Abstract
Production of the harmful carcinogenic aflatoxins by Aspergillus parasiticus and Aspergillus flavus has been postulated to be a mechanism to relieve oxidative stress. The msnA gene of A. parasiticus and A. flavus is the ortholog of Saccharomyces cerevisiae MSN2 that is associated with multi-stress response. Compared to wild type strains, the msnA deletion (∆msnA) strains of A. parasiticus and A. flavus exhibited retarded colony growth with increased conidiation. The ∆msnA strains also produced slightly higher amounts of aflatoxins and elevated amounts of kojic acid on mixed cereal medium. Microarray assays showed that expression of genes encoding oxidative stress defense enzymes, i.e., superoxide dismutase, catalase, and cytochrome c peroxidase in A. parasiticus ∆msnA, and the catalase A gene in A. flavus ∆msnA, was up-regulated. Both A. parasiticus and A. flavus ∆msnA strains produced higher levels of reactive oxygen species (ROS), and ROS production of A. flavus msnA addback strains was decreased to levels comparable to that of the wild type A. flavus. The msnA gene appears to be required for the maintenance of the normal oxidative state. The impairment of msnA resulted in the aforementioned changes, which might be used to combat the increased oxidative stress in the cells.
Keywords: Aspergillus, aflatoxin, kojic acid, oxidative stress, development
1. Introduction
In nature all living organisms react to unfavorable environmental conditions, such as high temperature, osmotic shock, oxidative damage and nutrient depletion via complex regulatory networks. These specific responses usually result from induction of a set of stress-associated genes whose expression is controlled by a common transcription factor. For example, in Saccharomyces cerevisiae a gene named MSN2 that encodes a C2H2-type zinc-finger regulator, Msn2p, is required for yeast cells to cope with a broad range of environmental and physiological stresses [1]. Msn2p mediates expression of a number of genes that are induced by stress conditions by binding to STRE (stress response element) motifs, CCCCT, which are located in the promoters of the regulated genes [2,3]. In Trichoderma atrovirde the expression of the MSN2 ortholog seb1 (stress response element binding) was increased under osmotic stress conditions [4]. Seb1 functions to up-regulate the glycerol dehydrogenase gene (gld1) whose product, Gld1, is required for glycerol biosynthesis to alleviate osmotic stress [5].
Conidiation and formation of sclerotia, hyphal aggregates that serve as the over-winter structure, are believed to be triggered by a hyperoxidant state (oxidative stress) in cells at late stages of fungal development [6,7]. These processes are often interrelated with production of secondary metabolites, such as aflatoxins [8,9,10] which may allow fungi to adapt to unique niches and life cycles. Jeon et al. [8] showed that deletion of the MSN2 ortholog (msnA) in Aspergillus nidulans resulted in enhanced asexual conidiation and production of sexual cleistothecia. Hence, they renamed the A. nidulansmsnA as nrdA (negative regulator of differentiation). Not all Aspergillus species have a natural sexual reproductive stage. Aspergillus parasiticus and Aspergillus flavus, the predominant producers of the aflatoxins, were thought, until recently, to possess only the asexual state, but under forced mating conditions in the laboratories strains of opposite mating types were able to undergo sexual reproduction [9,10]. The majority of A. parasiticus strains produce abundant conidia, but some strains, in addition to conidia, also produce large numbers of sclerotia on the same media [11]. A. flavus strains are morphologically diverse. Some strains produce predominantly conidia with a few large-sized sclerotia, and others produce copious tiny sclerotia along with a low number of conidia; the former is called L-strain and the latter S-strain [12].
In this study, we investigated the role of msnA in two morphologically distinct A. parasiticus strains and an L-strain A. flavus isolate. Deletion of msnA adversely affected vegetative growth and altered development as manifested by dense conidiation and the lack of sclerotial formation. Expression of oxidative stress defense genes and the production of kojic acid (5-hydroxy-2-(hydroxymethyl)-4-pyrone), a free radical scavenger, increased significantly in the A. parasiticus ∆msnA strains. Compared to respective wild-type strains, the ∆msnA strains of A. parasiticus and A. flavus produced increased levels of reactive oxygen species.
2. Materials and Methods
2.1. Fungal Strains and Media
Aspergillus parasiticus BN9∆ku70 [13] and RH∆ku70 [14] and A. flavus CA14PTs∆pyrG [15], a ∆ku70 strain sensitive to pyrithiamine, were the recipient strains used in the msnA gene knockout experiments. A. parasiticus BN9∆ku70 and A. flavus CA14PTs∆pyrG are aflatoxigenic and produce abundant conidia and a few sclerotia. RH∆ku70 accumulates O-methylsterigmatocystin (OMST) as the end product; it produces abundant sclerotia and conidia when grown in the dark. OMST-accumulating isolates have been found to account for about 2.6% of an A. parasiticus population in a southwestern Georgia peanut field [11]. Potato Dextrose Agar (PDA; EMD Chemicals Inc., Darmstadt, Germany) was used for fungal growth and production of conidia and sclerotia for enumeration. The mixed cereal agar (MCA, 5% Gerber® Mixed Grain Cereal, 1.5% agar) [16] was also used to promote sclerotial production.
2.2. Construction of the msnA Disruption Vector
TheEST of the A. flavus msnA gene (NAFAE55TH) was identified initially from the Aspergillus flavus Gene Index database at The Institute for Genomic Research (TIGR) based on S. cerevisiaeMSN2 and its homologue in A. nidulans (AN1652.2). The complete A. flavus msnA gene was subsequently obtained from the Aspergillus Comparative Database at Broad Institute (http://www.broadinstitute.org/annotation/genome/aspergillus_group/MultiHome.html). Restriction analysis of A. flavus msnA and flanking regions was carried out using the DNAMAN software (Lynnon Soft, Vandreuil, Quebec, Canada). The msnA disruption vector was constructed as follows: a 1.4-kb msnA 5’ and coding region and a 0.7-kb coding region near the 3’ end were amplified by PCR using primers msn5K: CTGTCTCCCGGTACCTTTGATCG and msn5P: GAGTATGCGCTGCAGCGCTGTCTC, and msn3P: GAGACAGCGCTGCAGCGCATACTC and msn3H: CGTGGGAAGCTTCATAGAGCAC, respectively. The PCR fragments after digestion were cloned sequentially into corresponding sites in pUC19. The 2.0-kb A. oryzaeptr selectable marker amplified from pPTR1 [17] was cloned into the PstI site of the above construct. This disruption vector construct, msnDV, was linearized by HindIII and KpnI to release the portion of pUC19 prior to fungal transformation.
2.3. Generation of msnA Disruption Strains of A. parasiticus and A. flavus
Preparation of protoplasts, ptr-based fungal transformation and selection of mutants were performed as previously described for A. parasiticus and A. flavus [14]. Homologous recombination is the primary event in fungi with the ku70-deficient background. The msnA gene disruption was confirmed by PCR with location-specific primers based on the expected genomic patterns generated by homologous recombination in the ∆ku70 genetic background. The primers used were P1: GACACAAGGTTCGTCGGTGACT and P2: GGTACTCGCGTCGCGATTA. PCR was carried out under the following conditions in a Perkin Elmer GeneAmp PCR System 2400. Twenty-five pmol of each primer and 10 ng genomic DNA were added to 25 μL Platinum Blue PCR Supermix (Invitrogen, Carlsbad, California, USA) and subject to 30 cycles consisting of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s and extension at 72 °C for 5 min.
2.4. Reintroduction of msnA into the A. flavus ∆msnA Strain
A genomic DNA fragment containing the full-length msnA gene including a 0.58 kb 5’UTR and a 0.24 kb 3’UTR was amplified by PCR using the Accuprime supermix (Invitrogen) with primers msn-E-pyrG, ATAGAATTCCCCGCGACTGTCCATTAGTC and msn-B-pyrG, ATAGGATCCTTTGTGAAGACCATGT. The PCR product was first cloned into the EcoR1 and BamH1 sites of pPG28 [18]. The A. nidulans autonomously-replicating sequence in the 5.2 kb HindIII fragment from pHELP1 [19] was cloned into the resulting construct. The circular plasmid was transformed into an A. flavus CA14∆msnA strain by the pyrG-based transformation protocol [15].
2.5. Colony Growth, Conidial Production, and Sclerotial Formation
The ∆msnA and the parental strains were point inoculated on PDA plates (100 × 15 mm) in triplicate and grown for five days at 30 °C for the determination of vegetative growth based on the colony diameter. For enumeration of conidia, two culture plugs were cored with Transfertube® (Spectrum, Houston, Texas, USA) from each of the three seven-day-old replicate PDA plates. The plugs were placed in a microfuge tube containing 0.5 mL ethanol to kill conidia and then vortexed 2 min with a Disruption Genie apparatus (ZYMO RESEARCH, Orange County, California, USA). Each sample was diluted 100-fold with a 0.01% Triton solution, and conidia were counted two to four times using a hemocytometer. The calculated numbers were the total conidia from the two agar plugs. Strains were cultured on PDA and MCA plates at 30 °C for a week for sclerotial formation.
2.6. Ultraviolet-Visible (UV-Vis) Spectrophotometry and Fourier Transform Infrared (FTIR) Spectroscopy
The ∆msnA strains produced an unknown diffusible pigment on MCA plates not observed on PDA plates. The water-soluble orange pigment was isolated from 25 MCA plates inoculated with A. parasiticus BN9∆msnA. The frozen and thawed cultures were filtered through No. 4 paper (Whatman, Piscataway, New Jersey, USA) and the filtrate passed through a 33mm Millex GP 0.22 µ syringe filter (Millipore, Billerica, Massachusetts, USA). The filtrate was stirred overnight with Amberlite XAD-2 resin (Rohm and Haas, Philadelphia, Pennsylvania, USA). The pigment was eluted from the XAD-2 resin with MeOH, and the solvent was removed under reduced pressure. The remaining solids were dissolved in water and applied to a Sephadex G-25 column (GE Healthcare, Piscataway, New Jersey, USA). The column was eluted with water, and the orange band was collected and lyophilized yielding 44 mg of the pigment. The absorbance spectrum between 350 and 700 nm for the pigment dissolved in water was recorded on an Agilent 8453 photodiode array UV-Vis spectrophotometer (Agilent, Santa Clara, California, USA). Fourier Transform Infrared (FTIR) analysis, which provides information about the chemical bonding and the molecular structure of a compound, was performed. The infrared spectrum of the mixture of 0.5% of the orange pigment in KBr was obtained by diffuse reflectance on a Nicolet 6700 FTIR (Thermo Scientific, Waltham, Massachusetts, USA).
2.7. Determination of Aflatoxins and Kojic Acid by HPLC
The amounts of aflatoxins and kojic acid produced on MCA plates in triplicate by four- and eight-day-old cultures of wild type and ∆msn strains of A. parasiticus BN9 (only kojic acid was determined for RH), and A. flavus CA14 were determined. For each sample, the entire contents of the Petri dish (100 × 15 mm, 25 mL per plate) were added to a Waring MC-2 blender cup and blended for 30 s with 20 mL hot water. The mixture was filtered through Whatman No. 4 paper and a 33 mm Millipore Millex GP 0.22μm syringe filter. The filtered extracts were analyzed for aflatoxins and kojic acid on a HPLC system (Agilent) consisting of a quaternary pump, autosampler, photodiode array detector, and fluorescence detector. The analyses were performed on a column of Intersil ODS-3, 5 μ, 4.6 × 250 mm (GL Sciences, Torrance, California, USA) at a flow rate of 1.0 mL/min. The injection volume was 20 μL. The mobile phase for aflatoxins was H2O:CH3CN:MeOH (45:25:30) and for kojic acid MeOH:0.1% H3PO4 (25:75), isocratic. Detection of aflatoxins was based on fluorescence at 365 nm excitation and 455 nm emission with “PHRED” postcolumn photochemical derivatization (Aura Industries, New York, New York, USA). Detection of kojic acid was based on UV absorption at 265 nm. The retention times of the standards for aflatoxins B1, B2, G1 and G2 were 10.6 min, 9.4 min, 8.7 min and 7.8 min, respectively, and for kojic acid the retention time was 5.4 min.
