Abstract
African trypanosomes, i.e. Trypanosoma brucei and related sub-species, are devastating human and animal pathogens that cause significant human mortality and limit sustained economic development in sub-Saharan Africa. Trypanosoma brucei is a highly motile protozoan parasite and coordinated motility is central to both disease pathogenesis in the mammalian host and parasite development in the tsetse fly vector. Since motility is critical for parasite development and pathogenesis, understanding unique aspects of the T. brucei flagellum may uncover novel targets for therapeutic intervention in African sleeping sickness. Moreover, studies of conserved features of the T. brucei flagellum are directly relevant to understanding fundamental aspects of flagellum and cilium function in other eukaryotes, making T. brucei an important model system. The T. brucei flagellum contains a canonical 9 + 2 axoneme, together with additional features that are unique to kinetoplastids and a few closely-related organisms. Until recently, much of our knowledge of the structure and function of the trypanosome flagellum was based on analogy and inference from other organisms. There has been an explosion in functional studies in T. brucei in recent years, revealing conserved as well as novel and unexpected structural and functional features of the flagellum. Most notably, the flagellum has been found to be an essential organelle, with critical roles in parasite motility, morphogenesis, cell division and immune evasion. This review highlights recent discoveries on the T. brucei flagellum.
Keywords: Flagellum, Cilium, Motility, Axoneme, Trypanosome, Dynein, Cytokinesis
1. Introduction
The eukaryotic flagellum is an evolutionarily “ancient” organelle. Flagella are represented in most eukaryotic lineages, from deep branching eukaryotes, e.g. Giardia, trichomonads and kinetoplastids, to crown eukaryotes, e.g. humans and other mammals. In all cases flagella have a conserved underlying structure and are usually assembled via the conserved process of intraflagellar transport (Rosenbaum, 2002). Thus, the combined evidence indicates that the flagellum arose very early in eukaryotic evolution and that organisms lacking flagella arose through loss of the organelle. The flagellum is also “old” as an object of study and is arguably the first organelle ever described (Satir, 1995). These biological nanomachines have captured the imagination of scientists and interested observers for over 300 years, since Antony Van Leeuwenhoek’s original description of “little legs” propelling the movement of microbes in 1675 (Dobell, 1958).
Flagella are structurally and functionally analogous to cilia and are most widely recognized as agents of cellular propulsion. Motile cilia and flagella drive the movement of many unicellular eukaryotes, including important human pathogens such as Trichomonas vaginalis, Giardia lamblia and Trypanosoma brucei, and are critical in the mating cycles of protozoa that cause malaria and toxoplasmosis (Ferguson, 2002; Vlachou et al., 2006). Cilia and flagella are also important for normal human physiology. For example, flagella drive sperm motility and motile cilia are critical for fluid movement across epithelial surfaces in the respiratory tract, brain ventricles, female reproductive tract and nodal cells of developing embyros (Ibanez-Tallon et al., 2003). Defects in these organelles lead to a wide variety of diseases and developmental defects (Badano et al., 2006). In addition to their obvious function in driving cell and fluid movement, flagella have recently been found to have an assortment of surprising functions beyond motility. For example, cilia and flagella are now recognized to play critical sensory and transport functions, and are important in cell cycle control. Several excellent reviews on mammalian cilia and flagella are available (Badano et al., 2006; Satir and Christensen, 2007), and this review will focus on recent discoveries in the African trypanosome, T. brucei.
African trypanosomes are devastating human and animal pathogens. Two sub-species, Trypanosoma brucei rhodesiense and Trypanosoma brucei gambiense are causative agents of the fatal human disease known as African sleeping sickness. This disease is always fatal if left untreated, and it is estimated that 500,000 new infections occur annually (Legros et al., 2002; Welburn and Odiit, 2002). Trypanosoma brucei is transmitted by the tsetse fly and alternates between bloodstream-form and insect-form life-cycle stages that are adapted to survive in the mammalian host and the insect vector, respectively. The importance of the flagellum for motility and for attachment to the tsetse fly salivary gland epithelium has been appreciated for many years (Vickerman, 1969, 1985; Vickerman and Preston, 1976). Indeed, the flagellum is responsible for the “undulating membrane” of bloodstream-form trypanosomes, which was one of the first described features of these parasites. Recent studies have revealed novel features of T. brucei flagellum structure, composition and function and these are outlined below. These discoveries are important not only from the standpoint of understanding trypanosome biology and the potential for uncovering novel drug targets, but also for advancing our understanding of fundamental aspects of eukaryotic flagellum structure and function.
2. Flagellum structure and composition
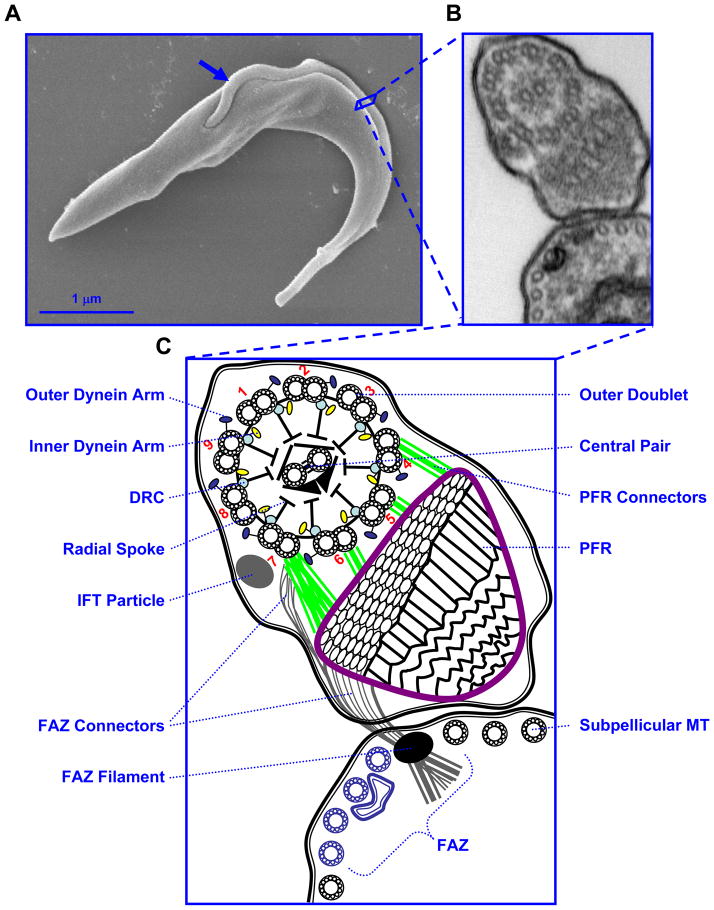
The T. brucei cell body is roughly cylindrical in shape with tapered anterior and posterior ends. Culture-adapted insect midgut (procyclic) and bloodstream life-cycle stages used most commonly in the laboratory are approximately 19 – 25 um long (Robinson et al., 1995; Tyler et al., 2001), although the length of the cell body varies considerably during the life-cycle, particularly in the tsetse fly (Van Den Abbeele et al., 1999). A single flagellum emerges from the basal body near the posterior end of the cell. Once the flagellum exits the cytoplasm, it is surrounded by its own membrane and is attached along most of its length to the cell body, tracing a left-handed helical path from posterior to anterior (Fig. 1). The distal end of the flagellum extends a short distance beyond the cell body. A specialized membrane domain termed the flagellar pocket forms from an invagination of the cell surface membrane where the proximal end of the flagellum emerges from the cytoplasm. The cell membrane, flagellar pocket membrane and flagellar membrane comprise three contiguous, but functionally distinct, membrane domains (Balber, 1990; Fridberg et al., 2007). Along the length of the cell, the flagellum and cell body are held in close apposition by a network of cytoskeletal and membranous connections that collectively make up the flagellum attachment zone (FAZ) (Vickerman, 1969; Kohl and Gull, 1998). Within the flagellum is a canonical “9 + 2” microtubule axoneme that drives flagellar movement. The flagellum also contains a large lattice-like structure, termed the paraflagellar rod (PFR), which runs parallel to the axoneme and is physically attached to both the axoneme and the FAZ.
Fig. 1.
The trypanosome flagellum. (A) Scanning electron micrograph of a procyclic trypanosome. An arrow indicates the single flagellum that is attached along the cell body. (B) Transmission electron micrograph, showing a cross-section of the flagellum and its attachment to the cell body as viewed from posterior toward anterior. (C) Schematic representation of the micrograph in B. Major flagellar sub-structures are indicated and outer doublet microtubules are numbered according to conventional nomenclature. The four specialized sub-pellicular microtubules that are part of the FAZ are indicated in blue. Abbreviations: DRC, dynein regulatory complex; IFT, intraflagellar transport; FAZ, flagellum attachment zone; PFR, paraflagellar rod.
2.1. Axoneme
As in most motile eukaryotic flagella, the trypanosome axoneme consists of nine outer doublet microtubules surrounding a pair of centrally located singlet microtubules. Radial spokes extend inward from each outer doublet toward the central pair. ATP-dependent dynein motor proteins attached to each doublet translocate along the adjacent doublet to generate the sliding forces that underlie flagellar movement. Connecting the outer doublets to one another are nexin links (Warner, 1976), which are thought to help maintain outer doublet organization (Lindemann and Kanous, 1997; Cibert, 2001) and may contribute to dynein regulation (Lindemann, 2004).
The ultrastructure of the trypanosome flagellum has been known for several decades, but only recently has the availability of genome sequences demonstrated that genes encoding axonemal proteins in other organisms are conserved in trypanosomes. This, combined with facile systems for RNA interference (RNAi) has spurred a burst in functional analyses of flagellar proteins in T. brucei. These studies have demonstrated conserved functions for several flagellum sub-complexes and provided functional analysis of some proteins that were previously defined only by their biochemical association with the flagellum. These efforts have also uncovered novel and unexpected aspects of flagellum architecture in trypanosomes. Specifically, functional studies in T. brucei have revealed a requirement for γ-tubulin in the nucleation of central pair microtubules (McKean et al., 2003), uncovered a novel family of “structural maintenance of chromosomes” (SMC) domain-containing proteins as potential components of the nexin linkages (Fig. 2) (Baron et al., 2007b) and demonstrated a role for parkin co-regulated proteins in outer doublet microtubule stability (Fig. 3) (Dawe et al., 2005). RNAi directed against conserved components of the radial spokes, central pair apparatus (Fig. 4), outer dynein arms (Fig. 5), dynein regulatory complex and protofilament ribbons demonstrated a requirement for each of these structures in T. brucei flagellar motility (Ralston et al., 2005, 2006; Branche et al., 2006; Baron et al., 2007a). See Table 1 for a complete list of these proteins, as well as other characterized components of the T. brucei flagellum.
