Abstract
Hair follicle formation depends on reciprocal epidermal-dermal interactions and occurs during skin development, but not in adult life. This suggests that the properties of dermal fibroblasts change during postnatal development. To examine this, we used a PdgfraEGFP mouse line to isolate GFP-positive fibroblasts from neonatal skin, adult telogen and anagen skin and adult skin in which ectopic hair follicles had been induced by transgenic epidermal activation of β-catenin (EF skin). We also isolated epidermal cells from each mouse. The gene expression profile of EF epidermis was most similar to that of anagen epidermis, consistent with activation of β-catenin signalling. By contrast, adult dermis with ectopic hair follicles more closely resembled neonatal dermis than adult telogen or anagen dermis. In particular, genes associated with mitosis were upregulated and extracellular matrix-associated genes were downregulated in neonatal and EF fibroblasts. We confirmed that sustained epidermal β-catenin activation stimulated fibroblasts to proliferate to reach the high cell density of neonatal skin. In addition, the extracellular matrix was comprehensively remodelled, with mature collagen being replaced by collagen subtypes normally present only in developing skin. The changes in proliferation and extracellular matrix composition originated from a specific subpopulation of fibroblasts located beneath the sebaceous gland. Our results show that adult dermis is an unexpectedly plastic tissue that can be reprogrammed to acquire the molecular, cellular and structural characteristics of neonatal dermis in response to cues from the overlying epidermis.
Keywords: Wnt, Dermis, Epidermis, Fibroblast, Niche, Stem cell, Mouse
INTRODUCTION
Stem cell behaviour is determined by the interplay between intrinsic transcriptional programmes and external signals from the local environment, or niche (Schofield, 1978; Fuchs et al., 2004; Watt and Driskell, 2010). How and when most mammalian stem cell niches form is little understood (Fuchs et al., 2004), yet this is crucial to defining the mechanisms by which stem cell activity is regulated during tissue regeneration and in disease.
One of the most prominent niche cell types is the fibroblast, a ubiquitous mesenchymal cell that plays a key role in constructing and maintaining the extracellular matrix (ECM) through the production of matrix proteins, metalloproteinases and inhibitors (Kalluri and Zeisburg, 2006). Fibroblasts are constituents of almost every tissue in the body, and are also active in the stroma of tumours (Kalluri and Zeisburg, 2006; Vermeulen et al., 2010). Their properties are known to change in different contexts. For example, there are differences between fibroblasts in developing and adult skin and between undamaged and wounded skin, including differences in cell migration and contractility and in growth factor and cytokine expression (Sandulache et al., 2007; Werner et al., 2007).
In skin, the formation of hair follicles from developing epidermis requires signals from fibroblasts in the underlying dermis (Millar, 2002; Fuchs, 2008; Yang and Cotsarelis, 2010). Hair follicle morphogenesis takes place during the late embryonic and early neonatal period (Millar, 2002; Fuchs, 2008), and adult skin does not normally give rise to new follicles. Nevertheless, hair follicle neogenesis can be induced in adult mouse skin in response to transgenic or wound-induced epidermal activation of Wnt/β-catenin (Lo Celso et al., 2004; Silva-Vargas et al., 2005; Ito et al., 2007). The new hair follicles have associated dermal papillae (DP) (Lo Celso et al., 2004; Silva-Vargas et al., 2005; Ito et al., 2007), implying crosstalk between Wnt-activated epidermis and dermis. The signalling events involved have barely been explored, partly owing to a lack of fibroblast-specific reagents for direct isolation of purified fibroblast populations.
We have now used PdgfraEGFP reporter mice to directly isolate pure populations of dermal fibroblasts, allowing their transcriptional profiling and functional characterisation. Our results demonstrate that the dermal niche that supports epidermal differentiation is not a fixed entity, but is capable of comprehensive remodelling in response to extrinsic cues.
MATERIALS AND METHODS
Mouse strains
Experiments were performed in accordance with the UK Government Animals (Scientific Procedures) Act 1986. PdgfraEGFP (PdgfraH2B-eGFP) mice (Hamilton et al., 2003) were obtained from Jackson Laboratories and maintained on a C57BL/6 CBA mixed background. The D2 line of K14ΔNβ-cateninER (K14β-catER) mice was used (Lo Celso et al., 2004).
K14β-catER mice were treated at 6-8 weeks of age, corresponding to the telogen (resting) phase of the hair cycle. To activate expression of the transgene, the back skin of mice was treated with 1.5 mg 4-OHT (Sigma) dissolved in 0.1 ml acetone. For transient activation of β-catenin mice were treated once, and for sustained activation mice were treated three times per week for 2 weeks. In those experiments all mice were analysed 2 weeks following the first treatment. In the timecourse experiment, mice were treated three times per week for 1, 2 or 3 weeks and then analysed 3 days following the final treatment. In some experiments, mice were injected with BrdU in PBS (50 mg/kg) 2 hours before sacrifice.
Gene expression profiling
Total RNA was purified from sorted cell populations using the Purelink Micro-to-Midi Total RNA Purification System Kit (Invitrogen), including an on-column DNase treatment step. RNA was isolated from Itgα6-positive epidermal keratinocytes and PdgfraEGFP-positive dermal fibroblasts from four different experimental groups.
Genome-wide expression profiling was carried out by the Paterson Institute Microarray Core Facility. The data are deposited in the NIH GEO repository under accession number GSE32966. RNA quality was assessed using an Agilent Bioanalyzer and assays were performed in triplicate using samples with RNA integrity number (RIN) values of ∼8. A NuGEN Pico Kit was used to generate cDNA from 5 ng input RNA samples and cDNA was hybridised to Affymetrix MG430.2A arrays. Array images produced by the Affymetrix PICR 3000 scanner were imported as CEL files into GeneSpring GX11 (Agilent) for analysis. RMA (robust multi-chip average) normalisation (baseline to median of all samples) was used.
Analyses were performed on genes selected for expression above the bottom twentieth percentile in all three samples within at least one of four experimental groups. Datasets derived from keratinocytes and from fibroblasts were treated as two separate experiments. To identify differentially expressed genes, we compared the four groups in each experiment using ANOVA followed by the Tukey HSD post-hoc test, with a P-value cut-off of 0.05. For each experiment, we constructed six lists of significantly regulated entities (ANOVA P<0.05) with a fold change of greater than 2 (supplementary material Tables S1, S2). These lists formed the basis of further analyses.
Quantitative (q) PCR
Total RNA was purified from sorted cell populations as described above and cDNA prepared using the Superscript III First-Strand Synthesis Supermix for qRT-PCR Kit (Invitrogen). qRT-PCR was carried out using the TaqMan Fast Universal PCR Master Mix and TaqMan probes and an ABI Prism HT7900 Sequence Detection System (Applied Biosystems). The following probes were used: Gapdh endogenous control (4352932E), Itga6 (Mm00434375_m1), Crabp1 (Mm00442776_m1), Krt14 (Mm00516876_m1), Krt10 (Mm03009921_m1), Ivl (Mm00515219_s1), Pdgfra (Mm01211694_m1), Vim (Mm01333430_m1), Col1a2 (Mm01165187_m1), Des (Mm00802455_m1) and Alx4 (Mm00431780_m1). Values were normalised to Gapdh expression using the ΔCT method (Jensen et al., 2009). Each assay was performed in triplicate on samples from four mice.