2.8. RNA Isolation and Probe Labeling
An aliquot of spore suspension was added to each Petri dish plate (100 × 15 mm) containing 30 mL potato dextrose broth (PDB, Merck, Darmstadt, Germany) to give a final concentration of 3 × 105 spores per milliliter. Four dishes were used for each strain. Stationary cultures were incubated at 30 °C in the dark for 4 days. Harvested mycelium were pooled and rinsed with sterilized distilled water and pulverized to a fine powder with a mortar and pestle in the presence of liquid nitrogen. Total RNA was prepared using TRIzol® reagent (Invitrogen) and treated with amplification grade DNase I followed by purification with an RNeasy Plant Mini kit (Qiagen, Valencia, California, USA).
Fluorescent dye Cy3 and Cy5 labeled probes were prepared using the indirect labeling method of aminoallyl-cRNA according to the protocol provided by TIGR. A total of 6 μg of aminoallyl-cRNA were needed in each probe labeling. The aminoallyl-cRNA was synthesized and amplified using an Amino Allyl MessageAmp™ II aRNA Amplification kit (Ambion, Austin, Texas, USA). Mono-reactive dyes Cy3 and Cy5 (Amersham, Piscataway, New Jersey, USA) were coupled respectively to aminoallyl-cRNA from wild type and mutant. The unincorporated free dyes were removed using the RNeasy MinElute cleanup kit (Qiagen).
2.9. Microarray Assays
The microarray used was Aspergillus flavus NRRL3357 27.6k oligo array [20]. A direct comparison design was applied, including Mutant/Wild type. Four technical replicates were used, including two dye swaps to compensate for cyanine dye effects. Following hybridization and washing according to the TIGR protocol, the microarray slides were scanned by a Genepix 4000B (Molecular Devices, Sunnyvale, California, USA), and the images were analyzed using GENEPIX 6.0 software.
Microarray data were normalized and analyzed using GeneSpring GX 10.0 software (Silicon Genetics, Redwood City, California, USA). Two criteria were used for selecting positive spots, (Signal-Background) mean > 500 unit as expression intensity filter, and at least two of the four replicates showing positive. These filters were imposed to remove genes with very minor differential expression or genes with little evidence for expression. Data normalization was performed using local regression LOWESS (locally weighted scatterplot smoothing). Differentially expressed genes were identified by performing a one-way ANOVA on the normalized data using a T test with no assumption of equal variance. The cutoff criteria in fold change analysis were set as P < 0.05 in significant difference and fold change >2.
2.10. Quantitative PCR
Quantitative real-time PCR (qPCR) was carried out in a 20-μL reaction volume with the SYBR Green Master Mix (Applied Biosystems, Foster City, California, USA) in a StepOneTM thermal cycler (Applied Biosystems). PCR conditions were as follows: an initial step of 95 °C for 10 min and 40 cycles with each cycle consisting of 95 °C for 15 s and 60 °C for 1 min. The primers sets used were designed by the PrimerExpress 3.0 software following the guidelines specified by Applied Biosystems. The specificity of the PCR products was confirmed by the melt curve analysis. The relative expression levels of the genes examined were determined by the relative standard curve method, in which standard curves based on 5-point, 10-fold serial dilutions were constructed for the endogenous (18S) and the target gene in each experiment using genomic DNA as the template.
2.11. Quantification of Reactive Oxygen Species (ROS)
ROS generation was assessed using the substrate 2’,7’-dichlorofluorescein diacetate (DCFH-DA; Sigma D6883). Oxidation of non-fluorescent DCFH-DA by ROS, such as H2O2 and the hydroxyl radical, yields the fluorescent product dichlorofluorescin (DCF). DCF fluorescence spectra is usually measured using excitation/emission wavelength of 490/525 nm. In this study, we used the OneStepPlus qPCR instrument (Applied Biosystems) that is able to measure dye fluorescence with the program set for the detection of SYBR®-DNA complex at excitation/emission wavelength of 488/522 nm. DCFH-DA stock solution (100×, 1 mM in dimethyl formamide) was prepared. Four agar plugs were cored from each PDA culture plate grown for 7 days at 30 °C. They were placed into a microfuge tube, and 1mL freshly made 1× PBS containing DCFH-DA at a final concentration of 1 μM was added. The reaction was allowed to continue at 37 °C for specified time periods (1, 5 or 24 hr). At the end of each time period, three replicates of 30 μL from each sample were loaded into the optical 48-well reaction plate. The program was set to hold at 37 °C and fluorescence measurements were taken for 6 cycles at 10 min intervals.
3. Results
3.1. Vegetative Growth, Conidial Production, and Sclerotial Formation of ∆msnA Strains
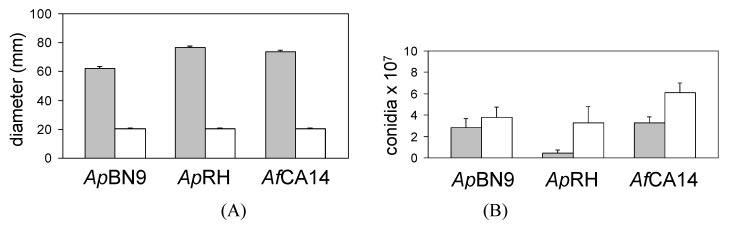
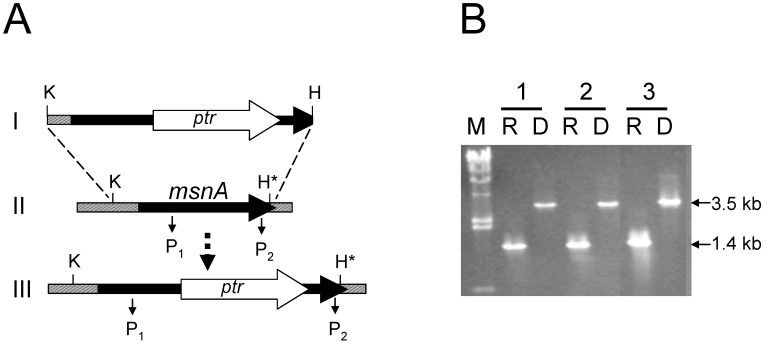
Disruption of msnA in A. parasiticus BN9 and RH, and A. flavus CA14 was confirmed by PCR based on expected genomic patterns (Figure S1). Colonies of the ∆msnA strains of A. parasiticus and A. flavus exhibited a restricted, densely-packed appearance on PDA plates. The radial colony growth of the ∆msnA strains was 2 to 3-fold less than that of the parental strains (Figure 1A). Compared to the parental strains the ∆msnA strains produced more conidia as estimated from an identical area (Figure 1B). The increase in conidiation of A. parasiticus BN9∆msnA (36%) was less than that of A. parasiticus RH∆msnA (>600%) and A. flavus CA14∆msnA (84%). Although A. parasiticus RH is a strain that produces many sclerotia, RH∆msnA was unable to produce any on PDA; BN9∆msnA and CA14∆msnA were also unable to produce sclerotia on the same medium.
Figure 1.
Effect of msnA disruption on colony size and conidial production. (A) Colony size after growth for five days at 30 °C. (B) Conidial production estimated from two cored agar plugs (see Materials and Methods 2.5). The gray bar represents the wild-type strain, and the clear bar represents the ∆msnA strain. Ap: A. parasiticus; Af: A. flavus.
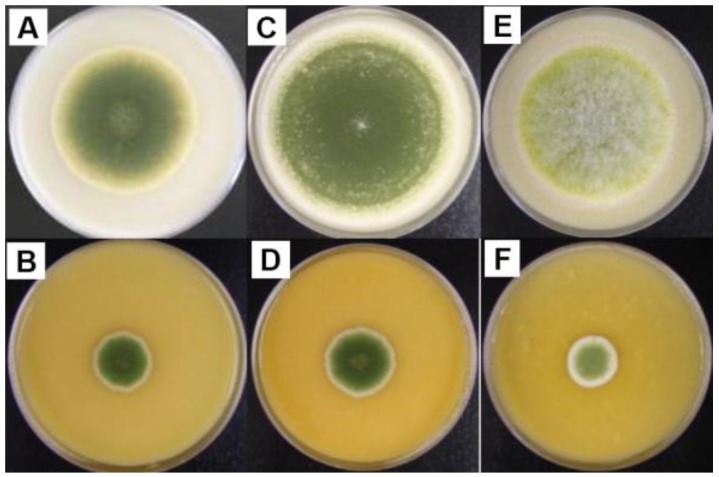
To further examine whether sclerotial formation of the three ∆msnA strains was abolished, we grew them on mixed cereal agar plates. All strains did not produce sclerotia on this medium either. They, however, produced a diffusible orange-colored substance that increased in intensity after prolonged incubation (Figure 2). In contrast, the orange-colored pigment was barely produced by the wild-type strains after the same 7-day period of growth.
3.2. Characterization of the Pigment by FTIR
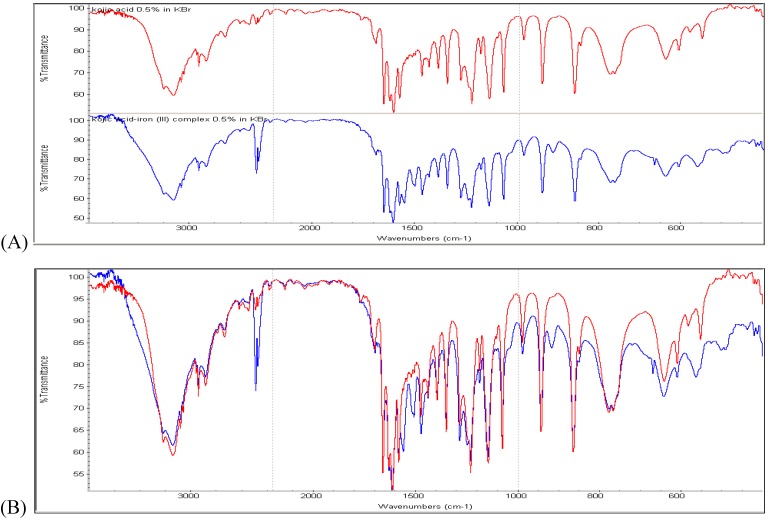
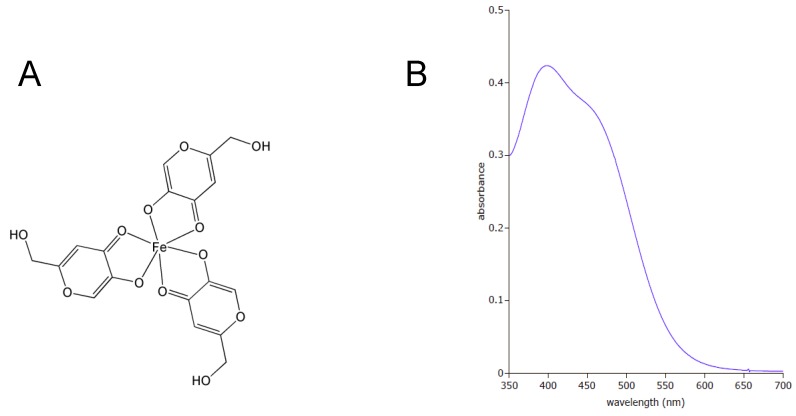
Based on the color we speculated that the orange pigment might be a complex derived from kojic acid, a metabolite commonly produced by some strains of A. flavus and closely related A. oryzae. The UV-Vis spectrum of the pigment purified from the A. parasiticus BN9∆msnA culture matched with the published spectrum of the complex of 3 kojic acid moieties per Fe(III) [21] and had a peak absorbance at 398 nm and a broad shoulder at 460 nm (Figure S2). The kojic acid in the complex was confirmed by FTIR analysis using kojic acid as the reference. The separate FTIR spectra of kojic acid and the pigment are shown in Figure 3A. The overlaid image indicates that the two spectra are nearly identical (Figure 3B); two extra peaks in the carbonyl region (1,500 to 1,700 cm−1) appear in the pigment and another observed difference is at about 2,550 cm−1.
Figure 2.
Culture morphology of A. parasiticus and A. flavus strains on MCA plates. (A) wild-type A. parasiticus BN9 (B) A. parasiticus BN9∆msnA (C) wild-type A. parasiticus RH; the white granules around the edge of the colony are sclerotia (D)A. parasiticus RH∆msnA (E) wild-type A. flavus CA14 (F) A. flavus CA14∆msnA.
