Introduction
The potential of using plasmin as a therapeutic agent in thrombotic disease is not new. In a 1960 symposium, Ambrus et al reported using plasmin, then called fibrinolysin, in 281 patients with a wide range of disorders, including ‘thrombophlebitis’, peripheral artery occlusion, coronary artery occlusion and pulmonary embolism. Patients with a somewhat tenuous association with coagulation as the principal pathologic mechanism (hyaline membrane disease, peritonitis, cystic fibrosis, inflammatory conditions and emphysema) were also included.1 Plasmin was produced by adding a plasminogen activator (PA) such as streptokinase or urokinase to plasminogen, after which the PA was removed by chemical processes. The ultimate purpose of the product was to dissolve fibrin without activating plasminogen after administration to patients.
Despite optimistic interpretations of results, it was soon realized that the intravenously - administered material was neutralized by antiplasmin before it reached the thrombus. Furthermore, positive clinical outcomes that were sometimes observed could well be attributed to the effect of contaminating PA in the product.2
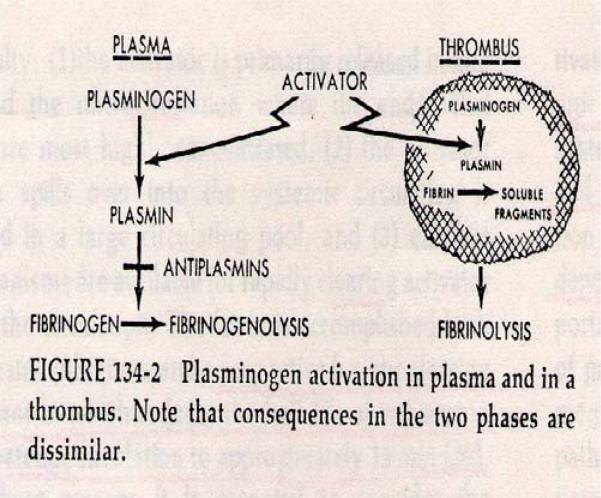
A conceptual framework of fibrinolytic activation and inhibition3 provided a foundation for understanding the actions of PA and plasmin in the blood and in a thrombus milieu.4 The schematic shown in Figure 1 illustrates how systemically administered plasmin is neutralized by inhibitors in plasma, precluding therapeutic benefit, while systemically administered PA would bind to a thrombus, be protected from inhibitor inactivation, and convert plasminogen to plasmin; the latter action would result in thrombolysis and, optimally, subsequent clinical benefit. Following this concept, PAs were used preferentially for the next 40 years with great success in thrombolytic therapy.5,6
Figure 1.
Schematic of fibrinolytic activation and inhibition in the circulation and in a thrombus, as proposed by Alkjaersig, Fletcher and Sherry.4 Reproduced with permission of The McGraw-Hill Companies from Sherry, S: Mechanisms of fibrinolysis. Hematology 1977 Edited by WJ Williams, E Beutler, AJ Erslev, RW Rundles. New York, McGraw-Hill: Section 7, Chapter 4 pp 1297.
Limitation of plasminogen activators
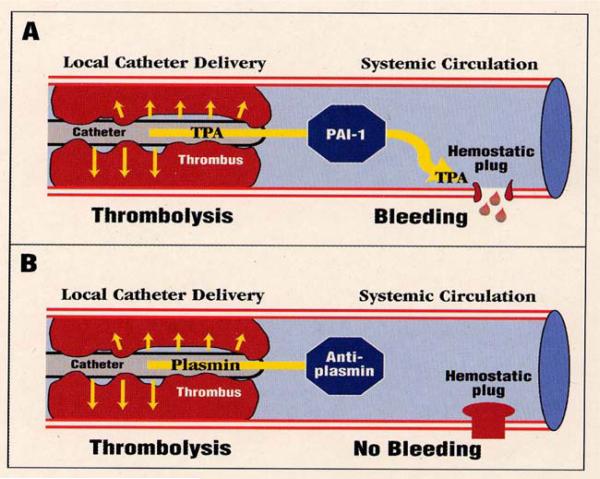
When PAs are administered systemically, to treat conditions such as myocardial infarction and deep-vein thrombosis, they activate plasminogen not only in thrombi but also in hemostatic plugs. This occurs because the high concentrations of PA required for therapeutic utility overwhelm plasminogen activator inhibitor type 1 (PAI-1) and circulate to sites of vascular injury (Figure 2A). Although the desired thrombolysis and vascular reperfusion occurs, unavoidable bleeding at sites of vascular injury can follow. Such bleeding complications do not occur in the absence of vascular injury or defect, and in a somewhat counterintuitive manner, the degree of derangement of coagulation assays does not predict the likelihood of bleeding events.7 Such bleeding episodes can follow local as well as systemic PA administration, since PA that escapes from the thrombus locale can reach and degrade hemostatic plugs. The most dangerous of such complications is intracranial hemorrhage, which can occur in approximately 1% of patients.5,6
Figure 2.