Histology and immunohistochemistry
Tissues were fixed in formal saline and embedded in paraffin. For frozen sections, tissues were placed in OCT compound (Tissue-Tek) and then frozen on dry ice. Sections of paraffin-embedded tissue were treated with xylene to remove wax and rehydrated using graded solutions of ethanol in water. Antigen retrieval was carried out by boiling for 20 minutes in citrate buffer (pH 6). For GFP staining, antigen retrieval was performed using a Proteinase K (IHC) Kit (Novocastra). Permeabilisation was performed using 0.5% Triton X-100 (Sigma) and non-specific antibody binding was blocked with PBS containing 3% BSA and 10% bovine serum. Frozen sections were thawed briefly at room temperature and then blocked as above. Incubation with primary antibodies was carried out overnight at 4°C and with fluorescent secondary antibodies for 2 hours at room temperature. Primary antibodies used were: rabbit anti-mouse Krt14 (Covance), chicken anti-GFP (Abcam), rat anti-BrdU (Abcam), rat anti-Pdgfrα (eBioscience), rabbit anti-Lef1 (Cell Signaling), goat anti-CDP (Cux1; Santa Cruz), goat anti-Lrig1 (R&D Systems), rabbit anti-vimentin (Cell Signaling), rabbit anti-Col11a1 (Abcam), goat anti-Alx4 (Santa Cruz), rabbit anti-perilipin A (Abcam) and goat anti-GFP (Abcam). Antibody staining was visualised using appropriate species-specific secondary antibodies conjugated to Alexa Fluor 488 or 555 (Invitrogen). Slides were mounted using ProLong Gold anti-fade reagent (Invitrogen) containing the nuclear counterstain 4′,6-diamidino-2-phenylindole (DAPI).
For Herovici’s stain, sections of paraffin-embedded tissue were treated with xylene to remove wax and rehydrated using graded solutions of ethanol in water. Sections were prestained for 5 minutes with Weigert’s Iron Haematoxylin, washed and then stained for 2 minutes with Herovici’s solution consisting of 50 ml Van Gieson’s solution, 50 ml 0.05% Methyl Blue solution, 10 ml glycerol and 0.5 ml lithium carbonate solution (sat. aq.). Slides were washed for 2 minutes with 1% acetic acid and then dehydrated using graded solutions of ethanol followed by xylene.
Cell isolation
Dissected back skin from neonatal or adult mice was scraped free of muscle and fat and incubated overnight at 4°C in 0.25% trypsin solution. The interfollicular epidermis was then scraped away to leave the dermis with associated embedded hair follicles. Neonatal dermis was minced and enzymatically dissociated using 0.4 mg/ml collagenase type I (Sigma) for 30 minutes at 37°C, with DNase I (20 U/ml) added for the final 5 minutes. Adult dermis was minced and dissociated for 20 minutes at 37°C using a mixture of 1.25 mg/ml collagenase type I (Invitrogen), 0.5 mg/ml collagenase type II (Worthington), 0.5 mg/ml collagenase type IV (Sigma), 0.1 mg/ml hyaluronidase IVS (Sigma) and 50 U/ml DNase I. Enzyme activity was neutralised by the addition of serum-containing medium and preparations were passed through a 70-μm cell sieve to remove debris. The resultant single-cell suspensions contained a mixture of fibroblasts, hair follicle keratinocytes and other cells.
Flow cytometry
Cells were labelled with the following primary antibodies: rat anti-Itgα6 (CD49f; Serotec), rat anti-CD45-PE (eBioscience), rat anti-CD31-PE (eBioscience) and rat anti-CD117-PE (c-Kit) (eBioscience). Non-conjugated primary antibodies were detected using chicken anti-rat Alexa Fluor 647 (Invitrogen). DAPI staining was used to exclude dead cells. Non-labelled cells and cells labelled with secondary antibody only were used as controls to set gates. An APC BrdU Flow Kit (BD Pharmingen) was used to perform BrdU staining. Flow cytometry analyses were carried out using a CyAn ADP Analyzer (Beckman Coulter). Sorting was performed using a MoFlo cell sorter (DakoCytomation). Re-analysis of sorted cells in each experiment showed that PdgfraEGFP-positive populations were of 97-100% purity and Itgα6-positive populations of 97-98% purity.
Image acquisition and processing
Microscopy was carried out using a Leica SP5 confocal microscope utilising 405, 488 and 561 nm lasers and Leica Application Suite version 8.2.2. Images were obtained using a 20× HCX PL APO CS dry objective with an L1 405/UV correction optic and a 63× HCX PL APO water-immersion objective with L8 405 and L10 UV correction optics (Leica). Images were optimised globally for brightness and contrast using Photoshop CS4 and assembled into figures with Illustrator CS4 (Adobe).
RESULTS
Direct isolation of dermal fibroblasts using PdgfraEGFP
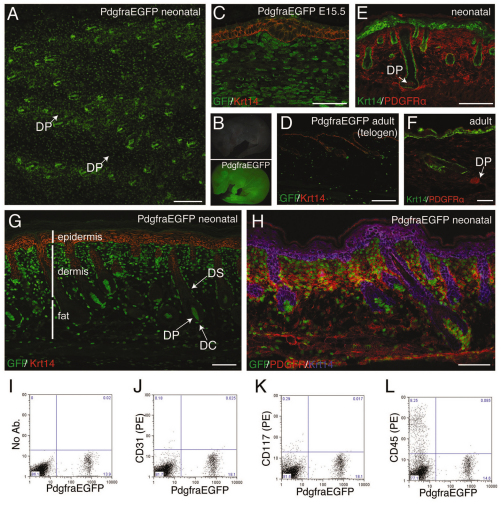
Platelet-derived growth factor receptor α (Pdgfrα) was previously reported to be a marker of the pre-DP dermal condensate in developing mouse skin (Karlsson et al., 1999). This led us to examine the skin of PdgfraEGFP mice, in which histone H2B-eGFP is expressed from the Pdgfra locus (Hamilton et al., 2003). In wholemounts of neonatal PdgfraEGFP back skin, GFP was expressed throughout the dermis and within the DP at the base of each developing follicle (Fig. 1A). Examination of neonatal mice with fluorescence illumination showed that GFP was expressed in the skin of all body regions (Fig. 1B). Immunohistochemistry of sections from developing and adult skin showed that GFP was absent from keratin 14 (Krt14)-positive epidermal keratinocytes but was expressed widely throughout the dermis (Fig. 1C,D). The same was true of endogenous Pdgfrα protein (Fig. 1E,F), although the endogenous receptor was expressed at the cell surface and in the cytoplasm, whereas GFP was expressed in the nucleus (Fig. 1G). Double labelling for GFP and Pdgfrα confirmed extensive coexpression (Fig. 1H).
Fig. 1.
Expression of PdgfraEGFP in developing and adult mouse skin. (A) Wholemount of neonatal back skin showing GFP fluorescence in dermal fibroblasts and dermal papilla. (B) Fluorescence images of neonatal PdgfraEGFP mouse (bottom) and wild-type littermate (top). (C-E,G) Paraffin sections of PdgfraEGFP back skin labelled with antibodies to GFP (green, C,D,G), Krt14 (red, C,D,G; green, E) or Pdgfrα (red, E). (F) Cryosection of back skin labelled with Pdgfrα (red) and Krt14 (green) antibodies. (H) Cryosection of back skin labelled with Pdgfrα (red), GFP (green) and Krt14 (blue) antibodies. (I-L) Flow cytometry of PdgfraEGFP adult dermal cells labelled with (I) no antibodies, (J) anti-CD31(PE), (K) anti-CD117(PE), or (L) anti-CD45(PE). DC, dermal cup; DP, dermal papilla; DS, dermal sheath. Scale bars: 150 μm in A; 50 μm in C; 200 μm in D,E,G,H; 40 μm in F.