Figure 3.
Characterization of the orange pigment by FTIR. (A) Spectrum of kojic acid: top panel red-line and spectrum of the pigment isolated from A. parasiticus BN9∆msnA: bottom panel blue-line. (B) Overlaid spectra.
3.3. Production of Aflatoxins and Kojic Acid by the ∆msnA Strains of A. parasiticus BN9 and A. flavus CA14
To determine how production of aflatoxins and kojic acid was affected by msnA disruption, we carried out a time-course quantitative determination. The normalized data (to growth area, Table 1) showed that the production of total aflatoxins and kojic acid increased from day 4 to day 8 in the parasiticus BN9∆msnA strain. The ∆msnA strain accumulated 50% more aflatoxins and 20-fold more kojic acid than the parental strain at day 8. The A. parasiticus strain RH is a blocked strain that accumulates O-methylsterigmatocystin as the end product. Although it does not produce aflatoxins, the derived RH∆msnA strain produced 10-fold more kojic acid. The A. flavus CA14∆msnA strain produced only small amounts of aflatoxins, but it produced four-fold more kojic acid than the parental strain.
Table 1.
Production of aflatoxins and kojic acid by ∆msnA strains on MCA.
| Strain(T)a | Colony(mm)b | AF(μg)c | KA(mg)c | ||||
|---|---|---|---|---|---|---|---|
| B1 | B2 | G1 | G2 | Total | |||
| a: T, days of growth. RH is a blocked strain and accumulates O-methylsterigmatocystin as the end product; it does not produce aflatoxins; b: Diameter averages from triplicate MCA plates; c: Data were normalized to an area with the radius of 1.0 cm; d: ND, not determined. | |||||||
| BN9(4) | 42 | 2.87 | 0.09 | 4.50 | 0.09 | 7.58 | 0.06 |
| BN∆msn (4) | 17 | 4.36 | 0.13 | 5.11 | 0.09 | 9.69 | 1.06 |
| BN9(8) | 75 | 5.36 | 0.18 | 7.18 | 0.17 | 12.90 | 0.05 |
| BN∆msn (8) | 28 | 9.31 | 0.31 | 10.04 | 0.24 | 19.81 | 1.19 |
| RH(4) | 42 | NDd | 0.10 | ||||
| RH∆msn (4) | 13 | ND | 1.17 | ||||
| RH(7) | 67 | ND | 0.12 | ||||
| RH∆msn (8) | 20 | ND | 1.38 | ||||
| CA14(4) | 35 | <0.01 | <0.01 | 0.18 | |||
| CA∆msn (4) | 13 | 0.02 | 0.02 | 0.83 | |||
| CA14(8) | 76 | <0.01 | <0.01 | 0.35 | |||
| CA∆msn (8) | 27 | 0.01 | 0.01 | 1.33 | |||
3.4. Microarray Profiling of Differentially Expressed Genes in the ∆msnA Strains of A. parasiticus and A. flavus
Microarray assays identified many differentially expressed genes in the ∆msnA strains of A. parasiticus BN9 and A. flavus CA14 (Table S1 and Table S2). For A. parasiticus, approximately 85% of the genes (0.005% saturation and 500 cutoff) were down-regulated; only 12 genes were up-regulated (°2-fold), and they included those oxidative stress defense genes encoding superoxide dismutase, catalase, and cytochrome c peroxidase. The up-regulation of these genes was confirmed by qPCR (Table 2). For A. flavus, approximately two thirds of the genes profiled were down-regulated; the genes up-regulated were diverse including many of those encoding hypothetical proteins, and only one up-regulated catalase A gene for the A. flavus ∆msnA strain was found.
Table 2.
Expression of oxidative stress defense genes in A. parasiticus BN9∆msnA.
| Enzyme | Gene Locus | Oligoprimer Sequence | Fold-of-Increase c |
|---|---|---|---|
| Primers used for 18S rDNA in qPCR are ttcctagcgagcccaacct and cccgccgaagcaactaag. | |||
| a: Broad Institute Aspergillus Comparative Database gene accession number (http://www.broadinstitute.org/annotation/genome/aspergillus_group/MultiHome.html); b: NCBI Entrez Gene accession number (http://www.ncbi.nlm.nih.gov/gene) (Table S1 and Table S2); c: Relative gene expression level ° S.D. The gene expression level of A. parasiticus BN9 is 1.00. | |||
| superoxide dismutase | AFL2G_10810.2 a | cgccggtactgacgacctt | 2.09 ° 0.06 |
| AFLA_099000 b | agcattgccagtcttcttgga | ||
| catalase | AFL2G_05806.2 | caggtggcttcgcgtccta | 2.30 ° 0.13 |
| AFLA_056170 | caggccgcgcttcttg | ||
| cytochrome c peroxidase | AFL2G_04481.2 | tcggtcgtgcccatcct | 2.38 ° 0.12 |
| AFLA_110690 | aagacagtagggctgaagttcca | ||
3.5. ROS Production by A. parasiticus and A. flavus ∆msnA Strains and by A. flavus msnA Addback Strains
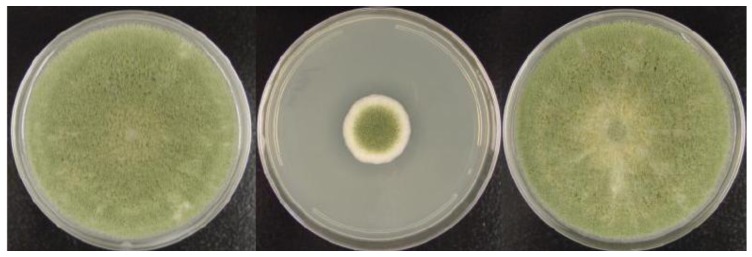
The recent generation of the A. flavus CA14-derived double mutant makes it possible to retransform a knockout strain, such as the ∆msnA strain, using a second selectable marker along with an intact gene to confirm gene function [15]. By this approach, the defects in colony growth were remediated in the A. flavus CA14∆msnA strain after the intact msnA genomic DNA was reintroduced (Figure S3).
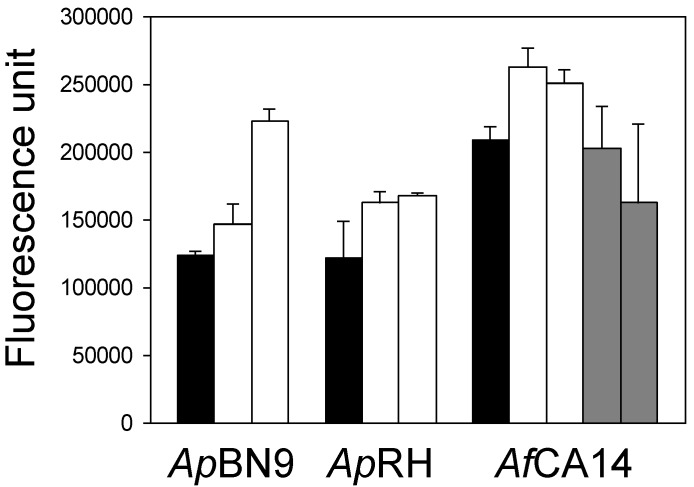
Figure 4.
Production of reactive oxygen species (ROS) by A. parasiticus and A. flavus strains. Solid bar: parental strain, clear bar: independent ∆msnA strains, and gray bar, independent msnA addback strains.
ROS production by the wild-type strains, ∆msnA strains of A. parasiticus and A. flavus, and by the A. flavus msnA addback strain was determined to see whether ROS levels correlated with observed changes in growth, development and secondary metabolite production. ROS production was found to fluctuate in the first 5 hr incubation assays (data not shown). Only after 24 hr incubation a more distinct pattern emerged; an increase in ROS production was seen in all ∆msnA strains (Figure 4). ROS production by the A. flavus msnA addback strains was decreased to levels comparable to that of the wild-type A. flavus.
4. Discussion
Our results show that msnA is required for normal colony growth and development of A. parasiticus and A. flavus. Similar findings have been reported for Trichoderma atrovidire [4] and A. nidulans [22]. The role of oxidative stress in conidiation has been implicated in A. nidulans [23]. Likewise, exposure of Neurospora crassa to antioxidants inhibits conidiation [24]. The N. crassa catalase-3 gene mutant that was sensitive to hydrogen peroxide produced six-times more conidia [25]. The increased production of conidia in the ∆msnA strains (Figure 1B) can be correlated with the increased levels of ROS (Figure 4) and suggests that conidiation is a response to increased levels of oxidative stress in the cells.
Sclerotial formation like conidiation has been correlated with oxidative stress, antioxidant effects, and antioxidant enzyme activities [26,27]. Sclerotia of A. parasiticus and A. flavus are considered to be a vestige of the sexual cleistothecia produced by other aspergilli [28]. The light responses of the two structures regulated by the common factor VeA [29,30,31] are similar. In the dark A. nidulans favors the formation of cleistothecia [32,33], and under light A. parasiticus and A. flavus suppress sclerotial production [34]. The ∆msnA strains of A. parasiticus and A. flavus are unable to produce sclerotia. This result was unexpected since the A. nidulans nrdA(msnA)-null strain produced enhanced levels of cleistothecia [22]. It suggests that subtle differences likely exist in the regulation of sclerotial and cleistothecial morphogenesis. Impairment of msnA like the disruption of laeA, a secondary metabolism regulatory gene [35], abolishes sclerotial formation but not conidiation, which shows that the two developmental processes are regulated differently.
Aflatoxin biosynthesis may be a defense mechanism against oxidative stress [36,37,38]. Studies have demonstrated that natural antioxidants, such as gallic acid, caffeic acid and eugenol can reduce aflatoxin production [39,40,41]. Several lines of evidence also have suggested a positive correlation between ROS accumulation and aflatoxin production by A. flavus and A. parasiticus [36,42]. Deletion of the A. parasiticus yapA gene, which encodes a transcription factor that mediates oxidative stress response, resulted in precocious ROS formation and increased aflatoxin biosynthesis [38]. Supporting this correlation we found that compared to respective parental strains, the ∆msnA strains produced more aflatoxins (Table 1) and had higher levels of ROS accumulation (Figure 4). Beside aflatoxins, FTIR assays confirm that the orange pigment is a kojic acid-iron complex (Figure 3). The peaks at 1500 and 1580 cm−1 in the spectrum of the pigment are consistent with the chelation of kojic acid with a metal through the carbonyl moiety (Figure 3B). Kojic acid is a scavenger of free radicals [43]. The highly elevated production levels (Table 1) suggest that the ∆msnA strains use the formation of kojic acid as a main detoxifying mechanism.
Microarray comparisons of ∆msnA to wild-type showed that genes encoding superoxide dismutase, catalase, and cytochrome c peroxidase in the A. parasiticus BN9∆msnA strain were up-regulated (Table 2). The result suggests that expression of these genes is probably needed to cope with increased oxidative stress in the cells. The generation of ROS is potentially deleterious. Superoxide dismutase converts superoxide to another ROS, hydrogen peroxide, probably to shunt the superoxide away from harmful lipid peroxidation to the cells [44] or from damages to DNA [45]. Catalase converts hydrogen peroxide to water and oxygen molecule. Like catalase, cytochrome c peroxidase takes reducing equivalents from cytochrome c and converts hydrogen peroxide to water. Different types of oxidative stress defense genes operate in the ∆msnA strains of A. parasiticus and A. flavus; this likely reflects respective physiological responses of each species in spite of their close genetic relatedness. The varied amounts of aflatoxins and/or kojic acid produced by respective ∆msnA strains also reflect intrinsic differences of the two species and within members of the same species, such as BN9 and RH.
5. Conclusions
This study suggests that the ∆msnA strains of A. parasiticus and A. flavus are not as capable as the wild-type strains in relieving oxidative stress and respond by up-regulating antioxidant enzyme genes as well as by increasing the production of conidia, aflatoxins, and kojic acid to alleviate the increased oxidative stress caused by the loss of msnA.
Supplementary Materials
Figure S1.