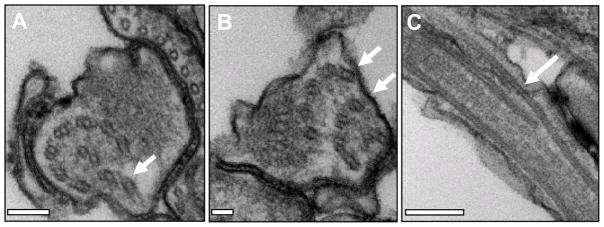
Fig. 2.
RNA interference (RNAi) knockdown of the conserved component of motile flagella 9 (CMF9) protein results in loss of inter-doublet connections. Transverse (A, B) and longitudinal (C) sections of procyclic cells in which CMF9 is knocked down with RNAi. White arrows point to misplaced outer doublets Scale bars are 100 nm (A), 50 nm (B) and 200 nm (C). Adapted from Baron et al., 2007b, with permission.
Fig. 3.
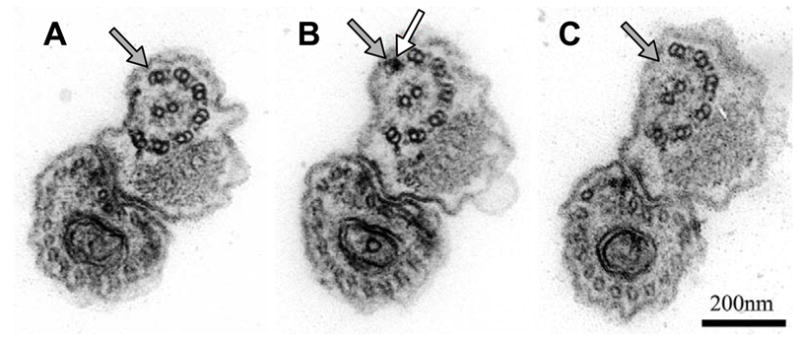
Simultaneous RNA interference (RNAi) knockdown of the parkin co-regulated genes PACRGA and PACRGB results in loss of outer doublets. Serial transverse sections (proceeding from posterior to anterior (A, B, C, respectively) of a procyclic cell in which both PACRGA and B have been knocked down with RNAi. Black arrows indicate the outer doublet that is lost toward the distal tip of the axoneme. The white arrow indicates the electron dense lumen of the A-tubule. Adapted from Dawe et al., 2005; reproduced with permission of the Company of Biologists.
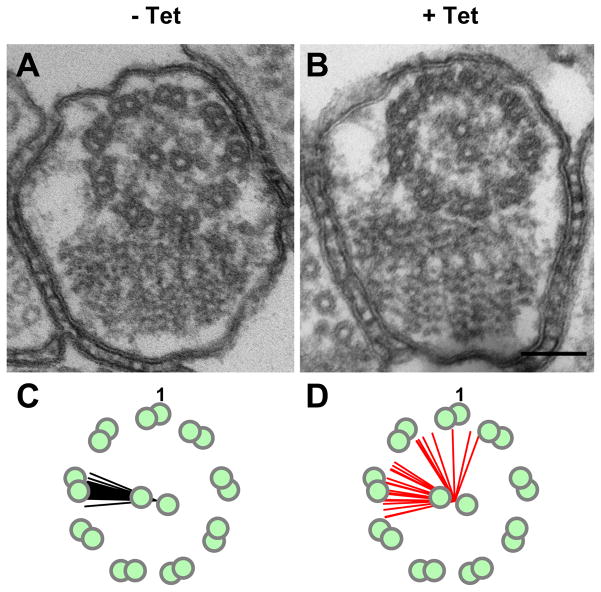
Fig. 4.
Central pair orientation is fixed in wild type cells and is dependent upon the central pair protein “paralyzed flagella 16” (PF16). (A, B) Transverse sections of PF16 knockdowns grown in the absence (−) or presence (+) of tetracycline to induce RNA interference knockdown of PF16. In control cells (-Tet) central pair microtubules lie in a plane that is roughly parallel to the paraflagellar rod, whereas in PF16 knockdown mutants (+ Tet) central pair orientation is variable. (C, D) Schematic with lines depicting the plane of central pair microtubules measured in control (C) and PF16 knockdown (D) cells, n = 26. Outer doublet number 1 is labeled for reference. Scale bar is 100 nm. Adapted from Ralston et al., 2006, with permission.
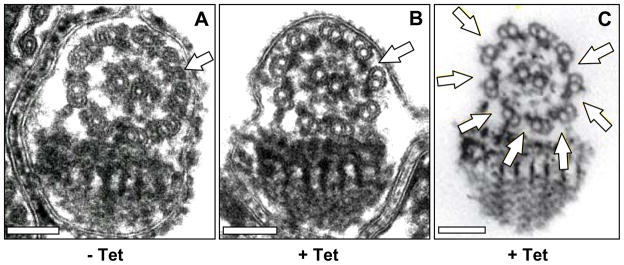
Fig. 5.
RNA interference (RNAi) knockdown of dynein light chain 1 (LC1) or axonemal dynein intermediate chain 1 (DNAI1) results in loss of outer dynein arms. Transverse sections of flagella (A, B) or flagellar cytoskeleton (C) from LC1 (A, B) or DNAI1 (C) knockdown lines grown in the absence (−) or presence (+) of tetracycline. Arrows point to positions of outer dynein arms, which are absent in LC1 and DNAI1 knockdowns. Scale bar is 100 nm. Adapted from Baron et al., 2007a (A); and Branche et al., 2006 (C), reproduced with permission of the Company of Biologists.
Table 1.
Characterized components of the Trypanosoma brucei flagellum. This table lists proteins for which experimental investigation has allowed protein assignment to specific flagellar functions or flagellar sub-complexes in T. brucei.
| Protein | Function and/or Location | Reference |
|---|---|---|
| Axoneme | ||
| CMF9 | OD Assembly/Maintenance | (Baron et al., 2007b) |
| CMF76b | OD Assembly/Maintenance | (Baron et al., 2007b) |
| Hydin | CP Maintenance/Orientation | (Dawe et al., 2007) |
| MBO2 | OD Component | (Broadhead et al., 2006) |
| PACRG-A | OD Assembly/Maintenance | (Dawe et al., 2005) |
| PACRG-B | OD Assembly/Maintenance | (Dawe et al., 2005) |
| PF16 | CP Orientation | (Branche et al., 2006; Ralston et al., 2006) |
| PF20 | CP Orientation | (Branche et al., 2006; Ralston et al., 2006) |
| Rib72 | OD Component | (Baron et al., 2007b) |
| RSP3 | RS Assembly/Maintenance | (Ralston et al., 2006) |
| Trypanin | DRC Component, Dynein Regulation | (Ralston et al., 2006) |
| γ-Tubulin | CP Assembly/Maintenance | (McKean et al., 2003) |
| Dyneins | ||
| DHC1b-1 | IFT | (Kohl et al., 2003; Adhiambo et al., 2005) |
| DHC1b-2 | IFT | (Kohl et al., 2003; Adhiambo et al., 2005)’ |
| DNAI1 | ODA | (Branche et al., 2006) |
| LC1 | ODA | (Baron et al., 2007a) |
| Tax-1/p38 | IDA | (Broadhead et al., 2006; Yamamoto et al., 2006) |
| Paraflagellar Rod | ||
| ADK-A | Adenylate Kinase | (Pullen et al., 2004; Ginger et al., 2005) |
| ADK-B | Adenylate Kinase | (Pullen et al., 2004; Ginger et al., 2005) |
| PDEB1 | cAMP Homeostasis | (Oberholzer et al., 2007) |
| PDEB2 | cAMP Homeostasis | (Oberholzer et al., 2007) |
| PFR1 | PFR Structural Protein | (Deflorin et al., 1994; Durand-Dubief et al., 2003) |
| PFR2 | PFR Structural Protein | (Schlaeppi et al., 1989; Bastin et al., 1998) |
| Tryp-CaM | Ca2+ binding | (Ridgley et al., 2000) |
| 5.20 | PFR component | (Woodward et al., 1994) |
| Flagellum Attachment Zone | ||
| FAZ1 | Flagellum Attachment | (Vaughan et al., 2008) |
| Fla1 | Flagellum Attachment | (LaCount et al., 2002) |
| Flagellar Membrane | ||
| ESAG4 | Adenylate Cyclase | (Paindavoine et al., 1992) |
| Intraflagellar Transport | ||
| Che2/IFT80 | IFT | (Davidge et al., 2006; Absalon et al., 2007a) |
| IFT20 | IFT, Flagellar Matrix | (Absalon et al., 2007a) |
| IFT52 | IFT, Flagellar Matrix | (Absalon et al., 2007a) |
| IFT57/55 | IFT | (Absalon et al., 2007a) |
| IFT88 | IFT | (Kohl et al., 2003; Absalon et al., 2007a) |
| IFT122 | IFT | (Absalon et al., 2007a) |
| IFT140 | IFT | (Absalon et al., 2007a) |
| IFT172 | IFT, Flagellar Matrix | (Absalon et al., 2007a) |
| PIFTA1 | IFT | (Absalon et al., 2007a) |
| PIFTB2 | IFT | (Absalon et al., 2007a) |
| PIFTC3 | IFT | (Absalon et al., 2007a) |
| PIFTD4 | IFT | (Absalon et al., 2007a) |
| PIFTF6 | IFT | (Absalon et al., 2007a) |
While many additional novel flagellar proteins have been identified through comparative genomics (Baron et al., 2007b) and proteomics (Broadhead et al., 2006), and have been linked to flagellar functions by mutant or RNAi analyses, their precise functions and locations are unknown. For a comprehensive inventory of flagellar proteins in a variety of organisms, the reader is referred to the ciliome database: www.ciliome.com (Inglis et al., 2006).
Abbreviations: OD: outer doublet; CP: central pair; RS: radial spokes; IFT: intraflagellar transport; ODA: outer dynein assembly; IDA: inner dynein assembly; PFR: paraflagellar rod; DRC: dynein regulatory complex.