Flow cytometry of PdgfraEGFP+ cells isolated from adult back skin showed that GFP+ cells were negative for CD31 (Pecam1), CD117 (c-Kit) and CD45 (Ptprc) (Fig. 1I-L). PdgfraEGFP+ cells did not therefore express markers of endothelial cells, melanocytes and haemopoietic cells, respectively.
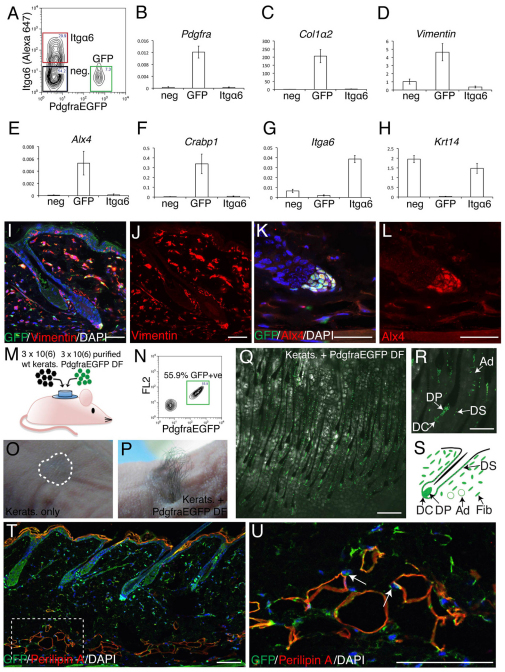
To confirm that PdgfraEGFP+ cells were indeed fibroblasts, we isolated three distinct populations from dissociated adult telogen back skin: GFP+ cells; Itgα6+ GFP– keratinocytes; and viable cells negative for both markers (Fig. 2A). qPCR analysis showed that the GFP-positive population was highly enriched for Pdgfra mRNA and also for mRNA of the skin fibroblast marker genes Col1a2 and vimentin (Vim) (Fig. 2B-D) (Kalluri and Zeisburg, 2006). GFP+ cells were enriched for the DP markers Alx4 and Crabp1 (Fig. 2E,F) and contained very low levels of mRNA corresponding to markers (Itga6 and Krt14) of undifferentiated keratinocytes (Fig. 2G,H). Immunohistochemistry of neonatal back skin confirmed that PdgfraEGFP+ cells coexpressed Vim and Alx4 (Fig. 2I-L).
Fig. 2.
PdgfraEGFP expression defines the mouse dermal fibroblast population. (A) Flow cytometry sort gates used to purify Itgα6+ cells (epidermal keratinocytes, red box), GFP+ cells (fibroblasts, green box), and Itgα6– GFP– cells (black box) from PdgfraEGFP adult telogen back skin. (B-H) qPCR of mRNA levels in sorted cell populations. Genes were normalised to Gapdh. Each bar represents the average of replicates from four mice (± s.e.). neg, GFP+, Itgα6+. (I-L) Paraffin sections of PdgfraEGFP back skin labelled with GFP (green) and Vim (red, I,J) or Alx4 (red, K,L) antibodies, and counterstained with DAPI (blue) to label nuclei. (M) Outline of grafting experiment. wt kerats, wild-type keratinocytes; DF, dermal fibroblasts. (N) Sort gates used to purify GFP+ cells (green box) from PdgfraEGFP neonatal dermis. (O,P) Hair growth at graft sites 30 days post-grafting. The graft site is outlined in O. (Q,R) Fluorescence/brightfield images of graft sites viewed from the dermal side. Note the refractile, lipid-filled adipocytes in Q. (S) The contribution of PdgfraEGFP+ cells to the different dermal compartments. DP, dermal papilla; DC, dermal cup; DS, dermal sheath; Ad, adipocyte; Fib, fibroblast. (T,U) Paraffin section of PdgfraEGFP back skin labelled with GFP (green) and perilipin A (red) antibodies, and counterstained with DAPI (blue) to label nuclei. The boxed region in T is enlarged in U. Note that perilipin A+ adipocytes have GFP+ nuclei (arrows). Scale bars: 50 μm in I-L; 150 μm in Q; 500 μm in R; 100 μm in T,U.
Col1a2 mRNA is a well-established marker of skin fibroblasts (Kalluri and Zeisberg, 2006). Since the GFP-positive population accounted for all of the detectable Col1a2 mRNA in the three cell populations, we conclude that PdgfraEGFP identifies the entire dermal fibroblast population. Furthermore, the lack of Krt14 mRNA establishes that the GFP-positive population does not contain keratinocytes.
In order to determine whether PdgfraEGFP+ fibroblasts could support hair follicle formation, we carried out skin reconstitution assays (Lichti et al., 2008) (Fig. 2M-P). Grafts of 3×106 neonatal keratinocytes alone (n=3) did not result in hair growth (Fig. 2O). However, grafts of keratinocytes combined with 3×106 flow-sorted PdgfraEGFP+ neonatal fibroblasts gave rise to hair in 3/3 grafts (Fig. 2N,P). GFP+ cells gave rise to all of the mesenchymal components of the hair follicle, including the DP, dermal cup and dermal sheath, and also to fibroblasts located outside the hair follicle and to adipocytes (Fig. 2Q-S). The expression of GFP in the fat layer of PdgfraEGFP back skin was confirmed by double labelling for GFP and perilipin A (perilipin 1) (Fig. 2T,U).
We conclude that PdgfraEGFP is expressed by all fibroblast subpopulations in mouse dermis and can be used as a tool for their direct isolation.
Gene expression profiling of purified fibroblasts and keratinocytes during hair follicle neogenesis
We hypothesized that fibroblasts from developing and adult skin would have distinct characteristics relating to the presence or absence of hair-inductive capacity. To explore this, we carried out gene expression profiling of keratinocytes and fibroblasts isolated from neonatal and adult skin. In addition, we profiled cells from adult K14ΔNβ-cateninER (K14β-catER) skin, in which the formation of ectopic follicles is induced by sustained epidermal activation of β-catenin using a 4-hydroxytamoxifen (4-OHT)-inducible transgene (Lo Celso et al., 2004). Applying 4-OHT six times over a 2-week period induces both ectopic follicles and anagen of existing follicles, whereas application of a single dose of 4-OHT results in anagen re-entry without ectopic follicle formation (Lo Celso et al., 2004; Van Mater et al., 2003) (supplementary material Fig. S1). Thus, by varying the number of 4-OHT treatments, we were able to distinguish gene regulation specific to anagen from gene regulation specifically associated with ectopic follicle formation.