Disruption of the msnA gene in A. parasiticus and A. flavus by the ptr selectable marker. (A) Diagram depicting the gene disruption event via double-crossover recombination. I: linearized portion of the disruption vector; II: genomic pattern of the recipient strain; III: expected genomic pattern after recombination. (B) PCR confirmation of genomic DNA patterns of the recipient, R, and the msnA disruptant, D. The primers used were P1 and P2. The DNA size markers (in kb) are lambda DNA/Hind III fragments. The three sets are 1: A. parasiticus BN9, 2: A parasiticus RH and 3: A. flavus CA14.
Figure S2.
Characterization of the orange pigment by ultraviolet-visible spectrophotometry. (A) Structure of the 3kojic acid-ferric iron (Fe+3) complex. (B) UV-Vis spectrum of the orange pigment.
Figure S3.
Colony morphology of A. flavus CA14∆msnA retransformed with the genomic msnA gene. Left: wild type, middle: CA14∆msnA, and right: msnA addback transformant. Cultures were grown at 30 °C for a week in the dark on PDA plates.
Table S1.
Genes differentially expressed in A. parasiticus BN9∆msnA.
| Gene ID (a) | Fold change | Regulation | Annotation |
|---|---|---|---|
| (a) NCBI Entrez Gene (http://www.ncbi.nlm.nih.gov/gene) accession number. | |||
| AFLA_003050 | 2.13 | down | RTA1 like protein |
| AFLA_003050 | 2.12 | down | RTA1 like protein |
| AFLA_003090 | 2.10 | down | hypothetical protein |
| AFLA_004690 | 2.12 | up | hypothetical protein |
| AFLA_004690 | 2.21 | up | hypothetical protein |
| AFLA_004940 | 8.07 | down | Glycolipid 2-alpha-mannosyltransferase family protein |
| AFLA_005520 | 3.38 | down | hypothetical protein |
| AFLA_006160 | 2.90 | down | hypothetical protein |
| AFLA_011970 | 2.22 | down | Major Facilitator Superfamily protein |
| AFLA_014260 | 3.08 | up | hydrophobin, putative |
| AFLA_014960 | 2.73 | down | hypothetical protein |
| AFLA_015300 | 2.03 | down | FMN-dependent dehydrogenase family protein |
| AFLA_015300 | 2.43 | down | FMN-dependent dehydrogenase family protein |
| AFLA_015550 | 4.26 | down | Sugar transporter family protein |
| AFLA_015780 | 5.24 | down | small oligopeptide transporter, OPT family protein |
| AFLA_017860 | 2.00 | up | hypothetical protein |
| AFLA_024250 | 2.01 | up | Amidase family protein |
| AFLA_027340 | 2.04 | down | Aha1 domain family, putative |
| AFLA_027340 | 2.16 | down | Aha1 domain family, putative |
| AFLA_028030 | 3.03 | down | conserved hypothetical protein |
| AFLA_029390 | 4.22 | down | HMG box family protein |
| AFLA_036040 | 2.18 | down | mitochondrial inner membrane translocase subunit (TIM17), putative |
| AFLA_036040 | 2.23 | down | mitochondrial inner membrane translocase subunit (TIM17), putative |
| AFLA_036980 | 2.76 | down | MOSC N-terminal beta barrel domain containing protein |
| AFLA_037160 | 2.40 | up | thiazole biosynthesis enzyme, putative |
| AFLA_037160 | 2.29 | up | thiazole biosynthesis enzyme, putative |
| AFLA_037290 | 2.10 | down | hypothetical protein |
| AFLA_037290 | 5.14 | down | hypothetical protein |
| AFLA_040140 | 2.02 | down | Major intrinsic protein |
| AFLA_040400 | 6.62 | down | hypothetical protein |
| AFLA_041930 | 2.93 | down | conserved hypothetical protein |
| AFLA_042970 | 3.98 | down | MIF4G domain containing protein |
| AFLA_042970 | 5.44 | down | MIF4G domain containing protein |
| AFLA_046230 | 4.20 | down | amino acid permease (Dip5), putative |
| AFLA_046400 | 4.57 | down | unknown-related |
| AFLA_046400 | 4.41 | down | DUF788 domain protein |
| AFLA_051570 | 3.88 | down | To ribosomal protein YmL36 precursormitochondrial |
| AFLA_052640 | 2.07 | down | PH domain containing protein |
| AFLA_056170 | 2.10 | up | mycelial catalase Cat1, putative |
| AFLA_056170 | 2.05 | up | mycelial catalase Cat1, putative |
| AFLA_056480 | 2.91 | down | glycosyl transferase, group 2 family protein |
| AFLA_056480 | 3.85 | down | glycosyl transferase, group 2 family protein |
| AFLA_057950 | 2.50 | up | hypothetical protein |
| AFLA_057950 | 2.33 | up | hypothetical protein |
| AFLA_059660 | 2.38 | down | Major intrinsic protein |
| AFLA_060050 | 3.07 | down | Amino acid permease family protein |
| AFLA_060050 | 3.99 | down | Amino acid permease family protein |
| AFLA_060090 | 3.87 | down | Major Facilitator Superfamily protein |
| AFLA_068610 | 2.92 | down | hypothetical protein |
| AFLA_070900 | 3.43 | down | hypothetical protein |
| AFLA_073580 | 2.96 | down | cell division control protein Cdc6, putative |
| AFLA_073800 | 2.07 | up | short chain dehydrogenase/reductase family, putative |
| AFLA_073800 | 2.10 | up | short chain dehydrogenase/reductase family, putative |
| AFLA_076860 | 2.38 | down | MOSC domain containing protein |
| AFLA_080910 | 2.98 | down | hypothetical protein |
| AFLA_080910 | 2.65 | down | hypothetical protein |
| AFLA_085250 | 2.03 | down | RNA recognition motif. (a.k.a. RRM, RBD, or RNP domain) protein, putative |
| AFLA_087740 | 3.09 | down | ANTH domain containing protein |
| AFLA_089240 | 2.98 | down | Amidase family protein |
| AFLA_089240 | 3.09 | down | Amidase family protein |
| AFLA_091600 | 3.23 | down | nascent polypeptide-associated complex (NAC) subunit, putative |
| AFLA_091980 | 4.26 | down | Ctr copper transporter family protein |
| AFLA_091980 | 3.38 | down | Ctr copper transporter family protein |
| AFLA_092600 | 2.54 | down | hypothetical protein |
| AFLA_094470 | 2.58 | down | To UV radiation resistance associated protein p63 |
| AFLA_096520 | 2.11 | down | hypothetical protein |
| AFLA_096570 | 4.88 | down | hypothetical protein |
| AFLA_097790 | 2.15 | down | To chloride-bicarbonate anion exchanger AE2, putative |
| AFLA_098050 | 3.28 | down | gamma-cysteine synthetase regulatory subunit, putative |
| AFLA_099000 | 2.07 | up | Cu, Zn superoxide dismutase SOD1, putative |
| AFLA_106370 | 2.46 | down | Conserved hypothetical ATP binding protein |
| AFLA_110690 | 2.04 | up | Peroxidase family protein |
| AFLA_110690 | 2.03 | up | Peroxidase family protein |
| AFLA_111740 | 2.83 | down | To SAC1 protein, putative |
| AFLA_111740 | 3.30 | down | To SAC1 protein, putative |
| AFLA_112180 | 2.50 | down | ATP-dependent RNA helicase, putative |
| AFLA_112420 | 2.59 | down | hypothetical protein |
| AFLA_112540 | 4.63 | down | hypothetical protein |
| AFLA_112540 | 5.17 | down | hypothetical protein |
| AFLA_114570 | 3.92 | down | conserved hypothetical protein |
| AFLA_118050 | 2.31 | down | POT family protein |
| AFLA_118050 | 2.47 | down | POT family protein |
| AFLA_119430 | 2.07 | down | Sec1 family protein |
| AFLA_120960 | 2.50 | down | hypothetical protein |
| AFLA_122110 | 2.32 | up | bifunctional catalase-peroxidase Cat2 |
| AFLA_123290 | 3.12 | down | hypothetical protein |
| AFLA_123290 | 2.35 | down | hypothetical protein |
| AFLA_124420 | 2.32 | down | amine oxidase, flavin-containing family protein |
| AFLA_127570 | 3.63 | down | hypothetical protein |
| AFLA_128560 | 3.84 | down | PrnA protein, putative |
| AFLA_131410 | 3.93 | down | PCI domain containing protein |
| AFLA_132310 | 2.45 | down | tRNA intron endonuclease, catalytic C-terminal domain containing protein |
| AFLA_132310 | 3.58 | down | tRNA intron endonuclease, catalytic C-terminal domain containing protein |
| AFLA_132750 | 2.47 | down | conserved hypothetical protein |
| AFLA_133810 | 3.05 | down | conserved hypothetical protein |
| AFLA_137510 | 4.23 | down | Emopamil binding protein |
| AFLA_138590 | 2.01 | up | cysteine-type peptidase, putative |
| AO090012000538 | 2.90 | down | predicted protein |
| AO090012000538 | 2.45 | down | predicted protein |
Table S2.
Genes differentially expressed in A. flavus CA14∆msnA.