Schematic illustration of the therapeutic use of catheter-delivered plasminogen activator (PA) or plasmin to induce therapeutic thrombolysis. PAs are not completely neutralized in the circulation, thereby allowing hemostatic plug disruption and bleeding (Panel A). Conversely, therapeutic dosages of plasmin are inhibited by antiplasmin, thereby avoiding such bleeding complications (Panel B).8 Reproduced with permission from Thrombosis and Haemostasis. Marder VJ, Landskroner K, Novokhatny V et al. Plasmin induces local thrombolysis without causing hemorrhage: a comparison with tissue plasminogen activator in the rabbit. Thromb Haemost 2001; 86: 739–745, Schattauer GmbH publications.
Hypothetical advantages of plasmin relative to t-PA
In contrast to PA, plasmin is quickly neutralized by antiplasmin in the circulation (Figure 2B), so plasmin cannot be used for thrombolytic therapy by the intravenous route. When plasmin is administered by catheter in close proximity to the thrombus, however, it binds to fibrin, is protected from antiplasmin inhibition, and induces thrombolysis.
Safety
After thrombolysis, plasmin that leaches off or bypasses a thrombus will be quickly neutralized by antiplasmin,9 thus avoiding the bleeding complications that accompany PA use.
Efficacy
Plasmin is a direct-acting agent, so there is no requirement for plasminogen substrate to effect thrombolysis.Unlike the situation with PA, plasmin is effective even for thrombi that have low concentrations of plasminogen, such as those that may exist in chronically or completely occluded vessels.
Trials of plasmin vs t-PA in animal models
Pre-clinical trials were carried out on rabbits on the premise that therapeutic plasmin (TAL-05-00018, Talecris BioTherapeutics, Research Triangle Park, NC) should effectively dissolve clots, but with less risk of bleeding than t-PA.
Efficacy: abdominal aorta thrombolysis model
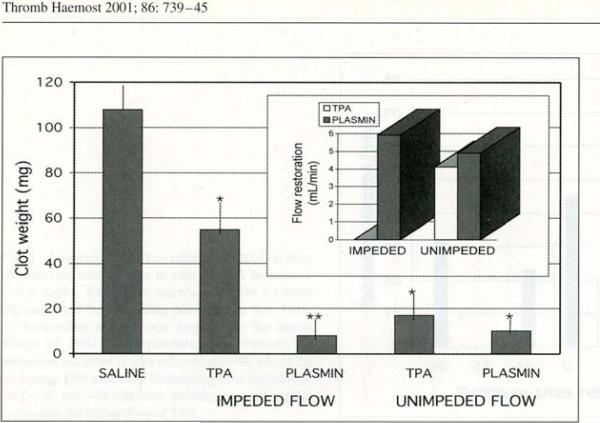
The distal abdominal aorta was isolated and a ligature applied, then thrombin was injected to occlude the vessel. Once the vessel was occluded the ligature was released and thrombolytic agent was administered by catheter through the renal artery. In one variation, aortic blood flow was impeded by a proximal ligature to prevent replenishment of plasminogen to the thrombus. In the unimpeded scenario, there was no proximal aortic ligature. Thrombolysis was measured by residual clot weight and by blood flow restoration.