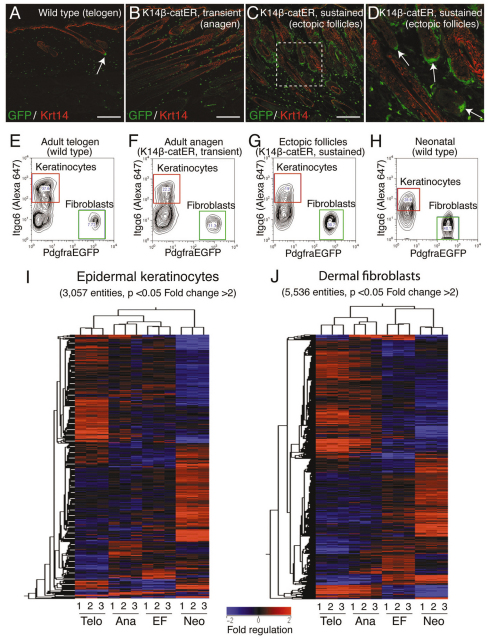
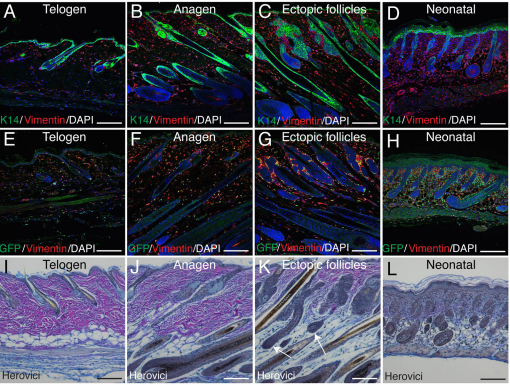
We isolated RNA from epidermal keratinocytes (Itgα6+) and dermal fibroblasts (PdgfraEGFP+) purified from adult telogen skin, adult anagen skin (K14β-catER, transient activation), adult anagen skin with ectopic follicles (EF) (K14β-catER, sustained activation) and neonatal skin (Fig. 3A-H). Examination of histological sections showed that ectopic follicles were encapsulated by GFP-expressing cells and that all ectopic DP were GFP+ (Fig. 3C,D).
Fig. 3.
Gene expression profiling of epidermal keratinocytes and dermal fibroblasts from PdgfraEGFP mice. (A-D) Paraffin sections of back skin labelled with GFP (green) and Krt14 (red) antibodies. The boxed region in C is enlarged in D. Arrows point to PdgfraEGFP+ fibroblasts associated with the DP of an original hair follicle (A) and ectopic follicles (D). Scale bars: 200 μm. (E-H) Flow cytometry sort gates used to isolate populations of Itgα6+ keratinocytes (red box) and PdgfraEGFP+ dermal fibroblasts (green box) for microarray analysis. (I,J) Heatmaps representing the hierarchical clustering (based on both entities and samples) of entities that are differentially regulated (P<0.05, fold change greater than 2) between at least one of six possible pairs of groups, in (I) epidermal keratinocytes and (J) dermal fibroblasts. Some genes are represented by multiple entities. Telo, ‘Telogen’ group (wild type); Ana, ‘Anagen’ group (K14β-catER, transient activation); EF, ‘Ectopic follicles’ group (K14β-catER, sustained activation); Neo, ‘Neonatal’ group. (I,J) Bar indicates fold regulation (baseline to median of all samples).
Gene expression profiling was performed on triplicate samples and datasets generated from epidermal keratinocytes and from dermal fibroblasts were treated as two separate experiments. For each experiment, we compared each pair of groups (a total of six pairs derived from four groups) to generate six lists of significantly regulated entities (ANOVA P<0.05, fold change greater than 2) (supplementary material Tables S1, S2). These lists formed the basis of further analysis.
In the keratinocyte array, a total of 3057 entities were significantly regulated (ANOVA P<0.05) by more than 2-fold within one or more of six possible pairs of groups (Fig. 3I). In the fibroblast array, the total was 5536 entities (Fig. 3J). Pairwise comparison of the ‘Telogen’ (adult wild-type) group with the ‘Ectopic follicles’ (K14β-catER, sustained activation) group revealed significant regulation of 1068 entities in keratinocytes and 3002 entities in dermal fibroblasts (supplementary material Tables S1, S2). Therefore, both keratinocytes and fibroblasts are subject to dynamic transcriptional regulation in different developmental states. Epidermal activation of β-catenin results in substantial transcriptional regulation of niche fibroblasts.
Reprogramming of the adult dermal fibroblast population to a neonatal state
We carried out hierarchical clustering of all significantly regulated entities (P<0.05, fold change greater than 2) based on both entities and samples (Fig. 3I,J). In the keratinocyte experiment, the most closely related experimental groups were ‘Anagen’ (K14β-catER, transient activation) and ‘Ectopic follicles’ (K14β-catER, sustained activation), and these two groups were more closely related to the ‘Telogen’ (adult wild-type) group than to the ‘Neonatal’ group, which formed a distinct cluster (Fig. 3I). Therefore, in epidermal keratinocytes the most important determinant of variance in transcriptional regulation was the age (neonatal or adult) of the mouse. The level of upregulation of a selection of genes previously reported to be positively regulated by the Wnt pathway was similar when ‘Anagen’, ‘Neonatal’ and ‘Ectopic follicles’ epidermal arrays were compared with ‘Telogen’ (supplementary material Table S3). The limited overlap in gene expression between the ‘Ectopic follicles’ and ‘Neonatal’ epidermal datasets might reflect, at least in part, the presence of pre-existing adult anagen follicles in the ‘Ectopic follicles’ sample.
In the fibroblast experiment, the adult ‘Telogen’ and ‘Anagen’ experimental groups were closely related. However, the adult ‘Ectopic follicles’ group was more closely related to the ‘Neonatal’ group than to either of the other adult groups (Fig. 3J). This result demonstrates that ectopic follicle formation in K14β-catER mice involves reprogramming of adult dermal fibroblasts to a state resembling neonatal fibroblasts.
Dynamic expression of DP signature genes
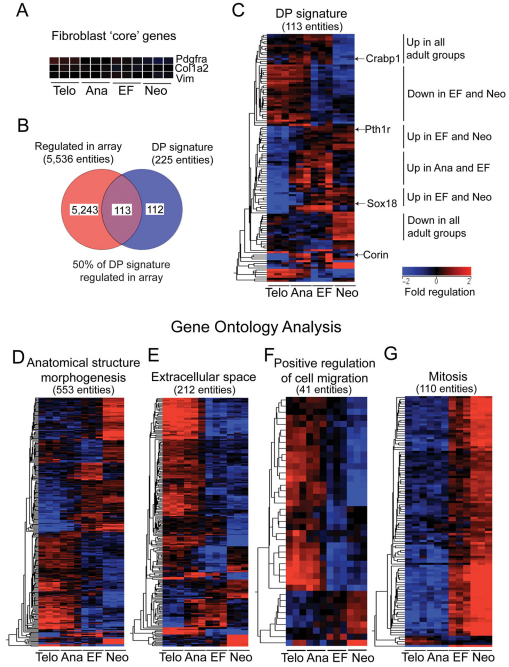
In the dermal fibroblast array, certain ‘core’ fibroblast genes, including Pdgfra, Col1a2 and Vim, were expressed at similar levels in all four experimental groups, showing that fibroblasts maintain essential characteristics that are independent of the developmental state (Fig. 4A). By contrast, expression of DP genes varied between experimental groups. Two hundred and twenty out of two hundred and twenty-five (98%) genes in a previously published DP signature list (Rendl et al., 2005) were detected above background levels in 3/3 samples in at least one of four experimental groups (not shown), consistent with the presence of DP cells within our cell isolates. Using a Venn diagram, the DP signature was overlaid onto the list of total regulated entities (5536). This showed that 113/225 (50%) of DP signature genes were significantly regulated (Fig. 4B).