Analysis of mutants lacking central pair proteins also led to the surprising finding that the central pair retains a fixed orientation relative to outer doublet microtubules in T. brucei (Fig. 4) (Ralston et al., 2005, 2006; Branche et al., 2006). This differs from Chlamydomonas reinhardtii, a well-established model system for flagellum studies, where the central pair rotates within the axoneme (Omoto et al., 1999; Mitchell, 2003). Rotation of the asymmetrically arranged central pair apparatus in C. reinhardtii has been hypothesized to play a role in activating distinct sets of dyneins on the outer doublets (Wargo and Smith, 2003; Smith and Yang, 2004). Thus, a non-rotating central pair in T. brucei may reflect or impose unique demands on dynein motor regulation. Subsequent studies demonstrated that a fixed orientation of central pair microtubules is conserved among other kinetoplastids (Gadelha et al., 2006) and that defects in central pair orientation arise in some, but not all, flagellar mutants (Gadelha et al., 2006; Baron et al., 2007b). Thus central pair orientation appears to be actively maintained and is not simply the consequence of constraints imposed by flagellar beat in trypanosomes. Interestingly, central pair orientation is also fixed relative to outer doublets in human axonemes, indicating that in some respects the trypanosome flagellum may provide advantages over C. reinhardtii as a model for human cilia.
2.2. Paraflagellar rod
One of the most peculiar structural features of the trypanosome flagellum is the presence of a large paracrystaline filament, the PFR, which extends alongside the axoneme from the flagellar pocket to the flagellum tip. Unlike the axoneme, which is broadly conserved among eukaryotes, the PFR is restricted to kinetoplastids, euglenoids and dinoflagellates. The PFR was first described 45 years ago (Vickerman, 1962b) and is required for normal motility (Bastin et al., 1998). However, its precise function is unknown and aside from its main structural components, PFR1 and PFR2 (Schlaeppi et al., 1989; Deflorin et al., 1994), and a few recently identified proteins (see below), its molecular composition remains to be determined.
Recently, several proteins affiliated with nucleotide metabolism have been found to be associated with the PFR. Three flagellar adenylate kinase proteins have been identified, with two of these (ADK-A and ADK-B) co-localizing with the PFR, and a third (ADK-E) localizing to the flagellum but not the PFR (Pullen et al., 2004; Ginger et al., 2005). Although adenylate kinase activity has not yet been demonstrated for these proteins, there appears to be adenylate kinase activity associated with the PFR since cytoskeletal extracts from the snl-2 mutant, which lacks the majority of the PFR, have reduced adenylate kinase activity compared with wild-type extracts (Pullen et al., 2004). There is precedence for flagellar adenylate kinases as the central pair component cpc1 of C. reinhardtii contains an adenylate kinase domain (Zhang and Mitchell, 2004). Two additional PFR-associated proteins involved in nucleotide metabolism, TbrPDEB1 and TbrPDEB2, were recently identified (Zoraghi and Seebeck, 2002; Oberholzer et al., 2007). These cAMP phosphodiesterases localize to the flagellum, though the majority of TbrPDEB2 localizes to punctuate spots in the cytoplasm (Oberholzer et al., 2007). Knockdown of flagellar ADKs or PDEBs did not impair growth of procyclic cells, but simultaneous ablation of PDEB1 and 2 was found to be lethal in bloodstream-form parasites (Zoraghi and Seebeck, 2002; Ginger et al., 2005; Oberholzer et al., 2007). The motility phenotypes of PDEB knockdown mutants have not been described. Overall, these new findings suggest that the PFR is not simply a passive structural support to the axoneme, but might serve as a scaffold for assembly of regulatory and metabolic proteins that contribute to flagellum function.
2.3. Flagellum attachment zone
Components of the T. brucei FAZ are also mostly unknown, although the ultrastructure of the FAZ has been characterized in detail (Vickerman, 1962a; Sherwin and Gull, 1989; Gull, 1999; Kohl et al., 1999). The cytoplasmic side of the FAZ is defined by an electron-dense filament of unknown composition, together with four specialized microtubules that associate with a membranous compartment. A network of filaments connects the FAZ filament in the cell body to both the axoneme and PFR within the flagellum, and there are likely to be membranous linkages in addition to these cytoskeletal linkages between the cell body and flagellum (Hutchings et al., 2002). Flagellum adhesion glycoprotein 1 (Fla1), the T. brucei homolog of Trypanosoma cruzi GP72, localizes to the FAZ and is required for flagellum attachment (el-Sayed et al., 1995; Nozaki et al., 1996; LaCount et al., 2000, 2002). Another FAZ component, FAZ1, was recently identified by screening an expression library with the monoclonal antibody L3B2 that recognizes the FAZ filament (Vaughan et al., 2008). FAZ1 is conserved amongst kinetoplastids and is required for normal FAZ assembly and flagellum attachment (Vaughan et al., 2008). Intriguingly, given the role of the FAZ in cytokinesis (see section 3.5), it is interesting that a T. brucei polo-like kinase homologue, TbPLK, has been localized to the FAZ (Kumar and Wang, 2006), although a separate study reported that TbPLK localizes to the cytoplasm (Hammarton et al., 2007).
2.4. Flagellar membrane and matrix
The flagellar membrane and soluble flagellar matrix in T. brucei represent mostly uncharted territory. A single flagellum membrane-specific protein, the ESAG4 adenylate cyclase, has been identified in T. brucei (Paindavoine et al., 1992), and a calcium-binding flagellar membrane protein has been characterized in T. cruzi (Engman et al., 1989; Buchanan et al., 2005). In the flagellar matrix, several components of the intraflagellar transport pathway (IFT20, IFT52, IFT172) have recently been shown to exhibit both a soluble flagellar pool and a detergent-insoluble basal body-associated pool (Absalon et al., 2007a), but no other soluble flagellar proteins have been identified. Thus we have very little knowledge of the proteins that make up the matrix or membrane. For reference, proteomic analysis of the C. reinhardtii flagellum revealed 146 proteins with four or more unique peptides in the membrane + matrix fraction (Pazour et al., 2005). Given that the flagellar membrane presents an interface with the host (Webster and Russell, 1993; Fridberg et al., 2007), flagellar membrane proteins are likely to include critical components of signal transduction pathways that coordinate flagellar movement in response to environmental cues. Clearly, this represents an untapped area of research that is rich with opportunity for important discoveries.
2.5. Flagellum biogenesis
Assembly of the T. brucei flagellum has been shown to be dependent on the conserved process of IFT (Rosenbaum, 2002; Kohl et al., 2003; Davidge et al., 2006). In this process, axoneme precursors are transported to the distal tip of the flagellum via IFT particles whose movement is powered by heterotrimeric kinesin (Kozminski et al., 1995). The DHC1b dynein then returns IFT particles to the base of the flagellum to reload with additional cargo (Pazour et al., 1999; Porter et al., 1999). Several conserved IFT components have now been identified and shown to function in T. brucei (Kohl et al., 2003; Davidge et al., 2006; Absalon et al., 2007a) and transport of an IFT52-GFP chimera has been directly visualized in live cells (Absalon et al., 2007a). Notably, since the trypanosome flagellum exhibits many unique structural features, these parasites may also possess unique IFT machinery. Indeed, trypanosomes possess two DHC1b homologues, while other eukaryotes possess only a single retrograde IFT motor (Adhiambo et al., 2005). Likewise, putative IFT particles observed by electron microscopy in procyclic T. brucei cells are found almost exclusively adjacent to axonemal doublets 3, 4, 7 and 8 (Absalon et al., 2007a), a preference that has not been reported in other organisms.
In addition to the axoneme, flagellum biogenesis in T. brucei must accommodate assembly of the PFR. Construction of the PFR is dependent upon IFT (Kohl et al., 2003), although whether this reflects a direct involvement of IFT or a requirement for the axoneme is not clear. Consistent with the involvement of an IFT-like system for PFR assembly, PFR subunits are added primarily to the distal tip of the growing PFR filament (Bastin et al., 1999) and RNAi knockdown of the PFR2 subunit results in accumulation of the PFR1 subunit at the distal tip of the flagellum (Bastin et al., 1998).
The T. brucei flagellum persists throughout the cell cycle and the cell is faced with the challenge of elaborating a new daughter flagellum, while retaining the old one and co-ordinating all of this with other cell cycle events. Thus, flagellar biogenesis in these parasites poses unique challenges to the organism and is an exciting area that awaits further investigation. Interestingly, studies of flagellum assembly in T. brucei have led to the discovery of a novel quality control checkpoint, which functions to ensure proper assembly of tubulin dimers as they are delivered into the flagellum (Stephan et al., 2007).
2.6. Flagellum composition revealed through genomic and proteomic studies
In addition to the novel and surprising structural features uncovered by functional analysis of individual genes, significant advances have been made through large-scale genomic and proteomic approaches (Broadhead et al., 2006; Baron et al., 2007b). A recent comparative genomics study identified 50 genes that are conserved in T. brucei and other eukaryotes with motile flagella, but are absent in organisms that contain only non-motile flagella or lack flagella entirely. Hence these genes represent core components of motile flagella (CMF) genes (Baron et al., 2007b). Of 50 T. brucei CMF genes identified, 30 were previously uncharacterized in any organism, providing an illustration of the number of surprises that await continued analysis of the trypanosome flagellum. RNAi knockdown of the 30 novel TbCMF genes, together with another 11 that had not previously been characterized in trypanosomes, demonstrated a motility function for the majority of these (Fig. 6). TbCMF proteins are expected to be axonemal, since they are conserved in organisms that lack a PFR or FAZ. Consistent with this, immunolocalization and biochemical fractionation demonstrated that several of these proteins are exclusively and stably associated with the flagellum, and ultrastructural analysis identified one family of novel TbCMF genes required for maintaining nexin linkages between axonemal outer doublets (Baron et al., 2007b).
Fig. 6.
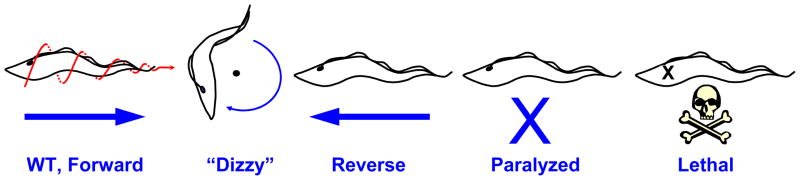
Importance of flagellum motility revealed by procyclic motility mutants. Schematic representations of motility phenotypes in procyclic Trypanosoma brucei flagellum mutants. Wild-type (WT) cell movement is helical with the flagellum tip leading. RNA interference knockdown of flagellar protein expression leads to a variety of motility phenotypes. “Dizzy” mutants, such as trypanin knockdown mutants (Hutchings et al., 2002), retain a vigorously beating flagellum, but are unable to move directionally, and instead tumble in place. “Reverse” motility is observed when subunits of the outer dynein motors, such as DNAI1 (Branche et al., 2006) or LC1 (Baron et al., 2007a) are targeted. These mutants exhibit a reverse, base-to-tip beat and move backward with the flagellum tip trailing. “Paralyzed” mutants, such as central pair and radial spoke mutants (Branche et al., 2006; Ralston et al., 2006) are incapable of movement at the cellular level. Some paralyzed mutants have flagella that are able to beat, while other mutants have more severe defects and are only capable of erratic twitching motions. “Lethal” defects arise in mutants with severe flagellar paralysis (Baron et al., 2007b).
