1 sensitizes human glioma cells to TRAIL-induced apoptosis.
U251 (A) and LN229 (B) cells were treated with the indicated concentrations of TRAIL following the transfection with a control or Beclin 1 siRNA for 72 h. The treated cells were collected and subjected to Western blot analysis with the pecific antibodies as indicated. Tubulin was used as a loading control. Results shown are the representative of three identical experiments.
Figure 6. Knock down of Beclin 1 enhances TRAIL-induced caspase activities in human glioma cells.
T98G (A), LN229 (B) and U251 (C) cells transfected with a control or Beclin 1 siRNA were treated with 200 ng/ml TRAIL for 24 h. Pan-caspase activities were measured using flow cytometry. Each point represents mean ± SD of three independent assays; *p < 0.05, **p < 0.01, t-test.
Figure 7. Introduction of survivin rescues the TRAIL-induced cell death in glioma cells with silencing of Beclin 1 expression.
T98G cells were co-transfected with a non-targeting siRNA and a control vector, or a Beclin 1 siRNAs and a control vector, or a Beclin 1 siRNA and a survivin expression vector for 48 h. (A) Cells were collected and subjected to Western blot with survivin antibody. Tubulin was used as a loading control. (B) Cells were treated with various concentrations of TRAIL and cell viability was determined using MTT assay, as described in Materials and Methods. Results shown are the mean ± SD of quadruplicate determinations from one of three identical experiments. *p < 0.05, t-test.
Previous studies have demonstrated the interaction between Beclin 1 and anti-apoptotic Bcl-2 family proteins [5-7]. In this study, we identified for the first time the interaction between Beclin 1 and survivin, a member of IAPs. As Beclin 1 is a key regulator of autophagy, the interaction between Beclin 1 and survivin might reflect a functional relationship between autophagy and apoptosis, two major cell death and survival mechanisms that impact a variety of physiologic or patho-physiologic processes.
Evasion of apoptosis is a hallmark of most malignant cells and contributes to insensitivity to various current cancer therapies [12]. Apoptosis resistance can be induced by a number of mechanisms, including aberrant expression of IAPs [13]. High expressions of IAPs have been observed in many human cancers [14], and are thus considered promising targets for cancer treatment. In this study, we have also demonstrated that interaction between Beclin 1 and survivin affects the sensitivity of human glioma cells to TRAIL-induced apoptosis. TRAIL is known to induce apoptosis in cancer cells with no or minimal toxicity on normal cells. However, glioma cells are often found to be resistant to TRAIL therapy. Combinations of TRAIL with other treatments such as Smac agonist and rapamycin have been shown to sensitize glioma cells to TRAIL-induced apoptosis [15, 16]. Also, inhibition of autophagy in tumor cells allows for the induction of significant apoptosis in response to TRAIL [17]. The results of this study present a possible new mechanism regulating the cross-talk between apoptosis and autophagy, and may help develop new strategies to sensitize tumor cells to apoptosis inducers such as TRAIL therapy. Taken together, our study provides evidence for a potentially important interaction between Beclin 1 and survivin and the therapeutic implication of this interaction in human tumor cells.
Acknowledgements
We are grateful to Dr. Dario Altieri (University of Massachusetts Medical School) for providing us with the human survivin expression vector. This work was supported by grants from the National Cancer Institute R01CA 135038 and R01CA 66077.