Fig. 4.
Sustained epidermal activation of β-catenin reprograms adult dermal fibroblasts toward a neonatal state. (A) Heatmap representations of three ‘core’ fibroblast genes expressed at similar levels in all four experimental groups. (B) Venn diagram constructed from two lists comprising (1) all significantly regulated entities (P<0.05, fold change greater than 2) in the dermal fibroblast array and (2) 225 DP signature genes (Rendl et al., 2005). (C) Hierarchical clustering (based on entities) of 113 regulated DP signature genes derived from the region of overlap in B. (D-G) GO analysis of significantly regulated genes in the dermal fibroblast array experiment. Hierarchical clustering was performed using genes grouped under three selected GO terms (from a total of 275 significantly enriched terms, P<0.05), as indicated. Some genes are represented by multiple entities. Scale bar represents fold regulation (baseline to median of all samples).
Hierarchical clustering of regulated DP signature genes revealed several distinct clusters (Fig. 4C). Some DP genes, such as Crabp1 (Collins and Watt, 2008), were highly expressed in all adult groups relative to the ‘Neonatal’ group. Consistent with a previous report (Enshell-Seijffers et al., 2008), the DP-specific serine protease Corin was highly upregulated in both the ‘Anagen’ group and ‘Ectopic follicles’ group (in which anagen follicles are also present) relative to the ‘Telogen’ group. Some DP genes were upregulated in both the ‘Ectopic follicles’ and ‘Neonatal’ groups, relative to the other two adult groups, implying a specific association with hair follicle neogenesis. These included Sox18 and Pth1r. Pth1r encodes the parathyroid hormone receptor, which activates β-catenin signalling through recruitment of Dishevelled (Romero et al., 2010) (Fig. 4C; supplementary material Table S2). We conclude that DP genes are expressed in all dermal preparations, but that the precise DP signature varies between different developmental states. The formation of ectopic follicles in adult skin involves the regulation of certain DP genes that are normally associated with hair follicle neogenesis during development.
Gene Ontology analysis of dermal fibroblast array datasets
Since DP genes represented only a small subset of the total genes regulated during dermal reprogramming (113/5536; 2%), we used Gene Ontology (GO) analysis to explore the list of total regulated entities (5536) in the dermal fibroblast array experiment. Two hundred and seventy-five GO terms satisfied the corrected P-value cut-off of 0.05. Of 5002 entities encompassed within at least one GO term, 31% were classified under ‘cellular component’, 31% under ‘biological process’ and 38% under ‘molecular function’.
Lists of genes associated with selected GO terms of interest were used to carry out hierarchical clustering (based on entities) (Fig. 4D-G). Many regulated genes fell within GO terms associated with developmental processes, such as ‘anatomical structure morphogenesis’ (Fig. 4D). In addition, numerous significant GO terms were associated with the ECM and secreted proteins, of which ‘extracellular space’ is represented as an example [see also the list of matrix metalloproteinase (MMP) genes in supplementary material Table S4]. There was a general downregulation of matrix and secreted proteins in both the ‘Ectopic follicles’ and ‘Neonatal’ groups (Fig. 4E). However, in both these groups a small number of genes encoding secreted proteins were strongly upregulated. These included the Wnt inhibitor Dkk1, which regulates hair follicle spacing (Sick et al., 2006), and Frzb, which promotes diffusion of Wnts through extracellular interactions (Mii and Taira, 2009). Also downregulated in the ‘Ectopic follicles’ and ‘Neonatal’ groups were many genes associated with the GO term ‘positive regulation of cell migration’ (Fig. 4F). Some of the most prominent groups of genes upregulated in both the ‘Ectopic follicles’ and ‘Neonatal’ groups were those associated with cell division, as exemplified by the GO term ‘mitosis’ (Fig. 4G). This implies an association of fibroblast proliferation with the formation of new hair follicles.
In summary, GO analysis indicates that key elements of dermal reprogramming during ectopic follicle formation are the general downregulation of genes associated with the ECM and cell migration and the strong upregulation of genes regulating mitosis.
Sustained epidermal activation of β-catenin results in comprehensive remodelling of the dermis
Gene expression profiling predicted that sustained epidermal activation of β-catenin stimulates dermal fibroblast proliferation and remodelling of the ECM. Labelling skin sections with antibodies to Vim or GFP confirmed that the number of dermal fibroblasts increased (Fig. 5A-H). The increase in the number of PdgfraEGFP+ cells was, as predicted, confined to the dermis (Fig. 5E-H).
Fig. 5.
Dermal reprogramming induced by sustained epidermal β-catenin activation. (A-H) Paraffin sections of PdgfraEGFP mouse back skin labelled with K14 (green, A-D), GFP (green, E-H) and Vim (red) antibodies, and stained with DAPI (blue) to label nuclei. Part of E is shown at higher magnification in Fig. 2I. (I-L) Paraffin sections stained using Herovici’s method to identify thick, mature collagen fibres (pink) and immature collagen fibrils (blue). Nuclei are stained grey/blue. Arrows in K indicate ectopic follicles. Scale bars: 200 μm.
To visualise the dermal ECM we performed classic histochemical staining using Herovici’s method (Fitzgerald et al., 1996), which stains mature collagen bright pink and immature collagen blue. Collagen in adult telogen and adult anagen skin was predominantly stained pink, consistent with the presence of a mature ECM undergoing relatively slow turnover during the hair cycle (Fig. 5I,J). Neonatal sections stained only weakly and the small amount of collagen present formed fine blue fibrils, consistent with an immature matrix (Fig. 5L). Sections from the ‘Ectopic follicles’ group also stained blue, and contained only sparse patches of mature pink collagen (Fig. 5K). Therefore, the mature matrix had been replaced with new, immature collagen (Fig. 5I-L).
Sustained epidermal activation of β-catenin stimulates dermal fibroblast proliferation
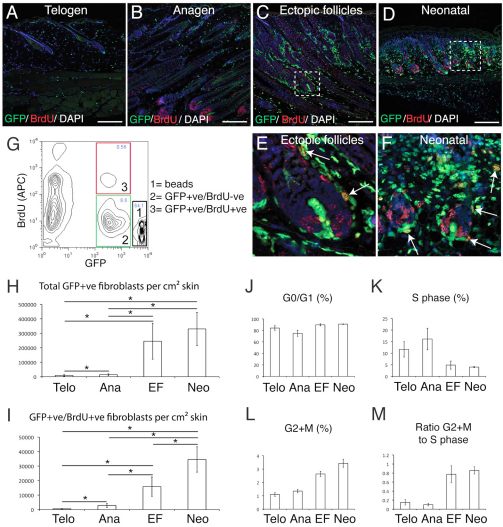
In light of the upregulation of genes associated with cell division (Fig. 4G; supplementary material Table S2) and the increased number of Vim+ and PdgfraEGFP+ fibroblasts (Fig. 5A-H) associated with the sustained epidermal activation of β-catenin, we predicted that fibroblast proliferation would be stimulated. When mice were injected with BrdU to label S-phase cells, there was a marked increase in GFP+ BrdU+ cells in the dermis following sustained activation of β-catenin, to a level that was comparable with neonatal dermis (Fig. 5A-F).