The genomic analysis of Baron and colleagues also revealed that several flagellar genes are represented by families of two or more related sequences and that these gene families are often expanded in T. brucei. These trypanosome-specific gene expansions include components of the dynein regulatory complex, protofilament ribbons, dynein light chains and three novel protein families. Analysis of individual mutants and immunolocalization studies indicate that in at least some instances, this does not simply reflect functional redundancy (Baron et al., 2007b; K.L. Hill, unpublished observation). Trypanosomes also contain two homologues of the retrograde IFT molecular motor, DHC1b, which has a single orthologue in other eukaryotes. Trypanosome-specific expansion of flagellum gene families may reflect unique aspects of trypanosome flagellar beat, or may be a consequence of unique constraints imposed by the unusual architecture of the trypanosome flagellum.
Our inventory of trypanosome flagellar proteins was greatly expanded by proteomic analysis of salt-extracted flagella (Broadhead et al., 2006). This important study identified 331 proteins that constitute a T. brucei-extracted flagellum proteome (TbEFP). These proteins may be components of the axoneme, PFR, FAZ or basal body, which were all present in the sample used for analysis. Of the TbEFP dataset, 63% were kinetoplastid-specific and 26% were conserved only among flagellated eukaryotes. RNAi knockdown indicated a flagellum function for eight TbEFP proteins while three others, including PFR2, had previously been shown to contribute to flagellum function (Bastin et al., 1998; Dawe et al., 2005). Interestingly, 238 of these proteins were annotated as hypothetical and there was a relatively small overlap (~50 proteins) between the trypanosome dataset and extracted axonemal proteomes from other organisms. This again emphasizes the unexplored nature of the trypanosome flagellar apparatus. These studies are also important from the standpoint of drug development, since trypanosome-specific flagellar proteins may represent novel targets for therapeutic intervention (see section 3.7).
An important outcome from functional genomic and proteomic studies in T. brucei was the identification of human disease gene candidates. Many human homologues of TbCMF and TbEFP genes have been directly implicated in human disease, or map to loci that are linked to diseases having known or suspected underpinnings of ciliary dysfunction (Broadhead et al., 2006; Baron et al., 2007b). For many of these genes, the works by Baron et al. and Broadhead et al. represent, to our knowledge, the first time that the connection to human disease has been made and, in some cases, the first functional characterization. For example, while it was known that protofilament ribbon protein 72 (Rib72) was biochemically associated with flagellar protofilament ribbons (Patel-King, 2002; Ikeda and Kamiya, 2003), and that mutations in Rib72 are associated with juvenile myoclonic epilepsy (Suzuki et al., 2004; King, 2006), studies in T. brucei demonstrated a requirement for Rib72 in flagellar motility (Baron et al., 2007b). Similarly, mutations in hydin induce hydrocephalus and studies in T. brucei were among the first to show a specific flagellum function, demonstrating that hydin knockdown leads to defects in the positioning and formation/stability of the axonemal central pair (Broadhead et al., 2006; Dawe et al., 2007; Lechtreck and Witman, 2007). In another recent work, it was shown that two T. brucei homologues of a gene implicated in male sterility (Lorenzetti et al., 2004), PACRG, are required for outer doublet stability in T. brucei (Fig. 3) (Dawe et al., 2005). Thus, studies in T. brucei have added to the repertoire of genes implicated in human ciliary diseases and have provided demonstration that these genes have bona fide flagellum functions. Trypanosoma brucei is therefore emerging as an extremely powerful experimental system for probing candidate disease genes. It will become even more valuable as the era of gene discovery comes to a close and the focus turns to functional analysis.
3. Flagellum function
The T. brucei flagellum is a multifunctional organelle with many critical roles, some of which are well-established and some of which have only recently come to light. The flagellum has long been known for its role in driving parasite motility, which impacts both disease pathogenesis and transmission (Hill, 2003), and facilitating host cell attachment in the salivary glands of the tsetse fly vector (Vickerman, 1985; Vickerman et al., 1988). RNAi knockdown studies have now firmly established the central importance of the T. brucei flagellum and flagellar motility in other processes, including cell morphogenesis, cell division and immune evasion. Intriguingly, there is a dichotomy in the requirement for the flagellum during cell division between procyclic and bloodstream life-cycle stages, implying developmental regulation of flagellum function.
3.1. Host cell attachment
Parasite attachment to host cells in the tsetse salivary gland is mediated by the flagellum and is a defining step in the developmental cycle of T. brucei and other salivarian trypanosomes. Although very little new information is available regarding host cell attachment, a brief discussion is provided to emphasize the need for more effort directed at understanding the underlying molecular and structural mechanisms. Parasite attachment to the salivary gland epithelium evokes extensive remodeling of the flagellar membrane and structural elements to establish intimate interdigitations between the parasite flagellum and tsetse salivary gland epithelial cells (Tetley and Vickerman, 1985; Vickerman, 1985; Tetley et al., 1987; Vickerman et al., 1988). These morphogenic events are associated with the final stages of parasite development into mammalian-infective trypomastigotes, including acquisition of the variant surface glycoprotein (VSG) coat (Tetley and Vickerman, 1985; Vickerman, 1985; Tetley et al., 1987; Vickerman et al., 1988). Attachment to the salivary gland via the flagellum is therefore of obvious importance for parasite survival and pathogenesis. The molecular mechanisms and signaling events that underlie morphological changes and developmental transformation of the parasite upon host cell attachment are unknown and demand further attention.
3.2. Motility and flagellar beat
The T. brucei flagellum wraps around the cell body in a left-handed helix and flagellar beat causes the entire cell to rotate as it moves forward in an auger-like motion (Hill, 2003). The genus name Trypanosoma is in fact derived from the Greek words for auger, “trypanon”, and cell, “soma”. Until recently, we knew very little about the molecular mechanisms of flagellar beat in T. brucei and this was almost exclusively derived by extrapolation from other systems, with little or no direct experimental evidence from trypanosomes. While some aspects of axonemal motility can reasonably be expected to be conserved in T. brucei, other features are unique and warrant direct examination. For example, in addition to the structural peculiarities discussed above, the T. brucei flagellum moves rapidly in three dimensions, producing a spiral waveform with beat parameters that vary significantly along the length of the axoneme. Hence, flagellar beat in these parasites is highly dynamic and considerably more complex than in most other systems where it has been studied. Moreover, the dominant waveform in T. brucei and other trypanosomatids is a tractile beat that initiates at the tip of the flagellum and propagates toward the base (Walker, 1961; Walker and Walker, 1963), which is opposite of the base-to-tip beat observed in most other flagella. This tractile beat is disrupted intermittently with a brief base-to-tip beat, although this is not sustained in wild-type trypanosomes (Branche et al., 2006; Baron et al., 2007a).
Control of flagellar beat requires co-ordinate regulation of several thousand dynein motors having distinct subunit composition and functionality, which are arrayed along the inner and outer face of axonemal doublet microtubules (Porter and Sale, 2000). RNAi knockdown of two separate dynein subunits have revealed that outer dynein motors are required for the tip-to-base beat that is a hallmark of T. brucei flagella (Branche et al., 2006; Baron et al., 2007a). Branche and colleagues (Branche et al., 2006) targeted the outer dynein intermediate chain DNAI1, while Baron et al. targeted the outer dynein light chain LC1 (Baron et al., 2007a). In both cases, loss of outer dynein motors (Fig. 5) resulted in loss of the tip-to-base beat, emergence of a sustained base-to-tip beat, and loss of forward motility. Reverse beat in LC1 mutants drove cell movement backward, with the flagellum tip trailing. These results provide clues into mechanisms controlling beat direction. They also illuminate an interesting contrast between trypanosomes and Chlamydomonas, where loss of outer arm dynein prevents flagella from assuming a symmetric base-to-tip beat (Kamiya and Okamoto, 1985). In other words, in one organism (trypanosomes), loss of the outer dyneins allows only symmetric base-to-tip beating, whereas in the other organism (Chlamydomonas), loss of outer dyneins prevents this beat form. Therefore, the data suggest that there are specialized functions of outer dynein motors in T. brucei.
There are also species-specific differences in the systems that regulate dynein activity. Ralston and co-workers (Ralston et al., 2005, 2006) recently showed that dynein regulation in T. brucei is mediated at least in part by a conserved dynein regulatory complex (DRC), which was originally discovered through genetic suppressor analysis in C. reinhardtii (Huang et al., 1982; Piperno et al., 1992; Gardner et al., 1994). The importance of the DRC is evidenced by the tumbling phenotype and loss of directional motility in DRC mutants (Hutchings et al., 2002; Rupp and Porter, 2003). Interestingly, although the DRC subunit trypanin is broadly conserved in flagellated eukaryotes (Hill et al., 2000; Ralston et al., 2005; Ralston and Hill, 2006), kinetoplastids are unique in having two trypanin-related proteins (Ralston et al., 2005, 2006). Recent data indicate that these proteins are not functionally redundant (K.L. Hill, unpublished observation), and it is reasonable to expect that constraints imposed by the unusual architecture or motility demands of the trypanosome flagellum may lead to unique requirements for dynein regulation in trypanosomes.
3.3. Endocytosis and immune evasion
The flagellar pocket is the sole site of endocytosis and secretion in T. brucei (Webster and Russell, 1993). The flagellar pocket is intimately associated with the flagellum and the external entrance to the flagellar pocket lumen is demarcated by close contacts between the flagellar and flagellar pocket membranes. Therefore, it has long been speculated that flagellar beating may influence trafficking through the flagellar pocket. Until recently however, experimental evidence in support of this idea has been lacking. Engstler and colleagues have now directly demonstrated that normal flagellar beat is necessary for uptake of surface Ig complexed with VSG in bloodstream-form T. brucei (Engstler et al., 2007). Since clearance of VSG-Ig protein complexes from the parasite surface is part of an integrated strategy for avoiding destruction by the host immune system, these studies suggest that flagellar motility is necessary for immune evasion by trypanosomes and establishment of a persistent infection in the mammalian host.