| Gene ID | Fold change | Regulation | Annotation |
|---|---|---|---|
| a: NCBI Entrez Gene (http://www.ncbi.nlm.nih.gov/gene) accession number. | |||
| AFLA_001010 | 4.06 | up | hypothetical protein |
| AFLA_002840 | 3.43 | down | conserved hypothetical protein |
| AFLA_002840 | 4.83 | down | conserved hypothetical protein |
| AFLA_002860 | 2.62 | down | Oxidoreductase family, NAD-binding Rossmann fold containing protein |
| AFLA_002940 | 2.43 | down | Glycosyl hydrolase family 76 protein |
| AFLA_003980 | 2.51 | down | cation diffusion facilitator family transporter containing protein |
| AFLA_003980 | 2.38 | down | cation diffusion facilitator family transporter containing protein |
| AFLA_004420 | 2.22 | up | hypothetical protein |
| AFLA_007830 | 2.85 | up | hypothetical protein |
| AFLA_007830 | 3.00 | up | hypothetical protein |
| AFLA_007860 | 2.09 | up | Major Facilitator Superfamily protein |
| AFLA_007860 | 2.12 | up | Major Facilitator Superfamily protein |
| AFLA_010180 | 2.42 | up | hypothetical protein |
| AFLA_010180 | 2.35 | up | hypothetical protein |
| AFLA_011370 | 2.17 | up | hypothetical protein |
| AFLA_011370 | 2.23 | up | hypothetical protein |
| AFLA_011530 | 5.11 | up | hypothetical protein |
| AFLA_011530 | 5.76 | up | hypothetical protein |
| AFLA_011560 | 2.49 | up | Phosphoesterase family protein |
| AFLA_011560 | 2.43 | up | Phosphoesterase family protein |
| AFLA_012030 | 2.10 | down | DUF895 domain membrane protein, putative |
| AFLA_012030 | 2.08 | down | DUF895 domain membrane protein, putative |
| AFLA_012050 | 2.68 | down | N-acetylglucosamine-6-phosphate deacetylase family protein |
| AFLA_012050 | 2.47 | down | N-acetylglucosamine-6-phosphate deacetylase family protein |
| AFLA_012080 | 2.74 | down | glucosamine-6-phosphate deaminase, putative |
| AFLA_012080 | 2.74 | down | glucosamine-6-phosphate deaminase, putative |
| AFLA_013060 | 2.88 | down | expressed protein, putative |
| AFLA_013060 | 2.83 | down | expressed protein, putative |
| AFLA_013740 | 2.46 | up | acid phosphatase SurE family protein |
| AFLA_013740 | 2.45 | up | acid phosphatase SurE family protein |
| AFLA_014210 | 3.22 | down | Major Facilitator Superfamily protein |
| AFLA_014210 | 3.78 | down | Major Facilitator Superfamily protein |
| AFLA_014510 | 4.89 | down | Formate/nitrite transporter family protein |
| AFLA_014510 | 4.33 | down | Formate/nitrite transporter family protein |
| AFLA_015780 | 4.29 | down | small oligopeptide transporter, OPT family protein |
| AFLA_015780 | 3.91 | down | small oligopeptide transporter, OPT family protein |
| AFLA_015800 | 2.25 | down | conserved hypothetical protein |
| AFLA_017750 | 3.37 | down | conserved hypothetical protein |
| AFLA_017750 | 2.75 | down | conserved hypothetical protein |
| AFLA_018790 | 3.16 | down | nitrate transporter (nitrate permease), putative |
| AFLA_018790 | 3.21 | down | nitrate transporter (nitrate permease), putative |
| AFLA_019510 | 2.60 | up | conserved hypothetical protein |
| AFLA_019510 | 3.04 | up | conserved hypothetical protein |
| AFLA_021000 | 2.16 | down | conserved hypothetical protein |
| AFLA_022370 | 2.08 | down | hypothetical protein |
| AFLA_023610 | 3.94 | down | hypothetical protein |
| AFLA_025130 | 4.27 | up | To blastomyces yeast phase-specific protein 1 |
| AFLA_025960 | 2.26 | down | Nucleoside transporter family protein |
| AFLA_025960 | 2.10 | down | Nucleoside transporter family protein |
| AFLA_026950 | 2.05 | down | acetyl-CoA-acetyltransferase, putative |
| AFLA_026950 | 2.22 | down | acetyl-CoA-acetyltransferase, putative |
| AFLA_028830 | 2.30 | up | FG-GAP repeat family protein |
| AFLA_028830 | 2.45 | up | FG-GAP repeat family protein |
| AFLA_028950 | 2.41 | down | Glycosyl hydrolase family 81 protein |
| AFLA_029000 | 2.03 | up | hypothetical protein |
| AFLA_029000 | 2.05 | up | hypothetical protein |
| AFLA_029970 | 3.69 | down | conserved hypothetical protein |
| AFLA_029970 | 3.51 | down | conserved hypothetical protein |
| AFLA_031380 | 3.64 | down | class V chitinase, putative |
| AFLA_031380 | 3.82 | down | class V chitinase, putative |
| AFLA_034140 | 3.10 | down | Major Facilitator Superfamily protein |
| AFLA_034140 | 2.88 | down | Major Facilitator Superfamily protein |
| AFLA_036370 | 2.37 | down | phosphoenolpyruvate carboxykinase (ATP), putative |
| AFLA_036370 | 2.24 | down | phosphoenolpyruvate carboxykinase (ATP), putative |
| AFLA_037820 | 3.75 | up | Hsp20/alpha crystallin family protein |
| AFLA_037820 | 3.81 | up | Hsp20/alpha crystallin family protein |
| AFLA_040140 | 2.07 | down | Major intrinsic protein |
| AFLA_040330 | 4.54 | down | Chitin binding Peritrophin-A domain containing protein |
| AFLA_040330 | 4.58 | down | Chitin binding Peritrophin-A domain containing protein |
| AFLA_041010 | 2.16 | up | hypothetical protein |
| AFLA_041010 | 2.16 | up | hypothetical protein |
| AFLA_041180 | 7.25 | down | hypothetical protein |
| AFLA_042000 | 2.01 | down | D-isomer specific 2-hydroxyacid dehydrogenase family protein, putative |
| AFLA_042360 | 2.07 | up | hypothetical protein |
| AFLA_042360 | 2.01 | up | hypothetical protein |
| AFLA_042540 | 2.38 | up | hypothetical protein |
| AFLA_043390 | 2.26 | down | hypothetical protein |
| AFLA_043390 | 2.13 | down | hypothetical protein |
| AFLA_044040 | 3.66 | down | hypothetical protein |
| AFLA_044720 | 3.39 | down | permease, cytosine/purines, uracil, thiamine, allantoin family protein |
| AFLA_044720 | 3.37 | down | permease, cytosine/purines, uracil, thiamine, allantoin family protein |
| AFLA_046620 | 2.91 | up | MAPEG family protein |
| AFLA_046620 | 2.66 | up | MAPEG family protein |
| AFLA_049470 | 3.22 | up | hypothetical protein |
| AFLA_049470 | 3.36 | up | hypothetical protein |
| AFLA_050070 | 2.19 | down | conserved hypothetical protein |
| AFLA_050940 | 2.08 | down | phenylalanyl-tRNA synthetase, beta subunit, putative |
| AFLA_053700 | 2.07 | up | hypothetical protein |
| AFLA_055550 | 2.75 | down | conserved hypothetical protein |
| AFLA_055550 | 2.55 | down | conserved hypothetical protein |
| AFLA_058030 | 2.77 | down | MFS transporter, putative |
| AFLA_058030 | 2.81 | down | MFS transporter, putative |
| AFLA_060260 | 2.32 | up | heat shock protein HSP30, putative |
| AFLA_062460 | 2.46 | down | non-classical export protein (Nce2), putative |
| AFLA_062460 | 2.66 | down | non-classical export protein (Nce2), putative |
| AFLA_063260 | 3.03 | down | Sic1.20-related |
| AFLA_063260 | 3.08 | down | Sic1.20-related |
| AFLA_063290 | 3.92 | down | hypothetical protein |
| AFLA_063290 | 4.09 | down | hypothetical protein |
| AFLA_063320 | 3.34 | down | hypothetical protein |
| AFLA_063320 | 3.74 | down | hypothetical protein |
| AFLA_065220 | 4.99 | up | hypothetical protein |
| AFLA_065220 | 4.93 | up | hypothetical protein |
| AFLA_065450 | 3.37 | down | Deuterolysin metalloprotease, putative |
| AFLA_065450 | 3.01 | down | Deuterolysin metalloprotease, putative |
| AFLA_065460 | 6.03 | down | hypothetical protein |
| AFLA_065460 | 7.02 | down | hypothetical protein |
| AFLA_065960 | 3.05 | up | fucose-specific lectin, putative |
| AFLA_065960 | 3.02 | up | fucose-specific lectin, putative |
| AFLA_066810 | 4.31 | up | To blastomyces yeast phase-specific protein 1 |
| AFLA_067640 | 2.15 | down | alternative NADH-dehydrogenase, putative |
| AFLA_067640 | 2.18 | down | alternative NADH-dehydrogenase, putative |
| AFLA_067770 | 2.62 | down | PQ loop repeat family protein |
| AFLA_067770 | 2.64 | down | PQ loop repeat family protein |
| AFLA_068600 | 2.89 | down | ammonium transporter MEAA, putative |
| AFLA_068600 | 2.90 | down | ammonium transporter MEAA, putative |
| AFLA_068790 | 2.23 | down | adenylylsulfate kinase, putative |
| AFLA_068790 | 2.27 | down | adenylylsulfate kinase, putative |
| AFLA_070070 | 2.09 | up | hypothetical protein |
| AFLA_070070 | 2.07 | up | hypothetical protein |
| AFLA_070470 | 2.02 | up | hypothetical protein |
| AFLA_074060 | 2.40 | down | R3H domain containing protein |
| AFLA_075190 | 2.96 | down | conserved hypothetical protein |
| AFLA_075190 | 2.94 | down | conserved hypothetical protein |
| AFLA_078210 | 2.37 | down | membrane protein-related |
| AFLA_078210 | 2.36 | down | membrane protein-related |
| AFLA_078900 | 3.11 | down | Glycosyl hydrolase family 20, catalytic domain containing protein |
| AFLA_078900 | 2.70 | down | Glycosyl hydrolase family 20, catalytic domain containing protein |
| AFLA_083890 | 2.60 | up | oxidoreductase, zinc-binding dehydrogenase family protein |
| AFLA_083890 | 2.59 | up | oxidoreductase, zinc-binding dehydrogenase family protein |
| AFLA_087630 | 2.96 | down | alpha, alpha-trehalose-phosphate synthase subunit, putative |
| AFLA_087630 | 2.36 | down | alpha, alpha-trehalose-phosphate synthase subunit, putative |
| AFLA_087750 | 2.96 | down | isopentenyl-diphosphate delta-isomerase, putative |
| AFLA_090690 | 2.25 | up | catalase A, putative |
| AFLA_090690 | 2.73 | up | catalase A, putative |
| AFLA_090970 | 2.24 | down | conserved hypothetical protein |
| AFLA_090970 | 2.09 | down | conserved hypothetical protein |
| AFLA_091260 | 2.08 | down | acetyltransferase, GNAT family protein |
| AFLA_091260 | 2.15 | down | acetyltransferase, GNAT family protein |
| AFLA_094630 | 2.11 | down | hypothetical protein |
| AFLA_094630 | 2.16 | down | hypothetical protein |
| AFLA_095460 | 2.39 | down | PBS lyase HEAT-like repeat family protein |
| AFLA_098380 | 3.39 | down | conidial hydrophobin RodA, putative |
| AFLA_098380 | 3.77 | down | conidial hydrophobin RodA, putative |
| AFLA_098700 | 2.54 | down | oxidoreductase, short chain dehydrogenase/reductase family protein |
| AFLA_098700 | 2.47 | down | oxidoreductase, short chain dehydrogenase/reductase family protein |
| AFLA_099050 | 3.83 | down | hypothetical protein |
| AFLA_099050 | 3.70 | down | hypothetical protein |
| AFLA_101780 | 2.41 | down | Oxidoreductase molybdopterin binding domain containing protein |
| AFLA_101780 | 2.20 | down | Oxidoreductase molybdopterin binding domain containing protein |
| AFLA_101800 | 3.81 | down | Glycosyl hydrolases family 18 protein |
| AFLA_101800 | 4.33 | down | Glycosyl hydrolases family 18 protein |
| AFLA_104350 | 8.01 | down | Dynamin central region family protein |
| AFLA_104350 | 6.90 | down | Dynamin central region family protein |
| AFLA_105630 | 3.94 | up | Cytochrome P450 family protein |
| AFLA_105630 | 3.82 | up | Cytochrome P450 family protein |
| AFLA_109030 | 3.01 | down | To nucleotide exsicion repair protein RAD7 |
| AFLA_109160 | 3.31 | down | isopentenyl-diphosphate delta-isomerase, putative |
| AFLA_110040 | 5.26 | down | blr7677-related |
| AFLA_110040 | 6.41 | down | blr7677-related |
| AFLA_112720 | 2.26 | down | diphosphomevalonate decarboxylase, putative |
| AFLA_112910 | 2.85 | up | hypothetical protein |
| AFLA_112910 | 2.89 | up | hypothetical protein |
| AFLA_113790 | 2.78 | down | hypothetical protein |
| AFLA_113790 | 2.39 | down | hypothetical protein |
| AFLA_115930 | 3.40 | up | hypothetical protein |
| AFLA_115930 | 3.22 | up | hypothetical protein |
| AFLA_119340 | 2.12 | up | Helix-loop-helix DNA-binding domain containing protein |
| AFLA_119340 | 2.29 | up | Helix-loop-helix DNA-binding domain containing protein |
| AFLA_125770 | 3.03 | down | hypothetical protein |
| AFLA_125770 | 3.25 | down | hypothetical protein |
| AFLA_127620 | 7.70 | down | New cDNA-based gene: (AO_CDS_042706, novel, updateIDs: 11597, [gene: novel_gene_1223, model: novel_model_1223]) |
| AFLA_127620 | 11.51 | down | New cDNA-based gene: (AO_CDS_042706, novel, updateIDs: 11597, [gene: novel_gene_1223, model: novel_model_1223]) |
| AFLA_129810 | 3.08 | down | cytoplasmic asparaginyl-tRNA synthetase, putative |
| AFLA_129810 | 2.94 | down | cytoplasmic asparaginyl-tRNA synthetase, putative |
| AFLA_130150 | 2.02 | down | NAD+ dependent glutamate dehydrogenase, putative |