We used flow cytometry to quantify the absolute numbers of GFP+ fibroblasts and proliferating GFP+ BrdU+ fibroblasts per unit area of back skin (Fig. 5G). In comparison to telogen skin, sustained epidermal activation of β-catenin (six 4-OHT treatments) led to a 27-fold increase in the total number of PdgfraEGFP+ fibroblasts/cm2, to a density approaching that of neonatal skin. Induction of anagen (one 4-OHT treatment) resulted in only a 1.5-fold increase. Similarly, the number of GFP+ BrdU+ fibroblasts/cm2 increased only modestly in response to anagen but 35-fold in response to sustained β-catenin activation, to about half the density in neonatal skin (Fig. 5A-I).
Cell cycle analysis of PdgfraEGFP+ cells showed an increased percentage of cells in the mitotic (G2+M) phases of the cell cycle for both the ‘Ectopic follicles’ and ‘Neonatal’ groups. The high ratio of G2+M to S-phase (BrdU+) cells implies an increased rate of cell division (Fig. 6J-M). Thus, the formation of ectopic follicles in adult skin involves dramatic proliferative expansion of dermal fibroblasts, resulting in a densely populated dermis.
Fig. 6.
Dermal remodelling is associated with an increase in fibroblast proliferation and population density. (A-F) Paraffin sections of mouse back skin labelled with GFP (green) and BrdU (red) antibodies and counterstained with DAPI. E and F are enlarged regions of C and D, respectively. Arrows point to proliferating PdgfraEGFP+ BrdU+ cells. Scale bars: 200 μm. (G) Flow cytometry gates used to quantify cell number relative to counting beads. (H,I) Absolute numbers per cm2 back skin of (H) total PdgfraEGFP+ cells or (I) proliferating PdgfraEGFP+ BrdU+ cells, under different experimental conditions. Asterisks denote a significant difference between two groups (t-test, P<0.05). (J-M) The percentage of PdgfraEGFP+ fibroblasts in (J) G0/G1, (K) S phase and (L) G2+M cell cycle stages; and (M) the ratio of cells in G2+M to cells in S phase. Average ± s.e. of 4-9 replicates.
We conclude that sustained epidermal activation of Wnt/β-catenin signalling stimulates fibroblast proliferation and leads to structural remodelling of the entire dermis, not only the regions closely associated with ectopic follicles.
Adult dermal reprogramming originates from a subpopulation of fibroblasts associated with the hair follicle junctional zone
In order to establish the timing and dynamics of dermal reprogramming, K14β-catER/PdgfraEGFP mice were analysed after 4-OHT treatment for 0 days (no 4-OHT), 7 days (three doses), 14 days (six doses) or 21 days (nine doses) (Fig. 7). Consistent with earlier work (Lo Celso et al., 2004), in 7 day-treated back skin the original hair follicles had initiated anagen re-entry but no ectopic follicles had formed. Ectopic follicles were present in 14 day- and 21 day-treated skin.
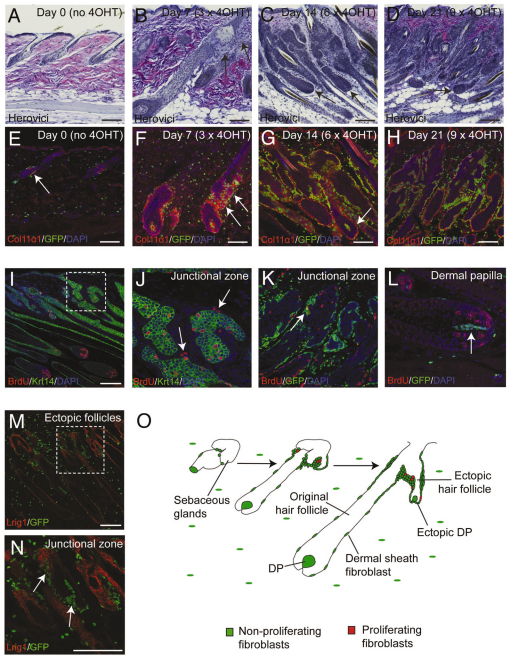
Fig. 7.
Remodelling of adult dermis originates from a population of fibroblasts in the hair follicle junctional zone. (A-L) Sections of K14β-catER mouse back skin treated with 4-OHT for 0, 7, 14 or 21 days as indicated or for 14 days (I-L). (A-D) Paraffin sections stained using Herovici’s method to identify mature (pink fibres) and immature (fine blue fibrils) collagen. (E-H) Frozen sections labelled with anti-Col11α1 (red) and showing GFP fluorescence (green). Nuclei were stained with DAPI (blue). (I-L) Paraffin sections stained for Krt14 (green) and BrdU (red). (M,N) Frozen section of ectopic follicle-forming K14β-catER back skin labelled with anti-Lrig1 (red) and showing GFP fluorescence (green). The boxed regions in I and M are enlarged in J and N, respectively. Arrows point to: (E) a small population of PdgfraEGFP+ fibroblasts associated with the telogen junctional zone; (B,F) regions of recently regenerated collagen at the junctional zone; (C,D,G,N) ectopic follicles; (J,K) BrdU-labelled junctional zone fibroblasts; and (L) the DP of an original hair follicle, which is not labelled with BrdU. (O) The progressive remodelling of adult dermis originating from fibroblasts associated with the hair follicle junctional zone. Scale bars: 200 μm.
Remodelling of ECM was first evident in the dermis adjacent to the epidermal junctional zone. The junctional zone is the region between the sebaceous glands, hair follicle infundibulum and bulge, and is the location of Lrig1+ epidermal stem cells (Jensen et al., 2009). Herovici staining showed that at 7 days the dermis adjacent to the junctional zone already comprised fine blue collagen fibrils, consistent with ECM remodelling. At this stage, the rest of the deep dermis consisted of a mixture of mature pink and immature blue collagen fibres, suggestive of ongoing remodelling (Fig. 7A,B). By 14 days, the majority of the dermis contained immature blue fibrils, thereby resembling developing dermis (Fig. 7C). By 21 days, patches of mature pink collagen had started to reappear, indicating that the remodelled matrix had started to mature (Fig. 7D).
To support the conclusion that the composition of the dermal ECM was changing in response to epidermal β-catenin activation, we stained skin sections with an antibody against Col11a1, which is encoded by one of the ECM genes upregulated in the ‘Ectopic follicles’ and ‘Neonatal’ microarrays (supplementary material Table S2). In telogen skin, Col11a1 was detected only in a narrow region of ECM associated with PdgfraEGFP+ junctional zone fibroblasts (Fig. 7E). By 7 days, the Col11a1-positive region had expanded, and there was a corresponding increase in PdgfraEGFP+ fibroblasts (Fig. 7F, arrows). In 14 day- and 21 day-treated skin, ectopic follicles were completely encapsulated by sheaths of Col11a1 (Fig. 7G,H).
During the timecourse we observed a progressive accumulation of PdgfraEGFP+ fibroblasts in regions associated with ectopic follicle formation and in the junctional zone at the tops of hair follicles. This began with a small population of PdgfraEGFP+ fibroblasts in the junctional zone and expanded in close association with emerging ectopic follicles (Fig. 7E-H). To establish the origin of the expanded population of PdgfraEGFP+ cells, we labelled mice with BrdU. Proliferating PdgfraEGFP+ BrdU+ fibroblasts were predominantly associated with the junctional zone, were rarely seen in other regions of dermis, and never seen in DP (Fig. 7I-L). The identity of the junctional zone was confirmed by immunostaining for Lrig1 (Fig. 7M,N).