3.4. Cell morphogenesis
The trypanosome flagellum persists throughout the cell cycle and must be faithfully replicated and segregated along with other single copy organelles during cell division. Early indications that the flagellum itself might contribute to organelle positioning and cell morphogenesis came from structural studies showing close association between the basal body and the kinetoplast, suggesting that segregation of these single copy organelles might be obligately linked (Robertson, 1913; Simpson, 1968). Experimental evidence supporting this idea came from the demonstration that the basal body and kinetoplast are indeed physically interconnected and that pharmacological perturbation of basal body segregation blocks kinetoplast segregation (Robinson and Gull, 1991). These studies provided an important framework for considering the role of the cytoskeleton in mitochondrial genome segregation (Robinson and Gull, 1991; Robinson et al., 1995), though they did not directly implicate the flagellum as an active player in this process. Direct evidence for flagellum growth and flagellar beat in kinetoplast segregation has now come from RNAi studies in which several independent mutants with defective flagellum biogenesis or movement have a common defect in kinetoplast positioning (Dawe et al., 2005; Ralston et al., 2005, 2006; Branche et al., 2006; Absalon et al., 2007b). At present it is still unclear precisely how the flagellum effects kinetoplast segregation, but a model (Absalon et al., 2007b) has been proposed in which the elongating new flagellum, which is tethered at the growing end by the flagellar connector (Moreira-Leite et al., 2001), exerts a reaction force on the basal body, driving movement of the new basal body/kinetoplast away from the old one (Absalon et al., 2007b). Given the central importance of the flagellum and flagellar motility to trypanosome biology, it seems advantageous that segregation of the mitochondrial genome is linked to flagellum formation, maintenance and function.
The discovery of a novel mobile connector between the old and new flagellum, termed the flagellar connector(FC) (Moreira-Leite et al., 2001), suggests that flagellum influence on positioning of organelles extends beyond the kinetoplast. The flagellar connector is a mobile, tripartite cytoskeletal structure that connects the tip of the new flagellum to the old flagellum as it grows in parallel to this old flagellum. Hence, the old flagellum defines the placement of the new flagellum and by extension is suspected to impart positional information that ensures correct polarity and organization of the daughter cell. The FC has been visualized by electron microscopy (Moreira-Leite et al., 2001) and antibody staining (Briggs et al., 2004), however neither the protein components of the FC nor the mechanism for FC movement are currently known. Interestingly, the FC appears to be specific to the procyclic life-cycle stage of T. brucei, since it has not been observed in the bloodstream-form stage or in other trypanosomatids (Briggs et al., 2004).
3.5. Cell division
African trypanosomes divide through binary fission. The cleavage furrow forms at the anterior end of the cell, between the new and old flagellum. The furrow then advances toward the cell posterior, dividing the cell into two daughter cells that are oriented with their flagella facing in opposite directions just before final cell separation occurs (Fig. 7) (Sherwin and Gull, 1989). It has previously been suggested that the flagellum and FAZ provide positional and directional cues for cleavage furrow formation (Robinson et al., 1995). RNAi knockdown of genes required for flagellum biogenesis, structure and movement have now provided direct evidence in support of this hypothesis. In a seminal study by Bastin and colleagues (Kohl et al., 2003), RNAi knockdown of IFT genes in procyclic cells not only showed that IFT is required for flagellum biogenesis in T. brucei, but also demonstrated that the flagellum is an essential organelle in these parasites. IFT knockdown halts new flagellum growth, resulting in cells with a new flagellum and FAZ that are shorter than normal. These cells initiate cytokinesis at a point that correlates with the position of the truncated FAZ filament, giving rise to progressively shorter cells and ultimately yielding aflagellate cells that are non-viable. Cells completely lacking flagella have disorganized organelles and abnormal cell shape. Therefore, in addition to influencing the position of intracellular organelles, the flagellum and FAZ define the position and direction of cleavage furrow ingression during cytokinesis (Fig. 8). Consistent with the importance of the FAZ in cytokinesis, RNAi knockdown of the FAZ proteins Fla-1 and FAZ1 lead to flagellum detachment and cytokinesis failure (LaCount et al., 2002; Vaughan et al., 2008).
Fig. 7.
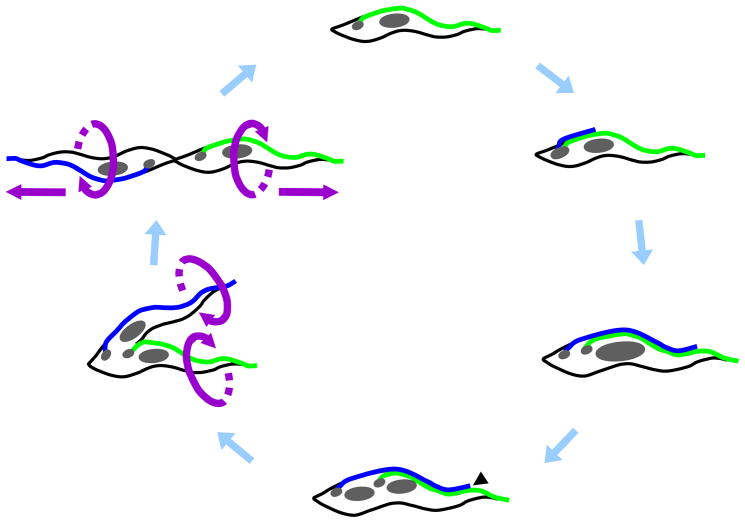
Cartoon depicting major stages of the Trypanosoma brucei cell division cycle in procyclic lifecycle stages. The cell cycle begins with replication of the basal body and initiation of new flagellum (blue) biogenesis. The new flagellum extends along a path defined by the old flagellum (green), while the basal bodies and associated kinetoplasts (small gray circles) migrate away from each other. In a closed mitotic cycle, the nucleus (large gray circle) is replicated and the new nucleus assumes a position between the new and old kinetoplast/basal body apparatus. Cytokinesis initiates at the tip of the new flagellum (black arrowhead) and cleavage furrow ingression proceeds to the cell posterior, separating the two daughter cells between the new and old flagellum. Ultimately, daughter cells are oriented in opposite directions, with their flagella exerting rotational and pulling forces that facilitate final cell separation.
Fig. 8.
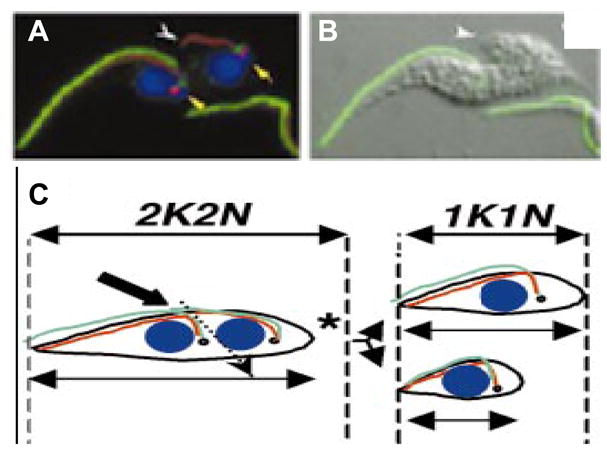
Flagellum attachment zone (FAZ) length defines cell cleavage site. (A, B) Immunofluorescence (A) and merged (B) image of a dividing procyclic cell following RNA interference knockdown of intraflagellar transport protein 88 (IFT88), showing the paraflagellar rod (green), FAZ (red) and nucleus (blue). (C) Cartoon depicting the result of improper cell cleavage in an IFT88 knockdown (left), resulting in one normal-sized daughter cell (right, top) and one short daughter cell (right, bottom). The site of cell cleavage corresponds to the end of the new FAZ filament (white arrowheads in A and B. Adapted from Kohl et al., 2003, with permission.
The potential to control cleavage furrow formation and cell size based on FAZ positioning has direct relevance to trypanosome development, since large variations in cell size occur as part of the natural life-cyle of T. brucei (Van Den Abbeele et al., 1999). For example, transformation from trypomastigotes to epimastigotes during development in the tsetse fly vector is associated with an assymetric cell division that yields one long and one short daughter cell (Van Den Abbeele et al., 1999). Recent studies have now shown that reduced cell size accompanying this differentiation is correlated with flagellum length and position in the newly forming daughter cell (Sharma et al., 2008). Since this asymmetric division occurs only after the parasite reaches specific host tissues, control of cell cleavage by the FAZ/flagellum is likely regulated in response to specific environmental cues.
3.6. Flagellar motility and cytokinesis
Perhaps even more surprising than the role for flagellum assembly and attachment in cleavage furrow formation was the finding that flagellar beating is required to complete cell division in procyclic cells (Fig. 9) (Ralston et al., 2005, 2006; Branche et al., 2006). RNAi knockdown of radial spoke and central pair proteins in the procyclic life-cycle stage leads to cells with full-length, attached flagella that are almost completely immotile (Ralston et al., 2005, 2006; Branche et al., 2006). These mutants fail in the final stage of cytokinesis, giving rise to daughter cells that remain connected at their extreme posterior ends (Ralston et al., 2005, 2006; Branche et al., 2006). Continued rounds of cytokinesis initiation in the absence of cell separation results in large, multicellular clusters. This cell division defect has now been observed in 33 independent motility mutants in the procyclic life-cycle stage (Ralston et al., 2005, 2006; Branche et al., 2006; Baron et al., 2007b) and the severity of the cell separation defect correlates with the severity of the motility defect (Baron et al., 2007b). Moreover, the block in cell division can be rescued by mechanical agitation (Ralston et al., 2005, 2006; Branche et al., 2006). The combined data therefore indicate that physical forces supplied by flagellar beating contribute to normal cytokinesis in procyclic cells. In wild-type T. brucei, newly replicated daughter cells are oriented with their flagella pointing in opposite directions and eventually pull apart (Fig. 7). Hence, the pulling and rotational forces provided by flagellar beating may provide a convenient way to drive the final steps of cell separation which, in trypanosomes, occurs in the absence of any detectable contractile ring. A similar phenomenon, termed rotokinesis, has been observed in Tetrahymena (Brown et al., 1999). Therefore, in addition to its role as a spatial and directional cue for directing cleavage furrow formation, the flagellum plays an active role in cell division in procyclic T. brucei cells.
Fig. 9.