| AFLA_133830 | 2.02 | down | oxidoreductase, zinc-binding dehydrogenase family protein |
| AFLA_134420 | 2.02 | up | Sugar transporter family protein |
| AFLA_139270 | 2.28 | up | aflNa/ hypD/ hypothetical protein |
| AFLA_139270 | 2.37 | up | aflNa/ hypD/ hypothetical protein |
| AFLA_139290 | 3.06 | up | aflMa/ hypE/ hypothetical protein |
| AFLA_139400 | 2.17 | up | aflCa/hypC/hypothetical protein |
| AFLA_139400 | 2.03 | up | aflCa/hypC/hypothetical protein |
| AO090120000447 | 6.76 | down | predicted protein |
| AO090120000447 | 5.18 | down | predicted protein |
References
- 1.Ruis H., Schuller C. Stress signaling in yeast. Bioessays. 1995;17:959–965. doi: 10.1002/bies.950171109. [DOI] [PubMed] [Google Scholar]
- 2.Schmitt A.P., McEntee K. Msn2p, a zinc finger DNA-binding protein, is the transcriptional activator of the multistress response in Saccharomyces cerevisia. Proc. Natl. Acad. Sci. USA. 1996;93:5777–5782. doi: 10.1073/pnas.93.12.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Treger J.M., Magee T.R., McEntee K. Functional analysis of the stress response element and its role in the multistress response of Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 1998;243:13–19. doi: 10.1006/bbrc.1997.8061. [DOI] [PubMed] [Google Scholar]
- 4.Peterbauer C.K., Litscher D., Kubicek C.P. The Trichoderma atroviride seb1 (stress response element binding) gene encodes an AGGGG-binding protein which is involved in the response to high osmolarity stress. Mol. Genet. Genom. 2002;268:223–231. doi: 10.1007/s00438-002-0732-z. [DOI] [PubMed] [Google Scholar]
- 5.Seidl V., Seiboth B., Karaffa L., Kubicek C.P. The fungal STRE-element-binding protein Seb1 is involved but not essential for glycerol dehydrogenase (gld1) gene expression and glycerol accumulation in Trichoderma atroviride during osmotic stress. Fungal Genet. Biol. 2004;41:1132–1140. doi: 10.1016/j.fgb.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 6.Aguirre J., Rios-Momberg M., Hewitt D., Hansberg W. Reactive oxygen species and development in microbial eukaryotes. Trends Microbiol. 2005;13:111–118. doi: 10.1016/j.tim.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 7.Georgiou C.D., Patsoukis N., Papapostolou I., Zervoudakis G. Sclerotial metamorphosis in filamentous fungi is induced by oxidative stress. Integ. Comp. Biol. 2006;46:691–712. doi: 10.1093/icb/icj034. [DOI] [PubMed] [Google Scholar]
- 8.Jeon M.-H., Hagiwara D., Yoshimi A., Abe K., Han D.-M., Chae S.-K. Analysis of nrdA, a negative regulator of differentiation in Aspergillus nidulans; Proceedings of 25thFungal Genetics Conference; Pacific Grove, CA, USA. 17-22 March 2009. [Google Scholar]
- 9.Horn B.W., Moore G.G., Carbone I. Sexual reproduction in Aspergillus flavus. Mycologia. 2009;101:423–429. doi: 10.3852/09-011. [DOI] [PubMed] [Google Scholar]
- 10.Horn B.W., Ramirez-Prado J.H., Carbone I. Sexual reproduction and recombination in the aflatoxin-producing fungus Aspergillus parasiticus. Fungal Genet. Biol. 2009;46:169–175. doi: 10.1016/j.fgb.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Horn B.W., Greene R.L., Sobolev V.S., Dorner J.W., Powell J.H., Layton R.C. Association of morphology and mycotoxin production with vegetative compatibility groups in Aspergillus flavus, A. parasiticus, and A. tamarii. Mycologia . 1996;88:574–587. doi: 10.2307/3761151. [DOI] [Google Scholar]
- 12.Cotty P.J. Virulence and cultural characteristics of two Aspergillus flavus strains pathogenic on cotton. Phytopathology. 1989;79:808–814. doi: 10.1094/Phyto-79-808. [DOI] [Google Scholar]
- 13.Ehrlich K.C., Scharfenstein L.L., Montalbano B.G., Chang P.-K. Are the genes nadA and norB involved in formation of aflatoxin G1? Int. J. Mol. Sci. 2008;9:1717–1729. doi: 10.3390/ijms9091717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang P.-K. A highly efficient gene-targeting system for Aspergillus parasiticus. Lett. Appl. Microbiol. 2008;46:587–592. doi: 10.1111/j.1472-765X.2008.02345.x. [DOI] [PubMed] [Google Scholar]
- 15.Chang P.-K., Scharfenstein L.L., Wei Q., Bhatnagar D. Development and refinement of a high-efficiency gene-targeting system for Aspergillus flavus. J. Microbiol. Methods. 2010;81:240–246. doi: 10.1016/j.mimet.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 16.McAlpin C.E., Wicklow D.T. Culture media and sources of nitrogen promoting the formation of stromata and ascocarps in Petromyces alliaceus (Aspergillus section Flavi) Can. J. Microbiol. 2005;51:765–771. doi: 10.1139/w05-057. [DOI] [PubMed] [Google Scholar]
- 17.Kubodera T., Yamashita N., Nishimura A. Pyrithiamine resistance gene (ptrA) of Aspergillus oryzae: cloning, characterization and application as a dominant selectable marker for transformation. Biosci. Biotechnol. Biochem. 2000;64:1416–1421. doi: 10.1271/bbb.64.1416. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi T., Chang P.-K., Matsushima K., Yu J., Abe K., Bhatnagar D., Cleveland T.E., Koyama Y. Nonfunctionality of Aspergillus sojae aflR in a strain of Aspergillus parasiticus with a disrupted aflR gene. Appl. Environ. Microbiol. 2002;68:3737–3743. doi: 10.1128/AEM.68.8.3737-3743.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gems D.H., Clutterbuck A.J. Co-transformation with autonomously-replicating helper plasmids facilitates gene cloning from an Aspergillus nidulans gene library. Curr. Genet. 1993;24:520–524. doi: 10.1007/BF00351716. [DOI] [PubMed] [Google Scholar]
- 20.Wan C., Sun C., Yu J., Niermann W., Fedorova N., Varga J. JCVI Aspergillus flavus NRRL3357 27.6k oligo array. [(Accessed on 6 January 2011)]. Available online: http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GPL7117.
- 21.McBryde W.A.E., Atkinson G.F. Spectrophotometric study of the reaction between iron(III) and kojic acid. Can. J. Chem. 1961;39:510–525. [Google Scholar]
- 22.Jeon M.-H., Hagiwara D., Yoshimi A., Abe K., Han D.-M., Chae S.-K. Analysis of nrdA, a negative regulator of differentiation in Aspergillus nidulans; Proceedings of 25th Fungal Genetics Conference; Pacific Grove, CA, USA. 17-22 March 2009. [Google Scholar]
- 23.Navarro R.E., Stringer M.A., Hansberg W., Timberlake W.E., Aguirre J. catA, a new Aspergillus nidulans gene encoding a developmentally regulated catalase. Curr. Genet. 1996;29:352–359. [PubMed] [Google Scholar]
- 24.Hansberg W., de Groot H., Sies H. Reactive oxygen species associated with cell differentiation in Neurospora crassa. Free Radic. Biol. Med. 1993;14:287–293. doi: 10.1016/0891-5849(93)90025-P. [DOI] [PubMed] [Google Scholar]
- 25.Michan S., Lledias F., Hansberg W. Asexual development is increased in Neurospora crassa cat-3-null mutant strains. Eukaryot. Cell. 2003;2:798–808. doi: 10.1128/EC.2.4.798-808.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Patsoukis N., Georgiou C.D. Effect of thiol redox state modulators on oxidative stress and sclerotial differentiation of the phytopathogenic fungus Rhizoctonia solani. Arch. Microbiol. 2007;188:225–233. doi: 10.1007/s00203-007-0237-6. [DOI] [PubMed] [Google Scholar]
- 27.Patsoukis N., Georgiou D.C. Thiol redox state and related enzymes in sclerotium-forming filamentous phytopathogenic fungi. Mycol. Res. 2008;112:602–610. doi: 10.1016/j.mycres.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 28.Geiser D.M., Timberlake W.E., Arnold M.L. Loss of meiosis in Aspergillus. Mol. Biol. Evol. 1996;13:809–817. doi: 10.1093/oxfordjournals.molbev.a025641. [DOI] [PubMed] [Google Scholar]
- 29.Calvo A.M., Bok J., Brooks W., Keller N.P. veA is required for toxin and sclerotial production in Aspergillus parasiticus. Appl. Environ. Microbiol. 2004;70:4733–4739. doi: 10.1128/AEM.70.8.4733-4739.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cary J.W., O'Brian G.R., Nielsen D.M., Nierman W., Harris-Coward P., Yu J., Bhatnagar D., Cleveland T.E., Payne G.A., Calvo A.M. Elucidation of veA-dependent genes associated with aflatoxin and sclerotial production in Aspergillus flavus by functional genomics. Appl. Microbiol. Biotechnol. 2007;76:1107–1118. doi: 10.1007/s00253-007-1081-y. [DOI] [PubMed] [Google Scholar]
- 31.Kim H., Han K., Kim K., Han D., Jahng K., Chae K. The veA gene activates sexual development in Aspergillus nidulans. Fungal Genet. Biol. 2002;37:72–80. doi: 10.1016/S1087-1845(02)00029-4. [DOI] [PubMed] [Google Scholar]
- 32.Mooney J.L., Yager L.N. Light is required for conidiation in Aspergillus nidulans. Genes Devel. 1990;4:1473–1482. doi: 10.1101/gad.4.9.1473. [DOI] [PubMed] [Google Scholar]
- 33.Purschwitz J., Muller S., Kastner C., Schoser M., Haas H., Espeso E.A., Atoui A., Calvo A.M., Fischer R. Functional and physical interaction of blue- and red-light sensors in Aspergillus nidulans. Curr. Biol. 2008;18:255–259. doi: 10.1016/j.cub.2008.01.061. [DOI] [PubMed] [Google Scholar]
- 34.Bennett J.W., Fernholz F.A., Lee L.S. Effect of light on aflatoxins, anthraquinones, and sclerotia in Aspergillus flavus and parasiticus. Mycologia. 1978;70:104–116. doi: 10.2307/3758691. [DOI] [PubMed] [Google Scholar]
- 35.Kale S.P., Milde L., Trapp M.K., Frisvad J.C., Keller N.P., Bok J.W. Requirement of LaeA for secondary metabolism and sclerotial production in Aspergillus flavus. Fungal Genet. Biol. 2008;45:1422–1429. doi: 10.1016/j.fgb.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narasaiah K.V., Sashidhar R.B., Subramanyam C. Biochemical analysis of oxidative stress in the production of aflatoxin and its precursor intermediates. Mycopathologia. 2006;162:179–189. doi: 10.1007/s11046-006-0052-7. [DOI] [PubMed] [Google Scholar]
- 37.Kim J.H., Campbell B.C., Yu J., Mahoney N., Chan K.L., Molyneux R.J., Bhatnagar D., Cleveland T.E. Examination of fungal stress response genes using Saccharomy cescerevisiae as a model system: targeting genes affecting aflatoxin biosynthesis by Aspergillus flavus Link. Appl. Microbiol. Biotechnol. 2005;67:807–815. doi: 10.1007/s00253-004-1821-1. [DOI] [PubMed] [Google Scholar]
- 38.Reverberi M., Zjalic S., Ricelli A., Punelli F., Camera E., Fabbri C., Picardo M., Fanelli C., Fabbri A.A. Modulation of antioxidant defense in Aspergillus parasiticus is involved in aflatoxin biosynthesis: a role for the ApyapA gene. Eukaryot. Cell. 2008;7:988–1000. doi: 10.1128/EC.00228-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jayashree T., Subramanyam C. Antiaflatoxigenic activity of eugenol is due to inhibition of lipid peroxidation. Lett. Appl. Microbiol. 1999;28:179–183. doi: 10.1046/j.1365-2672.1999.00511.x. [DOI] [PubMed] [Google Scholar]
- 40.Mahoney N., Molyneux R.J. Phytochemical inhibition of aflatoxigenicity in Aspergillus flavus by constituents of walnut (Juglans regia) J. Agric. Food Chem. 2004;52:1882–1889. doi: 10.1021/jf030812p. [DOI] [PubMed] [Google Scholar]
- 41.Kim J.H., Yu J., Mahoney N., Chan K.L., Molyneux R.J., Varga J., Bhatnagar D., Cleveland T.E., Nierman W.C., Campbell B.C. Elucidation of the functional genomics of antioxidant-based inhibition of aflatoxin biosynthesis. Int. J. Food Microbiol. 2008;122:49–60. doi: 10.1016/j.ijfoodmicro.2007.11.058. [DOI] [PubMed] [Google Scholar]
- 42.Reverberi M., Fabbri A.A., Zjalic S., Ricelli A., Punelli F., Fanelli C. Antioxidant enzymes stimulation in Aspergillus parasiticus by Lentinula edodes inhibits aflatoxin production. Appl. Microbiol. Biotechnol. 2005;69:207–215. doi: 10.1007/s00253-005-1979-1. [DOI] [PubMed] [Google Scholar]
- 43.Niwa Y., Akamatsu H. Kojic acid scavenges free radicals while potentiating leukocyte functions including free radical generation. Inflammation. 1991;15:303–315. doi: 10.1007/BF00917315. [DOI] [PubMed] [Google Scholar]
- 44.Halliwell B., Chirico S. Lipid peroxidation: its mechanism, measurement, and significance. Am. J. Clin. Nutr. 1993;57:715–724. doi: 10.1093/ajcn/57.5.715S. [DOI] [PubMed] [Google Scholar]
- 45.Keyer K., Gort A.S., Imlay J.A. Superoxide and the production of oxidative DNA damage. J. Bacteriol. 1995;177:6782–6790. doi: 10.1128/jb.177.23.6782-6790.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Genes differentially expressed in A. parasiticus BN9∆msnA.