The results showed that in the unimpeded model, t-PA and plasmin both effectively dissolved the clot and achieved equivalent blood-flow restoration (Figure 3). In the impeded model, which presumably restricted plasminogen supply, there was more residual clot with t-PA than with plasmin, and blood flow was restored with plasmin but not with t-PA.8
Figure 3.
Thrombolytic effect of tPA and plasmin (TAL-05-00018) in an impeded and unimpeded flow model.8 See text for details. Reproduced with permission from Thrombosis and Haemostasis. Marder VJ, Landskroner K, Novokhatny V et al. Plasmin induces local thrombolysis without causing hemorrhage: a comparison with tissue plasminogen activator in the rabbit. Thromb Haemost 2001; 86: 739–745, Schattauer GmbH publications.
Thus, plasmin induces thrombolysis in conditions of both unimpeded and impeded blood flow, but tPA-induced thrombolysis was limited if blood flow was impeded. The latter scenario is consistent with suboptimal efficacy under conditions of low or absent plasminogen content.
Safety: ear-puncture bleeding model
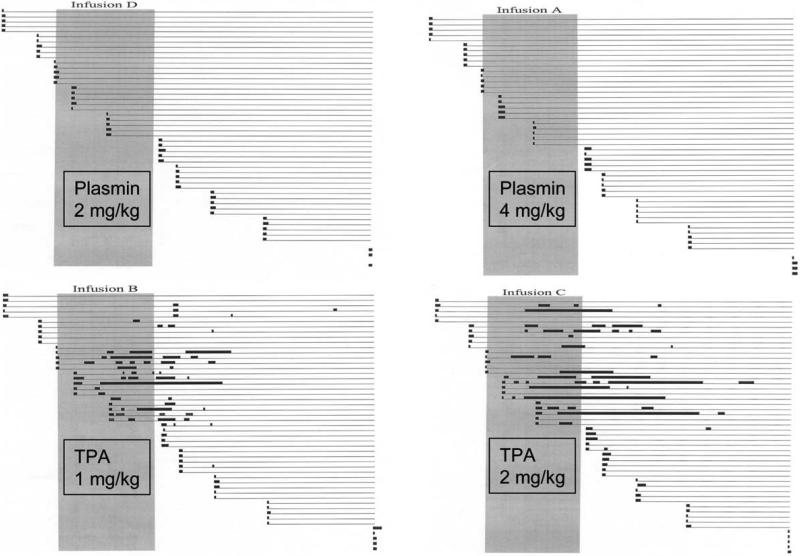
After isoflurane anesthesia, a double-lumen catheter was placed into the internal jugular vein for infusion of agent and blood sampling. Subjects were randomized to receive 60 minute infusions of 2 or 4 mg/kg of plasmin or 1 or 2 mg/kg t-PA, equivalent to 1- or 2-fold the therapeutic dose as documented by efficacy studies.8 Ear punctures were performed throughout the study beginning 30 minutes pre-infusion and ending 180 minutes post-infusion, primary bleeding times recorded, and sites observed for rebleeding without knowledge of the infused agent. Blood samples were taken at regular intervals for coagulation assays.
The results were striking, in that excessive bleeding, both primary and rebleeding episodes, occurred only in animals receiving t-PA, at both the 1 and 2 mg/kg dosages (see Figure 4). The rabbits that received plasmin showed normal primary bleeding times and no rebleeding.