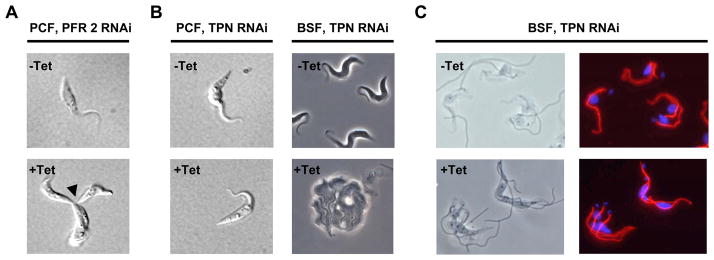
There is a dichotomy in the requirement of the flagellum in procyclic versus bloodstream form cells. (A) DIC images of uninduced (-Tet) and tetracycline-induced (+Tet) procyclic pfr2 knockdown mutants. Induced cells fail in the completion of cytokinesis, and accumulate as clusters of distinct cell bodies that remain physically attached at their posterior ends (arrowhead). (B) Phase-contrast images of uninduced and tetracycline-induced trypanin knockdown mutants in the procyclic (PCF) and bloodstream (BSF) lifecycle stages. Procyclic trypanin mutants are viable with little or no division defect, while bloodstream mutants fail in the initiation of cytokinesis and accumulate as amorphous masses with multiple flagella. (C) Immunofluorescence images of uninduced and tetracycline-induced bloodstream form trypanin mutants. Phase contrast images are shown in the left panels and staining for the FAZ (red) and DNA (blue) is shown in the right panels. Uninduced cells undergoing division have a maximum of two FAZ structures, which have a parallel configuration until cytokinesis is initiated. Induced cells have more than two parallel FAZ structures, which retain a parallel configuration, indicating that failure to initiate cytokinesis in these cells is not due to failed organelle replication or positioning. Adapted from Ralston et al., 2006 and Ralston and Hill, 2006, with permission.
3.7. Flagellar motility and cytokinesis in bloodstream-form cells
The vast majority of work on flagellum structure and function in T. brucei has been carried out in procyclic cells. Three important studies have now demonstrated that the requirement of the flagellar apparatus in cytokinesis extends to bloodstream-form cells (Fig. 9) (Branche et al., 2006; Broadhead et al., 2006; Ralston and Hill, 2006). Two studies used inducible RNAi to block expression of flagellar proteins in bloodstream-form cells. Ralston and Hill (2006) targeted the DRC subunit trypanin, while Broadhead and colleagues (Broadhead et al., 2006) targeted PFR2, MBO2, PACRG-A and two novel proteins within the TbEFP dataset. In a third study, Branche and colleagues targeted PFR2 for transient knockdown by transfection of double-stranded RNA (dsRNA) (Branche et al., 2006). In all cases these flagellar proteins were found to be essential. This was a surprising result, since none of these proteins are essential in the procyclic life cycle stage (Bastin et al., 1998; Hutchings et al., 2002; Broadhead et al., 2006). The terminal phenotype in all mutants was striking (Fig. 9b). Mutants failed to initiate cytokinesis but continued through multiple rounds of organelle replication, accumulating as large, amorphous masses with multiple kinetoplasts, flagella and nuclei. In at least one case, the kinetics of phenotype progression was shown to correlate with the extent of knockdown (Ralston and Hill, 2006). In procyclic T. brucei cytokinesis defects can arise from abnormal formation and/or positioning of the flagellum, FAZ or kinetoplast/basal body complex (see sections 3.4 and 3.5). However, in the work by Ralston, each of these events was examined and found to occur as expected (Fig. 9c) (Ralston and Hill, 2006). Importantly, Broadhead and colleagues showed that bloodstream-form flagellar mutants were endocytically active (Broadhead et al., 2006). Therefore, the cytokinesis defect and lethality in bloodstream-form flagellum mutants is not due to failed organelle replication or positioning or a block in endocytosis. Rather, the phenotype is a direct consequence of perturbing flagellum structure and/or function.
The above studies demonstrate that the requirement for flagellar apparatus in cytokinesis is a common feature of procyclic and bloodstream-form T. brucei. Nonetheless, there are important differences between procyclic and bloodstream-form flagellum mutants that must be considered. Firstly, bloodstream-form cells are more sensitive to flagellum defects, since none of the proteins examined in bloodstream-form cells are essential in procyclic cells (Fig. 9b). Moreover, PACRG-A knockdown produces no discernable phenotype in procyclic cells (Dawe et al., 2005), but is lethal in the bloodstream-form (Broadhead et al., 2006). In the case of trypanin, procyclic mutants are viable with little or no effect on cell doubling even though trypanin is undetectable (Hutchings et al., 2002), while bloodstream-form cells die when protein levels are reduced two to four-fold. Second, mechanical agitation rescues the cytokinesis defect of procyclic mutants (Ralston et al., 2005, 2006; Branche et al., 2006) but not of bloodstream-form mutants (Griffiths et al., 2007; K.L. Hill, unpublished observation). Third, the terminal phenotype of flagellar mutants is different between life cycle stages (Fig. 9a and 9b). Procyclic mutants fail during the progression and/or completion of cell cleavage. Bloodstream-form mutants ultimately fail to initiate cytokinesis. These results suggest life-cycle stage-specific regulation of flagellum function and emphasize the emerging concept (Hammarton et al., 2003; Tu and Wang, 2004) that there is a dichotomy with respect to factors that control cell division in bloodstream-form and insect-form African trypanosomes.
The combined data indicate that the flagellum and perhaps flagellar motility itself is essential in bloodstream-form T. brucei. Note however, that while these studies show that flagellar proteins are essential, they do not demonstrate that motility itself is essential. For example, it is possible that lethality in bloodstream flagellar mutants results from a structural defect, rather than a defect in motility, per se. This is more than a remote possibility for several reasons. Firstly, procyclic trypanin mutants have an actively beating flagellum (Hutchings et al., 2002) and PACRG-A procyclic mutants were reported to show no discernable motility defect (Dawe et al., 2005). Yet, both of these mutants were inviable in the bloodstream stage. Therefore, lethality in bloodstream-form mutants does not strictly correlate with motility defects in the corresponding procyclic mutants. Second, most of the mutants examined are known to produce structural changes in flagellum sub-structures, as directly shown in T. brucei, or in the analogous C. reinhardtii mutants. For example, PFR2 mutants disrupt the PFR, trypanin mutants disrupt theDRC, and MBO2 mutants loose the beak structure within the lumen outer doublet microtubules. Two other proteins targeted in bloodstream-form cells, LC1 and Tax1, are dynein subunits (Yamamoto et al., 2006; Baron et al., 2007b). Work in T. brucei (Branche et al., 2006; Baron et al., 2007a) and C. reinhardtii (Sakato and King, 2004) demonstrate that loss of dynein subunits causes disruption of the entire axonemal dynein arm. Since detailed ultrastructural analysis has not been completed on any of the bloodstream-form mutants, the structural consequences of knockdown in this life cycle stage is unknown. Finally, since there is an emerging link between flagellum assembly/disassembly and cell cycle control in other organisms, it is possible that perturbing fidelity of the flagellum, rather than motility itself, triggers a cytokinesis checkpoint. Therefore, it remains to be determined whether or not flagellar motility is essential in bloodstream-form trypanosomes. Clearly, many more exciting discoveries can be expected from continued investigation of the connection between the flagellar apparatus and cell division in these deadly pathogens.
Regardless of the precise reason for lethality in bloodstream-form flagellum mutants, the above studies raise the very exciting possibility that the flagellum might be exploited as a novel target for therapeutic intervention in African sleeping sickness. The flagellum is comprised of an estimated 500 –600 proteins (Pazour et al., 2005; Merchant et al., 2007), including many with enzymatic activity and/or small ligand binding sites. Such small molecule-binding proteins make up a large fraction of proteins considered to be good drug targets (Hopkins and Groom, 2002). Therefore, an essential role for flagellar proteins in bloodstream-form T. brucei greatly enhances the potential of this organelle as a novel drug target. In order to exploit this potential, it will be critical to determine the precise structural and enzymatic features of flagellar proteins and flagellar sub-complexes in these parasites.
4. Summary and future considerations
The flagellum of African trypanosomes is now established as an essential and multifunctional organelle with critical roles in host cell attachment, cell motility cell morphogenesis, cell division and immune evasion. Moreover, all flagellum structural proteins so far examined have been found to be essential in the bloodstream life-cycle stage, indicating that the flagellum may represent a rich source of novel targets for therapeutic intervention in African sleeping sickness. Much work is still needed to capitalize on this possibility and to determine the exact nature of the requirement for the flagellum and flagellar proteins in trypanosome cell division and cell polarity determination. Further surprises almost certainly await continued investigation, particularly in the area of flagellar membrane and matrix composition, IFT and flagellar biogenesis. It is therefore likely that this old dog may still have a few new tricks to teach us.