Abbreviation
- IAP
inhibitor of apoptosis protein
- TRAIL
tumor necrosis factor-related apoptosis-inducing ligand
- CHX
cycloheximide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Structured summary: MINT-7969366: Beclin-1 (uniprotkb:Q14457) physically interacts (MI:0915) with survivin (uniprotkb:O15392) by anti tag coimmunoprecipitation (MI:0007) MINT-7968986, MINT-7969161: survivin (uniprotkb:O15392) physically interacts (MI:0915) with Beclin-1 (uniprotkb:Q14457) by anti bait coimmunoprecipitation (MI:0006)
References
- 1.LaCasse EC, Mahoney DJ, Cheung HH, Plenchette S, Baird S, Korneluk RG. IAP-targeted therapies for cancer. Oncogene. 2008;27:6252–75. doi: 10.1038/onc.2008.302. [DOI] [PubMed] [Google Scholar]
- 2.Altieri DC. Survivin, cancer networks and pathway-directed drug discovery. Nat Rev Cancer. 2008;8:61–70. doi: 10.1038/nrc2293. [DOI] [PubMed] [Google Scholar]
- 3.Ulasov IV, Tyler MA, Zhu ZB, Han Y, He TC, Lesniak MS. Oncolytic adenoviral vectors which employ the survivin promoter induce glioma oncolysis via a process of beclin-dependent autophagy. Int J Oncol. 2009;34:729–42. doi: 10.3892/ijo_00000199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liang C, Feng P, Ku B, Dotan I, Canaani D, Oh BH, Jung JU. Autophagic and tumour suppressor activity of a novel Beclin 1-binding protein UVRAG. Nat Cell Biol. 2006;8:688–99. doi: 10.1038/ncb1426. [DOI] [PubMed] [Google Scholar]
- 5.Boya P, Kroemer G. Beclin 1: a BH3-only protein that fails to induce apoptosis. Oncogene. 2009;28:2125–7. doi: 10.1038/onc.2009.83. [DOI] [PubMed] [Google Scholar]
- 6.Erlich S, et al. Differential interactions between Beclin 1 and Bcl-2 family members. Autophagy. 2007;3:561–8. doi: 10.4161/auto.4713. [DOI] [PubMed] [Google Scholar]
- 7.Pattingre S, et al. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell. 2005;122:927–39. doi: 10.1016/j.cell.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 8.Guha M, Plescia J, Leav I, Li J, Languino LR, Altieri DC. Endogenous tumor suppression mediated by PTEN involves survivin gene silencing. Cancer Res. 2009;69:4954–8. doi: 10.1158/0008-5472.CAN-09-0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman WH, Biade S, Zilfou JT, Chen J, Murphy M. Transcriptional repression of the anti-apoptotic survivin gene by wild type p53. J Biol Chem. 2002;277:3247–57. doi: 10.1074/jbc.M106643200. [DOI] [PubMed] [Google Scholar]
- 10.Zhao J, Tenev T, Martins LM, Downward J, Lemoine NR. The ubiquitin-proteasome pathway regulates survivin degradation in a cell cycle-dependent manner. J Cell Sci. 2000;113(Pt 23):4363–71. doi: 10.1242/jcs.113.23.4363. [DOI] [PubMed] [Google Scholar]
- 11.Siegelin MD, Reuss DE, Habel A, Herold-Mende C, von Deimling A. The flavonoid kaempferol sensitizes human glioma cells to TRAIL-mediated apoptosis by proteasomal degradation of survivin. Mol Cancer Ther. 2008;7:3566–74. doi: 10.1158/1535-7163.MCT-08-0236. [DOI] [PubMed] [Google Scholar]
- 12.Brown JM, Attardi LD. The role of apoptosis in cancer development and treatment response. Nat Rev Cancer. 2005;5:231–237. doi: 10.1038/nrc1560. [DOI] [PubMed] [Google Scholar]
- 13.Deveraux QL, Reed JC. IAP family proteins-suppressors of apoptosis. Genes Dev. 1999;13:239–252. doi: 10.1101/gad.13.3.239. [DOI] [PubMed] [Google Scholar]
- 14.Wang R, Lin F, Wang X, Gao P, Dong K, Zou AM, Cheng SY, Wei SH, Zhang HZ. Silencing livin gene expression to inhibit proliferation and enhance chemosensitivity in human cells. Cancer Gene Ther. 2008;15:402–412. doi: 10.1038/cgt.2008.16. [DOI] [PubMed] [Google Scholar]
- 15.Panner A, James CD, Berger MS, Pieper RO. mTOR controls FLIPS translation and TRAIL sensitivity in glioblastoma multiforme cells. Mol Cell Biol. 2005;25:8809–23. doi: 10.1128/MCB.25.20.8809-8823.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fulda S, Wick W, Weller M, Debatin KM. Smac agonists sensitize for Apo2L/TRAIL- or anticancer drug-induced apoptosis and induce regression of malignant glioma in vivo. Nat Med. 2002;8:808–15. doi: 10.1038/nm735. [DOI] [PubMed] [Google Scholar]
- 17.Han J, Hou W, Goldstein LA, Lu C, Stolz DB, Yin XM, Rabinowich H. Involvement of protective autophagy in TRAIL resistance of apoptosis-defective tumor cells. J Biol Chem. 2008;283:19665–77. doi: 10.1074/jbc.M710169200. [DOI] [PMC free article] [PubMed] [Google Scholar]