| Gene ID (a) | Fold change | Regulation | Annotation |
|---|---|---|---|
| (a) NCBI Entrez Gene (http://www.ncbi.nlm.nih.gov/gene) accession number. | |||
| AFLA_003050 | 2.13 | down | RTA1 like protein |
| AFLA_003050 | 2.12 | down | RTA1 like protein |
| AFLA_003090 | 2.10 | down | hypothetical protein |
| AFLA_004690 | 2.12 | up | hypothetical protein |
| AFLA_004690 | 2.21 | up | hypothetical protein |
| AFLA_004940 | 8.07 | down | Glycolipid 2-alpha-mannosyltransferase family protein |
| AFLA_005520 | 3.38 | down | hypothetical protein |
| AFLA_006160 | 2.90 | down | hypothetical protein |
| AFLA_011970 | 2.22 | down | Major Facilitator Superfamily protein |
| AFLA_014260 | 3.08 | up | hydrophobin, putative |
| AFLA_014960 | 2.73 | down | hypothetical protein |
| AFLA_015300 | 2.03 | down | FMN-dependent dehydrogenase family protein |
| AFLA_015300 | 2.43 | down | FMN-dependent dehydrogenase family protein |
| AFLA_015550 | 4.26 | down | Sugar transporter family protein |
| AFLA_015780 | 5.24 | down | small oligopeptide transporter, OPT family protein |
| AFLA_017860 | 2.00 | up | hypothetical protein |
| AFLA_024250 | 2.01 | up | Amidase family protein |
| AFLA_027340 | 2.04 | down | Aha1 domain family, putative |
| AFLA_027340 | 2.16 | down | Aha1 domain family, putative |
| AFLA_028030 | 3.03 | down | conserved hypothetical protein |
| AFLA_029390 | 4.22 | down | HMG box family protein |
| AFLA_036040 | 2.18 | down | mitochondrial inner membrane translocase subunit (TIM17), putative |
| AFLA_036040 | 2.23 | down | mitochondrial inner membrane translocase subunit (TIM17), putative |
| AFLA_036980 | 2.76 | down | MOSC N-terminal beta barrel domain containing protein |
| AFLA_037160 | 2.40 | up | thiazole biosynthesis enzyme, putative |
| AFLA_037160 | 2.29 | up | thiazole biosynthesis enzyme, putative |
| AFLA_037290 | 2.10 | down | hypothetical protein |
| AFLA_037290 | 5.14 | down | hypothetical protein |
| AFLA_040140 | 2.02 | down | Major intrinsic protein |
| AFLA_040400 | 6.62 | down | hypothetical protein |
| AFLA_041930 | 2.93 | down | conserved hypothetical protein |
| AFLA_042970 | 3.98 | down | MIF4G domain containing protein |
| AFLA_042970 | 5.44 | down | MIF4G domain containing protein |
| AFLA_046230 | 4.20 | down | amino acid permease (Dip5), putative |
| AFLA_046400 | 4.57 | down | unknown-related |
| AFLA_046400 | 4.41 | down | DUF788 domain protein |
| AFLA_051570 | 3.88 | down | To ribosomal protein YmL36 precursormitochondrial |
| AFLA_052640 | 2.07 | down | PH domain containing protein |
| AFLA_056170 | 2.10 | up | mycelial catalase Cat1, putative |
| AFLA_056170 | 2.05 | up | mycelial catalase Cat1, putative |
| AFLA_056480 | 2.91 | down | glycosyl transferase, group 2 family protein |
| AFLA_056480 | 3.85 | down | glycosyl transferase, group 2 family protein |
| AFLA_057950 | 2.50 | up | hypothetical protein |
| AFLA_057950 | 2.33 | up | hypothetical protein |
| AFLA_059660 | 2.38 | down | Major intrinsic protein |
| AFLA_060050 | 3.07 | down | Amino acid permease family protein |
| AFLA_060050 | 3.99 | down | Amino acid permease family protein |
| AFLA_060090 | 3.87 | down | Major Facilitator Superfamily protein |
| AFLA_068610 | 2.92 | down | hypothetical protein |
| AFLA_070900 | 3.43 | down | hypothetical protein |
| AFLA_073580 | 2.96 | down | cell division control protein Cdc6, putative |
| AFLA_073800 | 2.07 | up | short chain dehydrogenase/reductase family, putative |
| AFLA_073800 | 2.10 | up | short chain dehydrogenase/reductase family, putative |
| AFLA_076860 | 2.38 | down | MOSC domain containing protein |
| AFLA_080910 | 2.98 | down | hypothetical protein |
| AFLA_080910 | 2.65 | down | hypothetical protein |
| AFLA_085250 | 2.03 | down | RNA recognition motif. (a.k.a. RRM, RBD, or RNP domain) protein, putative |
| AFLA_087740 | 3.09 | down | ANTH domain containing protein |
| AFLA_089240 | 2.98 | down | Amidase family protein |
| AFLA_089240 | 3.09 | down | Amidase family protein |
| AFLA_091600 | 3.23 | down | nascent polypeptide-associated complex (NAC) subunit, putative |
| AFLA_091980 | 4.26 | down | Ctr copper transporter family protein |
| AFLA_091980 | 3.38 | down | Ctr copper transporter family protein |
| AFLA_092600 | 2.54 | down | hypothetical protein |
| AFLA_094470 | 2.58 | down | To UV radiation resistance associated protein p63 |
| AFLA_096520 | 2.11 | down | hypothetical protein |
| AFLA_096570 | 4.88 | down | hypothetical protein |
| AFLA_097790 | 2.15 | down | To chloride-bicarbonate anion exchanger AE2, putative |
| AFLA_098050 | 3.28 | down | gamma-cysteine synthetase regulatory subunit, putative |
| AFLA_099000 | 2.07 | up | Cu, Zn superoxide dismutase SOD1, putative |
| AFLA_106370 | 2.46 | down | Conserved hypothetical ATP binding protein |
| AFLA_110690 | 2.04 | up | Peroxidase family protein |
| AFLA_110690 | 2.03 | up | Peroxidase family protein |
| AFLA_111740 | 2.83 | down | To SAC1 protein, putative |
| AFLA_111740 | 3.30 | down | To SAC1 protein, putative |
| AFLA_112180 | 2.50 | down | ATP-dependent RNA helicase, putative |
| AFLA_112420 | 2.59 | down | hypothetical protein |
| AFLA_112540 | 4.63 | down | hypothetical protein |
| AFLA_112540 | 5.17 | down | hypothetical protein |
| AFLA_114570 | 3.92 | down | conserved hypothetical protein |
| AFLA_118050 | 2.31 | down | POT family protein |
| AFLA_118050 | 2.47 | down | POT family protein |
| AFLA_119430 | 2.07 | down | Sec1 family protein |
| AFLA_120960 | 2.50 | down | hypothetical protein |
| AFLA_122110 | 2.32 | up | bifunctional catalase-peroxidase Cat2 |
| AFLA_123290 | 3.12 | down | hypothetical protein |
| AFLA_123290 | 2.35 | down | hypothetical protein |
| AFLA_124420 | 2.32 | down | amine oxidase, flavin-containing family protein |
| AFLA_127570 | 3.63 | down | hypothetical protein |
| AFLA_128560 | 3.84 | down | PrnA protein, putative |
| AFLA_131410 | 3.93 | down | PCI domain containing protein |
| AFLA_132310 | 2.45 | down | tRNA intron endonuclease, catalytic C-terminal domain containing protein |
| AFLA_132310 | 3.58 | down | tRNA intron endonuclease, catalytic C-terminal domain containing protein |
| AFLA_132750 | 2.47 | down | conserved hypothetical protein |
| AFLA_133810 | 3.05 | down | conserved hypothetical protein |
| AFLA_137510 | 4.23 | down | Emopamil binding protein |
| AFLA_138590 | 2.01 | up | cysteine-type peptidase, putative |
| AO090012000538 | 2.90 | down | predicted protein |
| AO090012000538 | 2.45 | down | predicted protein |
Table S2.
Genes differentially expressed in A. flavus CA14∆msnA.