Acknowledgments
We are grateful to Dr. Z. Pius Kabututu for the illustration in Fig. 1c and we thank all members of our laboratory, as well as two anonymous reviewers for thoughtful comments on the manuscript. Our apologies to those whose work was not covered in this review owing to space limitations. K. Ralston is the recipient of a USPHS National Research Service Award (GM07104) and a Dissertation Year Fellowship from the UCLA graduate division. Work in the authors’ laboratory is supported by grants from the National Institutes of Health (R01AI52348), the Arnold and Mabel Beckman Foundation and the Ellison Medical Foundation (ID-NS-0148–03).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Absalon S, Blisnick T, Kohl L, Toutirais G, Dore G, Julkowska D, Tavenet A, Bastin P. Intraflagellar Transport and Functional Analysis of Genes Required for Flagellum Formation in Trypanosomes. Mol Biol Cell. 2007a;19:929–944. doi: 10.1091/mbc.E07-08-0749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Absalon S, Kohl L, Branche C, Blisnick T, Toutirais G, Rusconi F, Cosson J, Bonhivers M, Robinson D, Bastin P. Basal body positioning is controlled by flagellum formation in Trypanosoma brucei. PLoS ONE. 2007b;2:e437. doi: 10.1371/journal.pone.0000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhiambo C, Forney JD, Asai DJ, LeBowitz JH. The two cytoplasmic dynein-2 isoforms in Leishmania mexicana perform separate functions. Mol Biochem Parasitol. 2005;143:216–225. doi: 10.1016/j.molbiopara.2005.04.017. [DOI] [PubMed] [Google Scholar]
- Badano JL, Mitsuma N, Beales PL, Katsanis N. The Ciliopathies: An Emerging Class of Human Genetic Disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- Balber AE. The pellicle and the membrane of the flagellum, flagellar adhesion zone, and flagellar pocket: functionally discrete surface domains of the bloodstream form of African trypanosomes. Crit Rev Immunol. 1990;10:177–201. [PubMed] [Google Scholar]
- Baron DM, Kabututu ZP, Hill KL. Stuck in reverse: loss of LC1 in Trypanosoma brucei disrupts outer dynein arms and leads to reverse flagellar beat and backward movement. J Cell Sci. 2007a;120:1513–1520. doi: 10.1242/jcs.004846. [DOI] [PubMed] [Google Scholar]
- Baron DM, Ralston KS, Kabututu ZP, Hill KL. Functional genomics in Trypanosoma brucei identifies evolutionarily conserved components of motile flagella. J Cell Sci. 2007b;120:478–491. doi: 10.1242/jcs.03352. [DOI] [PubMed] [Google Scholar]
- Bastin P, Sherwin T, Gull K. Paraflagellar rod is vital for trypanosome motility. Nature. 1998;391:548. doi: 10.1038/35300. [DOI] [PubMed] [Google Scholar]
- Bastin P, Pullen TJ, Sherwin T, Gull K. Protein transport and flagellum assembly dynamics revealed by analysis of the paralysed trypanosome mutant snl-1. J Cell Sci. 1999;112:3769–3777. doi: 10.1242/jcs.112.21.3769. [DOI] [PubMed] [Google Scholar]
- Branche C, Kohl L, Toutirais G, Buisson J, Cosson J, Bastin P. Conserved and specific functions of axoneme components in trypanosome motility. J Cell Sci. 2006;119:3443–3455. doi: 10.1242/jcs.03078. [DOI] [PubMed] [Google Scholar]
- Briggs LJ, McKean PG, Baines A, Moreira-Leite F, Davidge J, Vaughan S, Gull K. The flagella connector of Trypanosoma brucei: an unusual mobile transmembrane junction. J Cell Sci. 2004;117:1641–1651. doi: 10.1242/jcs.00995. [DOI] [PubMed] [Google Scholar]
- Broadhead R, Dawe HR, Farr H, Griffiths S, Hart SR, Portman N, Shaw MK, Ginger ML, Gaskell SJ, McKean PG, Gull K. Flagellar motility is required for the viability of the bloodstream trypanosome. Nature. 2006;440:224–227. doi: 10.1038/nature04541. [DOI] [PubMed] [Google Scholar]
- Brown JM, Hardin C, Gaertig J. Rotokinesis, a novel phenomenon of cell locomotion-assisted cytokinesis in the ciliate Tetrahymena thermophila. Cell Biol Int. 1999;23:841–848. doi: 10.1006/cbir.1999.0480. [DOI] [PubMed] [Google Scholar]
- Buchanan KT, Ames JB, Asfaw SH, Wingard JN, Olson CL, Campana PT, Araujo AP, Engman DM. A flagellum-specific calcium sensor. J Biol Chem. 2005;280:40104–40111. doi: 10.1074/jbc.M505777200. [DOI] [PubMed] [Google Scholar]
- Cibert C. Elastic extension and jump of the flagellar nexin links: a theoretical mechanical cycle. Cell Motil Cytoskeleton. 2001;49:161–175. doi: 10.1002/cm.1030. [DOI] [PubMed] [Google Scholar]
- Davidge JA, Chambers E, Dickinson HA, Towers K, Ginger ML, McKean PG, Gull K. Trypanosome IFT mutants provide insight into the motor location for mobility of the flagella connector and flagellar membrane formation. J Cell Sci. 2006;119:3935–3943. doi: 10.1242/jcs.03203. [DOI] [PubMed] [Google Scholar]
- Dawe HR, Farr H, Portman N, Shaw MK, Gull K. The Parkin co-regulated gene product, PACRG, is an evolutionarily conserved axonemal protein that functions in outer-doublet microtubule morphogenesis. J Cell Sci. 2005;118:5421–5430. doi: 10.1242/jcs.02659. [DOI] [PubMed] [Google Scholar]
- Dawe HR, Shaw MK, Farr H, Gull K. The hydrocephalus inducing gene product, Hydin, positions axonemal central pair microtubules. BMC Biol. 2007;5:33. doi: 10.1186/1741-7007-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deflorin J, Rudolf M, Seebeck T. The major components of the paraflagellar rod of Trypanosoma brucei are two similar, but distinct proteins which are encoded by two different gene loci. J Biol Chem. 1994;269:28745–28751. [PubMed] [Google Scholar]
- Dobell C. Antony van Leeuwenhoek and His Little Animals. Rusell and Russell; New York: 1958. [Google Scholar]
- Durand-Dubief M, Kohl L, Bastin P. Efficiency and specificity of RNA interference generated by intra- and intermolecular double stranded RNA in Trypanosoma brucei. Mol Biochem Parasitol. 2003;129:11–21. doi: 10.1016/s0166-6851(03)00071-9. [DOI] [PubMed] [Google Scholar]
- el-Sayed NM, Alarcon CM, Beck JC, Sheffield VC, Donelson JE. cDNA expressed sequence tags of Trypanosoma brucei rhodesiense provide new insights into the biology of the parasite. Mol Biochem Parasitol. 1995;73:75–90. doi: 10.1016/0166-6851(95)00098-l. [DOI] [PubMed] [Google Scholar]
- Engman DM, Krause KH, Blumin JH, Kim KS, Kirchhoff LV, Donelson JE. A novel flagellar Ca2+-binding protein in trypanosomes. J Biol Chem. 1989;264:18627–18631. [PubMed] [Google Scholar]
- Engstler M, Pfohl T, Herminghaus S, Boshart M, Wiegertjes G, Heddergott N, Overath P. Hydrodynamic Flow-Mediated Protein Sorting on the Cell Surface of Trypanosomes. Cell. 2007;131:505–515. doi: 10.1016/j.cell.2007.08.046. [DOI] [PubMed] [Google Scholar]
- Ferguson DJ. Toxoplasma gondii and sex: essential or optional extra? Trends Parasitol. 2002;18:355–359. [PubMed] [Google Scholar]
- Fridberg A, Buchanan KT, Engman DM. Flagellar membrane trafficking in kinetoplastids. Parasitol Res. 2007;100:205–212. doi: 10.1007/s00436-006-0329-2. [DOI] [PubMed] [Google Scholar]
- Gadelha C, Wickstead B, McKean PG, Gull K. Basal body and flagellum mutants reveal a rotational constraint of the central pair microtubules in the axonemes of trypanosomes. J Cell Sci. 2006;119:2405–2413. doi: 10.1242/jcs.02969. [DOI] [PubMed] [Google Scholar]
- Gardner LC, O'Toole E, Perrone CA, Giddings T, Porter ME. Components of a "dynein regulatory complex" are located at the junction between the radial spokes and the dynein arms in Chlamydomonas flagella. J Cell Biol. 1994;127:1311–1325. doi: 10.1083/jcb.127.5.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginger ML, Ngazoa ES, Pereira CA, Pullen TJ, Kabiri M, Becker K, Gull K, Steverding D. Intracellular positioning of isoforms explains an unusually large adenylate kinase gene family in the parasite Trypanosoma brucei. J Biol Chem. 2005;280:11781–11789. doi: 10.1074/jbc.M413821200. [DOI] [PubMed] [Google Scholar]
- Griffiths S, Portman N, Taylor PR, Gordon S, Ginger ML, Gull K. RNA interference mutant induction in vivo demonstrates the essential nature of trypanosome flagellar function during mammalian infection. Euk Cell. 2007;6:1248–1250. doi: 10.1128/EC.00110-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gull K. The cytoskeleton of trypanosomatid parasites. Annu Rev Microbiol. 1999;53:629–655. doi: 10.1146/annurev.micro.53.1.629. [DOI] [PubMed] [Google Scholar]
- Hammarton TC, Clark J, Douglas F, Boshart M, Mottram JC. Stage-specific differences in cell cycle control in Trypanosoma brucei revealed by RNA interference of a mitotic cyclin. J Biol Chem. 2003;278:22877–22886. doi: 10.1074/jbc.M300813200. [DOI] [PubMed] [Google Scholar]
- Hammarton TC, Kramer S, Tetley L, Boshart M, Mottram JC. Trypanosoma brucei Polo-like kinase is essential for basal body duplication, kDNA segregation and cytokinesis. Mol Microbiol. 2007;65:1229–1248. doi: 10.1111/j.1365-2958.2007.05866.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KL, Hutchings NR, Grandgenett PM, Donelson JE. T Lymphocyte triggering factor of African trypanosomes is associated with the flagellar fraction of the cytoskeleton and represents a new family of proteins that are present in several divergent eukaryotes. J Biol Chem. 2000;275:39369–39378. doi: 10.1074/jbc.M006907200. [DOI] [PubMed] [Google Scholar]
- Hill KL. Mechanism and biology of trypanosome cell motility. Euk Cell. 2003;2:200–208. doi: 10.1128/EC.2.2.200-208.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins AL, Groom CR. The druggable genome. Nat Rev Drug Discov. 2002;1:727–730. doi: 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- Huang B, Ramanis Z, Luck DJ. Suppressor mutations in Chlamydomonas reveal a regulatory mechanism for Flagellar function. Cell. 1982;28:115–124. doi: 10.1016/0092-8674(82)90381-6. [DOI] [PubMed] [Google Scholar]
- Hutchings NR, Donelson JE, Hill KL. Trypanin is a cytoskeletal linker protein and is required for cell motility in African trypanosomes. J Cell Biol. 2002;156:867–877. doi: 10.1083/jcb.200201036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez-Tallon I, Heintz N, Omran H. To beat or not to beat: roles of cilia in development and disease. Hum Mol Genet. 2003;12(Spec No 1):R27–35. doi: 10.1093/hmg/ddg061. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Brown JA, Yagi T, Norrander JM, Hirono M, Eccleston E, Kamiya RaL, RW Rib72, a conserved protein associated with the ribbon compartment of flagellar A-microtubules and potentially involved in the linkage between outer doublet microtubules. J Biol Chem. 2003;278:7725–7734. doi: 10.1074/jbc.M210751200. [DOI] [PubMed] [Google Scholar]