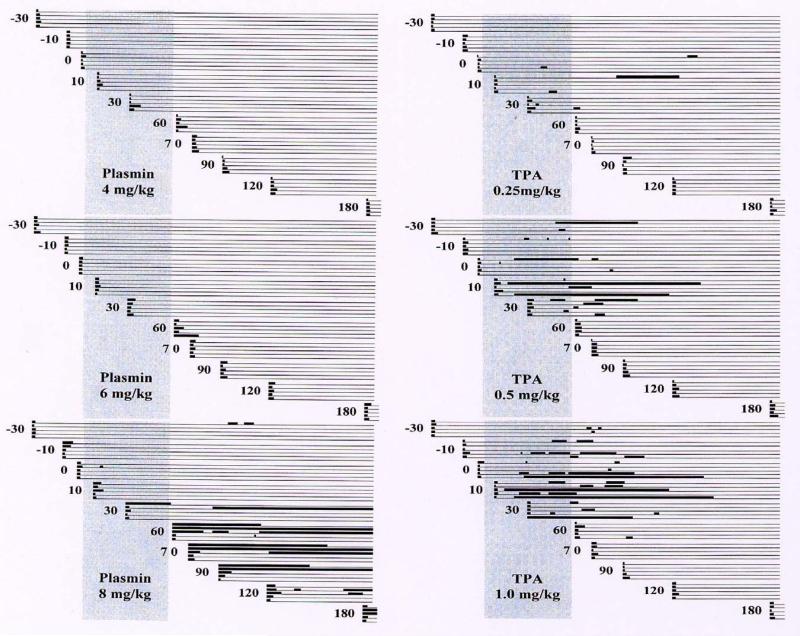
Figure 4.
Results of the ear-puncture bleeding model. In each infusion group, five animals each underwent 11 ear-puncture tests. Horizontal lines represent the bleeding course of an individual puncture site. The thick black dots at the left-hand end of each line represent the initial primary bleeding time, and subsequent heavy lines represent rebleeds. Faint lines indicate intervals of no bleeding.
These findings led to the following questions:
Is there a sub-therapeutic dose of t-PA that does not cause bleeding?
How much plasmin can be tolerated without causing bleeding?
To answer these questions a further study was carried out10 with increasing doses of plasmin (4, 6 and 8 mg/kg), representing dosages of 2-, 3-, and 4-fold the therapeutic dose, and decreasing doses of t-PA (1.0, 0.5 and 0.25 mg/kg), representing a full dose or 50% or 25% of the therapeutic dose (Figure 5).
Figure 5.
Effect of increasing dosages of plasmin and of decreasing dosages of t-PA on the ear puncture primary bleeding times and rebleeding.10 This research was originally published in Blood. Stewart D, Kong M, Novokhatny V. Distinct dose-dependent effects of plasmin and TPA on coagulation and hemorrhage. Blood 2003; 101: 3002–3007 © the American Society of Hematology.
The lower doses of t-PA showed less bleeding than the full therapeutic dose, in a dose–response relationship. Rebleeding still occurred even with the lowest dose, which corresponded to only 25% of that used for systemic treatment. Apparently, the hemorrhagic risk of t-PA parallels its thrombolytic potential, and any dosage of t-PA that carries potential thrombolytic benefit also carries a risk of hemorrhage.
Plasmin showed essentially normal primary bleeding times and no rebleeding at 4 mg/kg and only one self-limited episode of prolonged primary bleeding at the 6 mg/kg dose. At the highest dose of 8 mg/kg, however, extended bleeding episodes and rebleeding occurred, mostly at the end of or after completion of the 60 minute infusion.
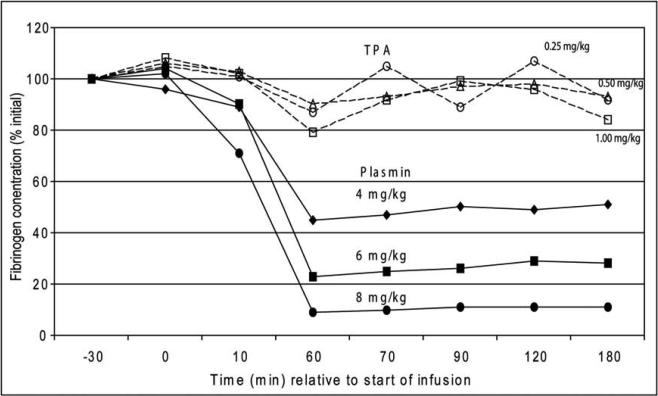
This effect was understood by measuring the fibrinogen concentration (Figure 6), relative to the administered dose of t-PA and plasmin. For t-PA, there was no significant decrease in fibrinogen, even at the highest dosage and even in animals with significant excess bleeding (Figure 5). This is consistent with results in patients suffering intracranial hemorrhage or cerebral infarction after receiving 100 mg t-PA for acute myocardial infarction, both groups showing the same plasma fibrinogen concentrations.11
Figure 6.