We conclude that remodelling of the dermis in response to the epidermal activation of β-catenin originates from a specific population of fibroblasts located adjacent to the hair follicle junctional zone. Proliferative expansion of junctional zone fibroblasts leads to both the breakdown of mature collagen and the expression of new ECM proteins.
DISCUSSION
Using PdgfraEGFP reporter mice to directly isolate dermal fibroblasts without subjecting them to a period in culture we have been able to carry out gene expression profiling of cells in their native state. We have shown that: (1) neonatal dermal fibroblasts have a gene expression profile that is distinct from that of adult dermal fibroblasts; (2) adult dermal fibroblasts can be reprogrammed to a neonatal transcriptional state through epidermal activation of β-catenin; (3) dermal reprogramming results in fibroblast proliferation and comprehensive remodelling of the ECM; and (4) the changes originate from a specific subpopulation of fibroblasts associated with the hair follicle junctional zone. Our results reveal a surprising ability of adult epidermis to extensively restructure its local environment. Our data show that epidermal stem cells and their dermal niche exist in a state of dynamic interdependence, with neither compartment having wholly fixed characteristics.
It has long been appreciated that during development an increase in dermal cell density precedes, and is required for, the formation of DP and induction of both hair follicles and feathers (Olivera-Martinez et al., 2004). Adult DP cells were never labelled by BrdU, which is consistent with earlier studies (Enshell-Seijffers et al., 2010; Chi et al., 2010; Horne and Jahoda, 1992). Our experiments identify the source of fibroblast renewal during β-catenin-driven reprogramming as a population of PdgfraEGFP+ cells associated with the hair follicle junctional zone (Jensen et al., 2009). We first identified these cells through their expression of Crabp1, a protein otherwise restricted to the DP (Collins and Watt, 2008). Fibroblasts in this location are normally quiescent and can retain a DNA label for many months (Morris and Potten, 1999).
We have previously reported that the junctional zone/sebaceous gland region is especially sensitive to ectopic follicle formation (Jensen et al., 2009; Baker et al., 2010). The close proximity of junctional zone fibroblasts makes them a strong candidate for the origin of new DP cells. The existence of the junctional zone as a common site of dermal reorganisation and ectopic hair follicle formation leads us to speculate that the altered mesenchymal niche formed there by epidermal β-catenin activation endows the adjacent epidermal cells with the ability to form ectopic hair follicles. Analysis of the keratinocyte microarrays identified a number of candidate fibroblast mitogens, including Fgf5, Fgf2, Tgfβ2 and Shh, that were upregulated in response to β-catenin activation (supplementary material Tables S1, S3, S5).
There is growing evidence for skin fibroblast heterogeneity (e.g. Driskell et al., 2009), reflecting, at least in part, different embryonic origins (Fliniaux et al., 2004) and body sites (Rinn et al., 2006). However, whether dermal fibroblasts are organised within a differentiation hierarchy, in which some cells have greater intrinsic proliferative and differentiation potential than others (Biernaskie et al., 2009) or whether proliferative heterogeneity is primarily determined through signals from the local microenvironment, remains to be investigated. It will also be interesting to compare, quantitatively, the ability of neonatal and reprogrammed adult fibroblasts to support hair growth in skin reconstitution assays.
The impact of epidermal Wnt/β-catenin on the dermal niche is important in light of the well-established role of Wnt signalling in epithelial cancers (Clevers, 2006). Epidermal activation of β-catenin led to the upregulation of a small subset of matrix genes including Col11a1, a prognostic marker of malignant stomach lesions (Zhao et al., 2009) and of colorectal cancers (Suceveanu et al., 2009). We speculate that the stroma of human sebaceous gland tumours bearing LEF1 mutations that inhibit Wnt signalling (Takeda et al., 2006) will have different characteristics to the stroma of pilomatricomas with activating β-catenin mutations (Chan et al., 1999). Given the normal role of Wnt signalling in many other adult epithelia, including intestine and lung, it will be interesting to determine whether the connective tissue stroma in other tissues can also be reprogrammed to an earlier developmental state and, if so, whether this is of significance for tumour progression.
Supplementary Material
Acknowledgments
We thank the Paterson Institute Microarray Facility for performing arrays; Rachael Walker, Margaret McLeish, Peter Humphreys and the CSCR Biological Resources Unit for excellent technical assistance; and Klaas Mulder, Emma Heath, Simon Broad and the other members of F.M.W.’s group for useful discussions and help with experiments.
Footnotes
Funding
C.A.C. was funded by a Herchel Smith Postdoctoral Research Fellowship and an Evans-Freke Next Generation Junior Research Fellowship. K.K. is the recipient of a PhD studentship from the UK Medical Research Council (MRC). We gratefully acknowledge financial support from the Wellcome Trust; the UK MRC; Cancer Research UK (CRUK); and the European Union. Deposited in PMC for release after 6 months.
Competing interests statement
The authors declare no competing financial interests.
Supplementary material
Supplementary material available online at http://dev.biologists.org/lookup/suppl/doi:10.1242/dev.064592/-/DC1
References
- Baker C. M., Verstuyf A., Jensen K. B., Watt F. M. (2010). Differential sensitivity of epidermal cell subpopulations to beta-catenin-induced ectopic hair follicle formation. Dev. Biol. 343, 40–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastide P., Darido C., Pannequin J., Kist R., Robine S., Marty-Double C., Bibeau F., Scherer G., Joubert D., Hollande F., et al. (2007). Sox9 regulates cell proliferation and is required for Paneth cell differentiation in the intestinal epithelium. J. Cell Biol. 178, 635–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biernaskie J., Paris M., Morozova O., Fagan M. B., Marra M., Pevny L., Miller F. D. (2009). SKPs derive from hair follicle precursors and exhibit properties of adult dermal stem cells. Cell Stem Cell 5, 610–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan E. F., Gat U., McNiff J. M., Fuchs E. (1999). A common human skin tumour is caused by activating mutations in β-catenin. Nat. Genet. 21, 410–413 [DOI] [PubMed] [Google Scholar]
- Chi W. Y., Enshell-Seijffers D., Morgan B. A. (2010). De novo production of dermal papilla cells during the anagen phase of the hair cycle. J. Invest. Dermatol. 130, 2664–2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clevers H. (2006). Wnt/β-catenin signaling in development and disease. Cell 127, 469–480 [DOI] [PubMed] [Google Scholar]
- Collins C. A., Watt F. M. (2008). Dynamic regulation of retinoic acid-binding proteins in developing, adult and neoplastic skin reveals roles for beta-catenin and Notch signalling. Dev. Biol. 324, 55–67 [DOI] [PubMed] [Google Scholar]