| Gene ID | Fold change | Regulation | Annotation |
|---|---|---|---|
| a: NCBI Entrez Gene (http://www.ncbi.nlm.nih.gov/gene) accession number. | |||
| AFLA_001010 | 4.06 | up | hypothetical protein |
| AFLA_002840 | 3.43 | down | conserved hypothetical protein |
| AFLA_002840 | 4.83 | down | conserved hypothetical protein |
| AFLA_002860 | 2.62 | down | Oxidoreductase family, NAD-binding Rossmann fold containing protein |
| AFLA_002940 | 2.43 | down | Glycosyl hydrolase family 76 protein |
| AFLA_003980 | 2.51 | down | cation diffusion facilitator family transporter containing protein |
| AFLA_003980 | 2.38 | down | cation diffusion facilitator family transporter containing protein |
| AFLA_004420 | 2.22 | up | hypothetical protein |
| AFLA_007830 | 2.85 | up | hypothetical protein |
| AFLA_007830 | 3.00 | up | hypothetical protein |
| AFLA_007860 | 2.09 | up | Major Facilitator Superfamily protein |
| AFLA_007860 | 2.12 | up | Major Facilitator Superfamily protein |
| AFLA_010180 | 2.42 | up | hypothetical protein |
| AFLA_010180 | 2.35 | up | hypothetical protein |
| AFLA_011370 | 2.17 | up | hypothetical protein |
| AFLA_011370 | 2.23 | up | hypothetical protein |
| AFLA_011530 | 5.11 | up | hypothetical protein |
| AFLA_011530 | 5.76 | up | hypothetical protein |
| AFLA_011560 | 2.49 | up | Phosphoesterase family protein |
| AFLA_011560 | 2.43 | up | Phosphoesterase family protein |
| AFLA_012030 | 2.10 | down | DUF895 domain membrane protein, putative |
| AFLA_012030 | 2.08 | down | DUF895 domain membrane protein, putative |
| AFLA_012050 | 2.68 | down | N-acetylglucosamine-6-phosphate deacetylase family protein |
| AFLA_012050 | 2.47 | down | N-acetylglucosamine-6-phosphate deacetylase family protein |
| AFLA_012080 | 2.74 | down | glucosamine-6-phosphate deaminase, putative |
| AFLA_012080 | 2.74 | down | glucosamine-6-phosphate deaminase, putative |
| AFLA_013060 | 2.88 | down | expressed protein, putative |
| AFLA_013060 | 2.83 | down | expressed protein, putative |
| AFLA_013740 | 2.46 | up | acid phosphatase SurE family protein |
| AFLA_013740 | 2.45 | up | acid phosphatase SurE family protein |
| AFLA_014210 | 3.22 | down | Major Facilitator Superfamily protein |
| AFLA_014210 | 3.78 | down | Major Facilitator Superfamily protein |
| AFLA_014510 | 4.89 | down | Formate/nitrite transporter family protein |
| AFLA_014510 | 4.33 | down | Formate/nitrite transporter family protein |
| AFLA_015780 | 4.29 | down | small oligopeptide transporter, OPT family protein |
| AFLA_015780 | 3.91 | down | small oligopeptide transporter, OPT family protein |
| AFLA_015800 | 2.25 | down | conserved hypothetical protein |
| AFLA_017750 | 3.37 | down | conserved hypothetical protein |
| AFLA_017750 | 2.75 | down | conserved hypothetical protein |
| AFLA_018790 | 3.16 | down | nitrate transporter (nitrate permease), putative |
| AFLA_018790 | 3.21 | down | nitrate transporter (nitrate permease), putative |
| AFLA_019510 | 2.60 | up | conserved hypothetical protein |
| AFLA_019510 | 3.04 | up | conserved hypothetical protein |
| AFLA_021000 | 2.16 | down | conserved hypothetical protein |
| AFLA_022370 | 2.08 | down | hypothetical protein |
| AFLA_023610 | 3.94 | down | hypothetical protein |
| AFLA_025130 | 4.27 | up | To blastomyces yeast phase-specific protein 1 |
| AFLA_025960 | 2.26 | down | Nucleoside transporter family protein |
| AFLA_025960 | 2.10 | down | Nucleoside transporter family protein |
| AFLA_026950 | 2.05 | down | acetyl-CoA-acetyltransferase, putative |
| AFLA_026950 | 2.22 | down | acetyl-CoA-acetyltransferase, putative |
| AFLA_028830 | 2.30 | up | FG-GAP repeat family protein |
| AFLA_028830 | 2.45 | up | FG-GAP repeat family protein |
| AFLA_028950 | 2.41 | down | Glycosyl hydrolase family 81 protein |
| AFLA_029000 | 2.03 | up | hypothetical protein |
| AFLA_029000 | 2.05 | up | hypothetical protein |
| AFLA_029970 | 3.69 | down | conserved hypothetical protein |
| AFLA_029970 | 3.51 | down | conserved hypothetical protein |
| AFLA_031380 | 3.64 | down | class V chitinase, putative |
| AFLA_031380 | 3.82 | down | class V chitinase, putative |
| AFLA_034140 | 3.10 | down | Major Facilitator Superfamily protein |
| AFLA_034140 | 2.88 | down | Major Facilitator Superfamily protein |
| AFLA_036370 | 2.37 | down | phosphoenolpyruvate carboxykinase (ATP), putative |
| AFLA_036370 | 2.24 | down | phosphoenolpyruvate carboxykinase (ATP), putative |
| AFLA_037820 | 3.75 | up | Hsp20/alpha crystallin family protein |
| AFLA_037820 | 3.81 | up | Hsp20/alpha crystallin family protein |
| AFLA_040140 | 2.07 | down | Major intrinsic protein |
| AFLA_040330 | 4.54 | down | Chitin binding Peritrophin-A domain containing protein |
| AFLA_040330 | 4.58 | down | Chitin binding Peritrophin-A domain containing protein |
| AFLA_041010 | 2.16 | up | hypothetical protein |
| AFLA_041010 | 2.16 | up | hypothetical protein |
| AFLA_041180 | 7.25 | down | hypothetical protein |
| AFLA_042000 | 2.01 | down | D-isomer specific 2-hydroxyacid dehydrogenase family protein, putative |
| AFLA_042360 | 2.07 | up | hypothetical protein |
| AFLA_042360 | 2.01 | up | hypothetical protein |
| AFLA_042540 | 2.38 | up | hypothetical protein |
| AFLA_043390 | 2.26 | down | hypothetical protein |
| AFLA_043390 | 2.13 | down | hypothetical protein |
| AFLA_044040 | 3.66 | down | hypothetical protein |
| AFLA_044720 | 3.39 | down | permease, cytosine/purines, uracil, thiamine, allantoin family protein |
| AFLA_044720 | 3.37 | down | permease, cytosine/purines, uracil, thiamine, allantoin family protein |
| AFLA_046620 | 2.91 | up | MAPEG family protein |
| AFLA_046620 | 2.66 | up | MAPEG family protein |
| AFLA_049470 | 3.22 | up | hypothetical protein |
| AFLA_049470 | 3.36 | up | hypothetical protein |
| AFLA_050070 | 2.19 | down | conserved hypothetical protein |
| AFLA_050940 | 2.08 | down | phenylalanyl-tRNA synthetase, beta subunit, putative |
| AFLA_053700 | 2.07 | up | hypothetical protein |
| AFLA_055550 | 2.75 | down | conserved hypothetical protein |
| AFLA_055550 | 2.55 | down | conserved hypothetical protein |
| AFLA_058030 | 2.77 | down | MFS transporter, putative |
| AFLA_058030 | 2.81 | down | MFS transporter, putative |
| AFLA_060260 | 2.32 | up | heat shock protein HSP30, putative |
| AFLA_062460 | 2.46 | down | non-classical export protein (Nce2), putative |
| AFLA_062460 | 2.66 | down | non-classical export protein (Nce2), putative |
| AFLA_063260 | 3.03 | down | Sic1.20-related |
| AFLA_063260 | 3.08 | down | Sic1.20-related |
| AFLA_063290 | 3.92 | down | hypothetical protein |
| AFLA_063290 | 4.09 | down | hypothetical protein |
| AFLA_063320 | 3.34 | down | hypothetical protein |
| AFLA_063320 | 3.74 | down | hypothetical protein |
| AFLA_065220 | 4.99 | up | hypothetical protein |
| AFLA_065220 | 4.93 | up | hypothetical protein |
| AFLA_065450 | 3.37 | down | Deuterolysin metalloprotease, putative |
| AFLA_065450 | 3.01 | down | Deuterolysin metalloprotease, putative |
| AFLA_065460 | 6.03 | down | hypothetical protein |
| AFLA_065460 | 7.02 | down | hypothetical protein |
| AFLA_065960 | 3.05 | up | fucose-specific lectin, putative |
| AFLA_065960 | 3.02 | up | fucose-specific lectin, putative |
| AFLA_066810 | 4.31 | up | To blastomyces yeast phase-specific protein 1 |
| AFLA_067640 | 2.15 | down | alternative NADH-dehydrogenase, putative |
| AFLA_067640 | 2.18 | down | alternative NADH-dehydrogenase, putative |
| AFLA_067770 | 2.62 | down | PQ loop repeat family protein |
| AFLA_067770 | 2.64 | down | PQ loop repeat family protein |
| AFLA_068600 | 2.89 | down | ammonium transporter MEAA, putative |
| AFLA_068600 | 2.90 | down | ammonium transporter MEAA, putative |
| AFLA_068790 | 2.23 | down | adenylylsulfate kinase, putative |
| AFLA_068790 | 2.27 | down | adenylylsulfate kinase, putative |
| AFLA_070070 | 2.09 | up | hypothetical protein |
| AFLA_070070 | 2.07 | up | hypothetical protein |
| AFLA_070470 | 2.02 | up | hypothetical protein |
| AFLA_074060 | 2.40 | down | R3H domain containing protein |
| AFLA_075190 | 2.96 | down | conserved hypothetical protein |
| AFLA_075190 | 2.94 | down | conserved hypothetical protein |
| AFLA_078210 | 2.37 | down | membrane protein-related |
| AFLA_078210 | 2.36 | down | membrane protein-related |
| AFLA_078900 | 3.11 | down | Glycosyl hydrolase family 20, catalytic domain containing protein |
| AFLA_078900 | 2.70 | down | Glycosyl hydrolase family 20, catalytic domain containing protein |
| AFLA_083890 | 2.60 | up | oxidoreductase, zinc-binding dehydrogenase family protein |
| AFLA_083890 | 2.59 | up | oxidoreductase, zinc-binding dehydrogenase family protein |
| AFLA_087630 | 2.96 | down | alpha, alpha-trehalose-phosphate synthase subunit, putative |
| AFLA_087630 | 2.36 | down | alpha, alpha-trehalose-phosphate synthase subunit, putative |
| AFLA_087750 | 2.96 | down | isopentenyl-diphosphate delta-isomerase, putative |
| AFLA_090690 | 2.25 | up | catalase A, putative |
| AFLA_090690 | 2.73 | up | catalase A, putative |
| AFLA_090970 | 2.24 | down | conserved hypothetical protein |
| AFLA_090970 | 2.09 | down | conserved hypothetical protein |
| AFLA_091260 | 2.08 | down | acetyltransferase, GNAT family protein |
| AFLA_091260 | 2.15 | down | acetyltransferase, GNAT family protein |
| AFLA_094630 | 2.11 | down | hypothetical protein |
| AFLA_094630 | 2.16 | down | hypothetical protein |
| AFLA_095460 | 2.39 | down | PBS lyase HEAT-like repeat family protein |
| AFLA_098380 | 3.39 | down | conidial hydrophobin RodA, putative |
| AFLA_098380 | 3.77 | down | conidial hydrophobin RodA, putative |
| AFLA_098700 | 2.54 | down | oxidoreductase, short chain dehydrogenase/reductase family protein |
| AFLA_098700 | 2.47 | down | oxidoreductase, short chain dehydrogenase/reductase family protein |
| AFLA_099050 | 3.83 | down | hypothetical protein |
| AFLA_099050 | 3.70 | down | hypothetical protein |
| AFLA_101780 | 2.41 | down | Oxidoreductase molybdopterin binding domain containing protein |
| AFLA_101780 | 2.20 | down | Oxidoreductase molybdopterin binding domain containing protein |
| AFLA_101800 | 3.81 | down | Glycosyl hydrolases family 18 protein |
| AFLA_101800 | 4.33 | down | Glycosyl hydrolases family 18 protein |
| AFLA_104350 | 8.01 | down | Dynamin central region family protein |
| AFLA_104350 | 6.90 | down | Dynamin central region family protein |
| AFLA_105630 | 3.94 | up | Cytochrome P450 family protein |
| AFLA_105630 | 3.82 | up | Cytochrome P450 family protein |
| AFLA_109030 | 3.01 | down | To nucleotide exsicion repair protein RAD7 |
| AFLA_109160 | 3.31 | down | isopentenyl-diphosphate delta-isomerase, putative |
| AFLA_110040 | 5.26 | down | blr7677-related |
| AFLA_110040 | 6.41 | down | blr7677-related |
| AFLA_112720 | 2.26 | down | diphosphomevalonate decarboxylase, putative |
| AFLA_112910 | 2.85 | up | hypothetical protein |
| AFLA_112910 | 2.89 | up | hypothetical protein |
| AFLA_113790 | 2.78 | down | hypothetical protein |
| AFLA_113790 | 2.39 | down | hypothetical protein |
| AFLA_115930 | 3.40 | up | hypothetical protein |
| AFLA_115930 | 3.22 | up | hypothetical protein |
| AFLA_119340 | 2.12 | up | Helix-loop-helix DNA-binding domain containing protein |
| AFLA_119340 | 2.29 | up | Helix-loop-helix DNA-binding domain containing protein |
| AFLA_125770 | 3.03 | down | hypothetical protein |
| AFLA_125770 | 3.25 | down | hypothetical protein |
| AFLA_127620 | 7.70 | down | New cDNA-based gene: (AO_CDS_042706, novel, updateIDs: 11597, [gene: novel_gene_1223, model: novel_model_1223]) |
| AFLA_127620 | 11.51 | down | New cDNA-based gene: (AO_CDS_042706, novel, updateIDs: 11597, [gene: novel_gene_1223, model: novel_model_1223]) |
| AFLA_129810 | 3.08 | down | cytoplasmic asparaginyl-tRNA synthetase, putative |
| AFLA_129810 | 2.94 | down | cytoplasmic asparaginyl-tRNA synthetase, putative |
| AFLA_130150 | 2.02 | down | NAD+ dependent glutamate dehydrogenase, putative |
| AFLA_133830 | 2.02 | down | oxidoreductase, zinc-binding dehydrogenase family protein |
| AFLA_134420 | 2.02 | up | Sugar transporter family protein |
| AFLA_139270 | 2.28 | up | aflNa/ hypD/ hypothetical protein |
| AFLA_139270 | 2.37 | up | aflNa/ hypD/ hypothetical protein |
| AFLA_139290 | 3.06 | up | aflMa/ hypE/ hypothetical protein |
| AFLA_139400 | 2.17 | up | aflCa/hypC/hypothetical protein |
| AFLA_139400 | 2.03 | up | aflCa/hypC/hypothetical protein |
| AO090120000447 | 6.76 | down | predicted protein |
| AO090120000447 | 5.18 | down | predicted protein |