Effect of increasing doses of t-PA and plasmin on fibrinogen levels.10 This research was originally published in Blood. Stewart D, Kong M, Novokhatny V. Distinct dose-dependent effects of plasmin and TPA on coagulation and hemorrhage. Blood 2003; 101: 3002–3007 © the American Society of Hematology.
In contradistinction, the fibrinogen levels decreased in rabbits receiving plasmin, in proportion to the dose administered. However, hemostasis was maintained so long as the plasma fibrinogen remained above 20% of initial values, and bleeding appeared when fibrinogen was essentially and completely depleted (at 8 mg/kg, about 4-fold the therapeutic dose). These data suggest that a plasmin dose of 2 mg/kg will have thrombolytic efficacy, but that plasma fibrinogen levels will be maintained sufficiently to allow complete safety from bleeding complications (see Figures 4 and 5).
Stroke: Middle cerebral artery (MCA) ischemia
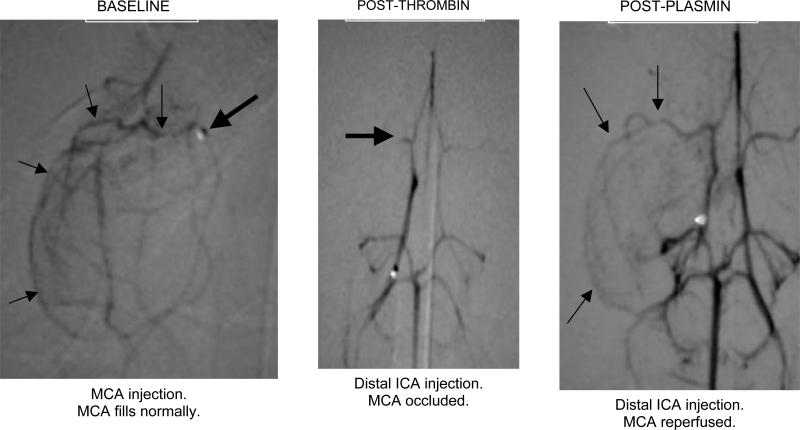
There is a need for an improved model of ischemic stroke, as existing models have indiscriminate occlusion of target arteries, variable ischemic territory, and typically absent angiographic documentation. To this end, we have developed a stroke model in the rabbit to better reflect the typical distribution of ischemic stroke in humans, namely, the middle cerebral artery, using routine angiography to document vessel occlusion and reperfusion.12 After femoral artery isolation, a microcatheter was used to identify and enter the distal internal cerebral artery (ICA) bifurcation into the middle and anterior cerebral arteries. MCA occlusion was consistently achieved by local thrombin infusion, resulting in focal ischemia, after which plasmin was administered to the site (Figure 7).
Figure 7.
Middle cerebral artery (MCA) occlusion in the rabbit model of ischemic stroke.12 The baseline study (left panel) shows the tip of the microcatheter (heavy arrow) and course of the MCA (light arrows). After local infusion of thrombin, only the M1 segment of the MCA is filled by contrast (middle panel, heavy arrow). Following catheter delivery of plasmin (2 mg) to the distal ICA, the MCA is reperfused (light arrows, right panel). Reproduced with permission from Jahan R, Stewart D, Vinters HV, et al. Middle cerebral artery occlusion in the rabbit using selective angiography: application for assessment of thrombolysis. Stroke, in press. Lippincott, Williams & Wilkins.
Of 6 animals receiving 1-3 mg plasmin, all demonstrated rapid reperfusion, occurring as soon as 10 minutes after infusion of the larger dosages.