- Driskell R. R., Giangreco A., Jensen K. B., Mulder K. W., Watt F. M. (2009). Sox2-positive dermal papilla cells specify hair follicle type in mammalian epidermis. Development 136, 2815–2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enshell-Seijffers D., Lindon C., Morgan B. A. (2008). The serine protease Corin is a novel modifier of the Agouti pathway. Development 135, 217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enshell-Seijffers D., Lindon C., Kashiwagi M., Morgan B. A. (2010). Beta-catenin activity in the dermal papilla regulates morphogenesis and regeneration of hair. Dev. Cell 18, 633–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrach S., Ambler C. A., Lo Celso C., Hozumi K., Watt F. M. (2006). Jagged 1 is a β-catenin target gene required for ectopic hair follicle formation in adult epidermis. Development 133, 4427–4438 [DOI] [PubMed] [Google Scholar]
- Fitzgerald A. M. P., Kirkpatrick J. J. R., Foo I. T. H., Naylor I. L. (1996). Human skin histology as demonstrated using Herovici’s stain: a guide for the improvement of dermal substitutes for use with cultured keratinocytes? Burns 22, 200–202 [DOI] [PubMed] [Google Scholar]
- Fliniaux I., Viallet J. P., Dhouailly D. (2004). Ventral vs. dorsal chick dermal progenitor specification. Int. J. Dev. Biol. 48, 103–106 [PubMed] [Google Scholar]
- Fuchs E. (2008). Skin stem cells: rising to the surface. J. Cell Biol. 180, 273–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E., Tumbar T., Guasch G. (2004). Socializing with the neighbors: stem cells and their niche. Cell 116, 769–778 [DOI] [PubMed] [Google Scholar]
- Hamilton T. G., Klinghoffer R. A., Corrin P. D., Soriano P. (2003). Evolutionary divergence of platelet-derived growth factor alpha signaling mechanisms. Mol. Cell. Biol. 23, 4013–4025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne K. A., Jahoda C. A. (1992). Restoration of hair growth by surgical implantation of follicular dermal sheath. Development 116, 563–571 [DOI] [PubMed] [Google Scholar]
- Ito M., Yang Z., Andl T., Cui C., Kim N., Millar S. E., Cotsarelis G. (2007). Wnt-dependent de novo hair follicle regeneration in adult mouse skin after wounding. Nature 447, 316–320 [DOI] [PubMed] [Google Scholar]
- Jensen K. B., Collins C. A., Nascimento E., Tan D. W., Frye M., Itami S., Watt F. M. (2009). Lrig1 expression defines a distinct multipotent stem cell population in mammalian epidermis. Cell Stem Cell 8, 427–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalluri R., Zeisberg M. (2006). Fibroblasts in cancer. Nat. Rev. Cancer 6, 392–401 [DOI] [PubMed] [Google Scholar]
- Karlsson L., Bondjers C., Betsholtz C. (1999). Roles for PDGF-A and sonic hedgehog in development of mesenchymal components of the hair follicle. Development 126, 2611–2621 [DOI] [PubMed] [Google Scholar]
- Lichti U., Anders J., Yuspa S. H. (2008). Isolation and short-term culture of primary keratinocytes, hair follicle populations and dermal cells from newborn mice and keratinocytes from adult mice for in vitro analysis and for grafting to immunodeficient mice. Nat. Protoc. 3, 799–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Celso C., Prowse D. M., Watt F. M. (2004). Transient activation of beta-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development 131, 1787–1799 [DOI] [PubMed] [Google Scholar]
- Mii Y., Taira M. (2009). Secreted Frizzled-related proteins enhance the diffusion of Wnt ligands and expand their signalling range. Development 136, 4083–4088 [DOI] [PubMed] [Google Scholar]
- Millar S. E. (2002). Molecular mechanisms regulating hair follicle development. J. Invest. Dermatol. 118, 216–225 [DOI] [PubMed] [Google Scholar]
- Morris R. J., Potten C. S. (1999). Highly persistent label-retaining cells in the hair follicles of mice and their fate following induction of anagen. J. Invest. Dermatol. 112, 470–475 [DOI] [PubMed] [Google Scholar]
- Olivera-Martinez I., Thélu J., Dhouailly D. (2004). Molecular mechanisms controlling dorsal dermis generation from the somitic dermomyotome. Int. J. Dev. Biol. 48, 93–101 [DOI] [PubMed] [Google Scholar]
- Rendl M., Lewis L., Fuchs E. (2005). Molecular dissection of mesenchymal-epithelial interactions in the hair follicle. PLoS Biol. 3, e331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinn J. L., Bondre C., Gladstone H. B., Brown P. O., Chang H. Y. (2006). Anatomic demarcation by positional variation in fibroblast gene expression programs. PLoS Genet. 2, e119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero G., Sneddon W. B., Yang Y., Wheeler D., Blair H. C., Friedman P. A. (2010). Parathyroid hormone receptor directly interacts with dishevelled to regulate β-catenin signaling and osteoclastogenesis. J. Biol. Chem. 285, 14756–14763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandulache V. C., Parekh A., Dohar J. E., Hebda P. A. (2007). Fetal dermal fibroblasts retain a hyperactive migratory and contractile phenotype under 2-and 3-dimensional constraints compared to normal adult fibroblasts. Tissue Eng. 13, 2791–2801 [DOI] [PubMed] [Google Scholar]
- Schofield R. (1978). The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells 4, 7–25 [PubMed] [Google Scholar]
- Sick S., Reinker S., Timmer J., Schlake T. (2006). WNT and DKK determine hair follicle spacing through a reaction-diffusion mechanism. Science 314, 1447–1450 [DOI] [PubMed] [Google Scholar]
- Silva-Vargas V., Lo Celso C., Giangreco A., Ofstad T., Prowse D. M., Braun K. M., Watt F. M. (2005). Beta-catenin and Hedgehog signal strength can specify number and location of hair follicles in adult epidermis without recruitment of bulge stem cells. Dev. Cell 9, 121–131 [DOI] [PubMed] [Google Scholar]
- Suceveanu A. I., Suceveanu A., Voinea F., Mazilu L., Mixici F., Adam T. (2009). Introduction of cytogenetic tests in colorectal cancer screening. J. Gastrointestin. Liver Dis. 18, 33–38 [PubMed] [Google Scholar]
- Takeda H., Lyle S., Lazar A. J., Zouboulis C. C., Smyth I., Watt F. M. (2006). Human sebaceous tumors harbor inactivating mutations in LEF1. Nat. Med. 12, 395–397 [DOI] [PubMed] [Google Scholar]
- Van Mater D., Kolligs F. T., Dlugosz A. A., Fearon E. R. (2003). Transient activation of beta-catenin signaling in cutaneous keratinocytes is sufficient to trigger the active growth phase of the hair cycle in mice. Genes Dev. 17, 1219–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeulen L., De Sousa E., Melo F., van der Heijden M., Cameron K., de Jong J. H., Borovski T., Tuynman J. B., Todaro M., Merz C., et al. (2010). Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 12, 468–476 [DOI] [PubMed] [Google Scholar]
- Watt F. M., Driskell R. R. (2010). The therapeutic potential of stem cells. Philos. Trans. R. Soc. Lond. B Biol. Sci. 365, 155–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner S., Krieg T., Smola H. (2007). Keratinocyte-fibroblast interactions in wound healing. J. Invest. Dermatol. 127, 998–1008 [DOI] [PubMed] [Google Scholar]
- Yang C. C., Cotsarelis G. (2010). Review of hair follicle dermal cells. J. Dermatol. Sci. 57, 2–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Zhou T., Li A., Yao H., He F., Wang L., Si J. (2009). A potential role of collagens expression in distinguishing between premalignant and malignant lesions in stomach. Anat. Rec. (Hoboken) 292, 692–700 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.