Conclusion
In summary, pre-clinical studies have demonstrated that plasmin is a suitable alternative to t-PA as it causes less bleeding but has equivalent or greater thrombolytic efficacy. In cases of thrombi with low concentrations of plasminogen, plasmin appears to have superior thrombolytic efficacy to t-PA. Plasmin should be an ideal agent for catheter-directed thrombolysis in situations such as thrombosed shunts and peripheral arterial grafts. Plasmin has also shown promise in a new ischemic stroke model that has been developed in the rabbit. This model has advantages over existing stroke models in that precise and reliable MCA occlusion can be achieved and documented by angiography. Successful thrombolysis and reperfusion has been achieved with catheter-directed administration of plasmin in this model.
Acknowledgments
Supported by Grant RO1 HL 074051 from the National Heart Lung and Blood Institute, NIH, Bethesda, MD, and by a grant from Talecris BioTherapeutics, RTP, NC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
This work was supported by grants No. RO 1 HL 07451 and T32 HL 66992 from the National Heart, Lung, and Blood Institute, and P50 NS 44378 from the National Institute of Neurological Diseases and Stroke, and from Talecris Biotherapeutics, Research Triangle Park, North Carolina. Dr Marder serves as a consultant to Talecris Biotherapeutics.
References
- 1.Ambrus JL, Ambrus CM, Sokal JE, et al. Clinical pharmacology of various types of fibrinolytic enzyme preparations. Am J Cardiol. 1960;6:462–475. [Google Scholar]
- 2.Douglas AS, McNicol GP. Thrombolytic therapy. Br Med Bull. 1964;20:228–232. doi: 10.1093/oxfordjournals.bmb.a070337. [DOI] [PubMed] [Google Scholar]
- 3.Christensen LR, MacLeod CM. A proteolytic enzyme of serum: characterization, activation and reaction with inhibitors. J Gen Physiol. 1945;28:559–583. doi: 10.1085/jgp.28.6.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherry S. Mechanisms of fibrinolysis. In: Williams WJ, Beutler E, Erslev AJ, Rundles RW, editors. Hematology. McGraw-Hill; New York: 1977. p. 1297. Section 7, Chapter 4. [Google Scholar]
- 5.Marder VJ, Sherry S. Thrombolytic therapy: current status (1). N Engl J Med. 1988;318:1512–1520. doi: 10.1056/NEJM198806093182306. [DOI] [PubMed] [Google Scholar]
- 6.Marder VJ, Sherry S. Thrombolytic therapy: current status (2). N Engl J Med. 1988;318:1585–1595. doi: 10.1056/NEJM198806163182406. [DOI] [PubMed] [Google Scholar]
- 7.Marder VJ. The use of thrombolytic agents: choice of patient, drug administration, laboratory monitoring. Ann Intern Med. 1979;90:802–808. doi: 10.7326/0003-4819-90-5-802. [DOI] [PubMed] [Google Scholar]
- 8.Marder VJ, Landskroner K, Novokhatny V, et al. Plasmin induces local thrombolysis without causing hemorrhage: a comparison with tissue plasminogen activator in the rabbit. Thromb Haemost. 2001;86:739–745. [PubMed] [Google Scholar]
- 9.Collen D. On the regulation and control of fibrinolysis. Thromb Haemost. 1980;43:77–89. [PubMed] [Google Scholar]
- 10.Stewart D, Kong M, Novokhatny V. Distinct dose-dependent effects of plasmin and TPA on coagulation and hemorrhage. Blood. 2003;101:3002–3007. doi: 10.1182/blood-2002-08-2546. [DOI] [PubMed] [Google Scholar]
- 11.Califf RM, Fortin DF, Tenaglia AN, Sane DC. Clinical risks of thrombolytic therapy. Am J Cardiol. 1992;69:12A–20A. doi: 10.1016/0002-9149(92)91168-4. [DOI] [PubMed] [Google Scholar]
- 12.Jahan R, Stewart D, Vinters HV, et al. Middle cerebral artery occlusion in the rabbit using selective angiography: application for assessment of thrombolysis. Stroke. 2008;39:1613–1615. doi: 10.1161/STROKEAHA.107.507376. [DOI] [PMC free article] [PubMed] [Google Scholar]