- Inglis PN, Boroevich KA, Leroux MR. Piecing together a ciliome. Trends Genet. 2006;22:491–500. doi: 10.1016/j.tig.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Kamiya R, Okamoto M. A mutant of Chlamydomonas reinhardtii that lacks the flagellar outer dynein arm but can swim. J Cell Sci. 1985;74:181–191. doi: 10.1242/jcs.74.1.181. [DOI] [PubMed] [Google Scholar]
- King SM. Axonemal protofilament ribbons, DM10 domains, and the link to juvenile myoclonic epilepsy. Cell Motil Cytoskeleton. 2006;63:245–253. doi: 10.1002/cm.20129. [DOI] [PubMed] [Google Scholar]
- Kohl L, Gull K. Molecular architecture of the trypanosome cytoskeleton. Mol Biochem Parasitol. 1998;93:1–9. doi: 10.1016/s0166-6851(98)00014-0. [DOI] [PubMed] [Google Scholar]
- Kohl L, Sherwin T, Gull K. Assembly of the paraflagellar rod and the flagellum attachment zone complex during the Trypanosoma brucei cell cycle. J Eukaryot Microbiol. 1999;46:105–109. doi: 10.1111/j.1550-7408.1999.tb04592.x. [DOI] [PubMed] [Google Scholar]
- Kohl L, Robinson D, Bastin P. Novel roles for the flagellum in cell morphogenesis and cytokinesis of trypanosomes. EMBO J. 2003;22:5336–5346. doi: 10.1093/emboj/cdg518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Beech PL, Rosenbaum JL. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J Cell Biol. 1995;131:1517–1527. doi: 10.1083/jcb.131.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P, Wang CC. Dissociation of cytokinesis initiation from mitotic control in a eukaryote. Euk Cell. 2006;5:92–102. doi: 10.1128/EC.5.1.92-102.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaCount DJ, Bruse S, Hill KL, Donelson JE. Double-stranded RNA interference in Trypanosoma brucei using head-to-head promoters. Mol Biochem Parasitol. 2000;111:67–76. doi: 10.1016/s0166-6851(00)00300-5. [DOI] [PubMed] [Google Scholar]
- LaCount DJ, Barrett B, Donelson JE. Trypanosoma brucei FLA1 is required for flagellum attachment and cytokinesis. J Biol Chem. 2002;277:17580–17588. doi: 10.1074/jbc.M200873200. [DOI] [PubMed] [Google Scholar]
- Lechtreck KF, Witman GB. Chlamydomonas reinhardtii hydin is a central pair protein required for flagellar motility. J Cell Biol. 2007;176:473–482. doi: 10.1083/jcb.200611115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legros D, Ollivier G, Gastellu-Etchegorry M, Paquet C, Burri C, Jannin J, Buscher P. Treatment of human African trypanosomiasis--present situation and needs for research and development. Lancet Infect Dis. 2002;2:437–440. doi: 10.1016/s1473-3099(02)00321-3. [DOI] [PubMed] [Google Scholar]
- Lindemann CB, Kanous KS. A model for flagellar motility. Int Rev Cytol. 1997;173:1–72. doi: 10.1016/s0074-7696(08)62475-4. [DOI] [PubMed] [Google Scholar]
- Lindemann CB. Testing the geometric clutch hypothesis. Biol Cell. 2004;96:681–690. doi: 10.1016/j.biolcel.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Lorenzetti D, Bishop CE, Justice MJ. Deletion of the Parkin coregulated gene causes male sterility in the quaking(viable) mouse mutant. Proc Natl Acad Sci USA. 2004;101:8402–8407. doi: 10.1073/pnas.0401832101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKean PG, Baines A, Vaughan S, Gull K. Gamma-tubulin functions in the nucleation of a discrete subset of microtubules in the eukaryotic flagellum. Curr Biol. 2003;13:598–602. doi: 10.1016/s0960-9822(03)00174-x. [DOI] [PubMed] [Google Scholar]
- Merchant SS, Prochnik SE, Vallon O, Harris EH, Karpowicz SJ, Witman GB, Terry A, Salamov A, Fritz-Laylin LK, Marechal-Drouard L, Marshall WF, Qu LH, Nelson DR, Sanderfoot AA, Spalding MH, Kapitonov VV, Ren Q, Ferris P, Lindquist E, Shapiro H, Lucas SM, Grimwood J, Schmutz J, Cardol P, Cerutti H, Chanfreau G, Chen CL, Cognat V, Croft MT, Dent R, Dutcher S, Fernandez E, Fukuzawa H, Gonzalez-Ballester D, Gonzalez-Halphen D, Hallmann A, Hanikenne M, Hippler M, Inwood W, Jabbari K, Kalanon M, Kuras R, Lefebvre PA, Lemaire SD, Lobanov AV, Lohr M, Manuell A, Meier I, Mets L, Mittag M, Mittelmeier T, Moroney JV, Moseley J, Napoli C, Nedelcu AM, Niyogi K, Novoselov SV, Paulsen IT, Pazour G, Purton S, Ral JP, Riano-Pachon DM, Riekhof W, Rymarquis L, Schroda M, Stern D, Umen J, Willows R, Wilson N, Zimmer SL, Allmer J, Balk J, Bisova K, Chen CJ, Elias M, Gendler K, Hauser C, Lamb MR, Ledford H, Long JC, Minagawa J, Page MD, Pan J, Pootakham W, Roje S, Rose A, Stahlberg E, Terauchi AM, Yang P, Ball S, Bowler C, Dieckmann CL, Gladyshev VN, Green P, Jorgensen R, Mayfield S, Mueller-Roeber B, Rajamani S, Sayre RT, Brokstein P, Dubchak I, Goodstein D, Hornick L, Huang YW, Jhaveri J, Luo Y, Martinez D, Ngau WC, Otillar B, Poliakov A, Porter A, Szajkowski L, Werner G, Zhou K, Grigoriev IV, Rokhsar DS, Grossman AR. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science. 2007;318:245–250. doi: 10.1126/science.1143609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DR. Orientation of the central pair complex during flagellar bend formation in Chlamydomonas. Cell Motil Cytoskeleton. 2003;56:120–129. doi: 10.1002/cm.10142. [DOI] [PubMed] [Google Scholar]
- Moreira-Leite FF, Sherwin T, Kohl L, Gull K. A trypanosome structure involved in transmitting cytoplasmic information during cell division. Science. 2001;294:610–612. doi: 10.1126/science.1063775. [DOI] [PubMed] [Google Scholar]
- Nozaki T, Haynes PA, Cross GA. Characterization of the Trypanosoma brucei homologue of a Trypanosoma cruzi flagellum-adhesion glycoprotein. Mol Biochem Parasitol. 1996;82:245–255. doi: 10.1016/0166-6851(96)02741-7. [DOI] [PubMed] [Google Scholar]
- Oberholzer M, Marti G, Baresic M, Kunz S, Hemphill A, Seebeck T. The Trypanosoma brucei cAMP phosphodiesterases TbrPDEB1 and TbrPDEB2: flagellar enzymes that are essential for parasite virulence. FASEB J. 2007;21:720–731. doi: 10.1096/fj.06-6818com. [DOI] [PubMed] [Google Scholar]
- Omoto CK, Gibbons IR, Kamiya R, Shingyoji C, Takahashi K, Witman GB. Rotation of the central pair microtubules in eukaryotic flagella. Mol Biol Cell. 1999;10:1–4. doi: 10.1091/mbc.10.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paindavoine P, Rolin S, Van Assel S, Geuskens M, Jauniaux JC, Dinsart C, Huet G, Pays E. A gene from the variant surface glycoprotein expression site encodes one of several transmembrane adenylate cyclases located on the flagellum of Trypanosoma brucei. Mol Cell Biol. 1992;12:1218–1225. doi: 10.1128/mcb.12.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel-King RS, Benashski SE, King SM. A bipartite Ca2+-regulated nucleoside-diphosphate kinase system within the Chlamydomonas flagellum. The regulatory subunit p72. J Biol Chem. 2002;277:34271–34279. doi: 10.1074/jbc.M204137200. [DOI] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Witman GB. The DHC1b (DHC2) Isoform of Cytoplasmic Dynein Is Required for Flagellar Assembly. J Cell Biol. 1999;144:473–481. doi: 10.1083/jcb.144.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Agrin N, Leszyk J, Witman GB. Proteomic analysis of a eukaryotic cilium. J Cell Biol. 2005;170:103–113. doi: 10.1083/jcb.200504008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, Mead K, Shestak W. The inner dynein arms I2 interact with a "dynein regulatory complex" in Chlamydomonas flagella. J Cell Biol. 1992;118:1455–1463. doi: 10.1083/jcb.118.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ME, Bower R, Knott JA, Byrd P, Dentler W. Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in Chlamydomonas. Mol Biol Cell. 1999;10:693–712. doi: 10.1091/mbc.10.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ME, Sale WS. The 9 + 2 axoneme anchors multiple inner arm dyneins and a network of kinases and phosphatases that control motility. J Cell Biol. 2000;151:F37–42. doi: 10.1083/jcb.151.5.f37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullen TJ, Ginger ML, Gaskell SJ, Gull K. Protein targeting of an unusual, evolutionarily conserved adenylate kinase to a eukaryotic flagellum. Mol Biol Cell. 2004;15:3257–3265. doi: 10.1091/mbc.E04-03-0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston KS, Lerner AG, Hill KL. Flagellar motility is essential for cytokinesis in Trypanosoma brucei and is modulated by an evolutionarily conserved dynein regulatory system (Meeting Abstract) Am J Trop Med Hyg Suppl. 2005;73:365. doi: 10.1128/EC.5.4.696-711.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston KS, Hill KL. Trypanin, a component of the flagellar Dynein regulatory complex, is essential in bloodstream form African trypanosomes. PLoS Pathog. 2006;2:e101. doi: 10.1371/journal.ppat.0020101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston KS, Lerner AG, Diener DR, Hill KL. Flagellar motility contributes to cytokinesis in Trypanosoma brucei and is modulated by an evolutionarily conserved dynein regulatory system. Eukaryot Cell. 2006;5:696–711. doi: 10.1128/EC.5.4.696-711.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridgley E, Webster P, Patton C, Ruben L. Calmodulin-binding properties of the paraflagellar rod complex from Trypanosoma brucei. Mol Biochem Parasitol. 2000;109:195–201. doi: 10.1016/s0166-6851(00)00246-2. [DOI] [PubMed] [Google Scholar]
- Robertson M. Notes on the life-history of Trypanosoma gambiense, with a brief reference to the cycles of Trypanosoma nanum and Trypanosoma pecorum in Glossina palpalis Philosophical Transactions of the Royal Society, Ser. B. 1913;B203:161–184. [Google Scholar]
- Robinson DR, Gull K. Basal body movements as a mechanism for mitochondrial genome segregation in the trypanosome cell cycle. Nature. 1991;352:731–733. doi: 10.1038/352731a0. [DOI] [PubMed] [Google Scholar]
- Robinson DR, Sherwin T, Ploubidou A, Byard EH, Gull K. Microtubule polarity and dynamics in the control of organelle positioning, segregation, and cytokinesis in the trypanosome cell cycle. J Cell Biol. 1995;128:1163–1172. doi: 10.1083/jcb.128.6.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum JL, Witman GB. Intraflagellar Transport. Nat Rev Mol Cell Biol. 2002;3:813–825. doi: 10.1038/nrm952. [DOI] [PubMed] [Google Scholar]
- Rupp G, Porter ME. A subunit of the dynein regulatory complex in Chlamydomonas is a homologue of a growth arrest-specific gene product. J Cell Biol. 2003;162:47–57. doi: 10.1083/jcb.200303019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakato M, King SM. Design and regulation of the AAA+ microtubule motor dynein. J Struct Biol. 2004;146:58–71. doi: 10.1016/j.jsb.2003.09.026. [DOI] [PubMed] [Google Scholar]
- Satir P. Landmarks in cilia research from Leeuwenhoek to us. Cell Motil Cytoskel. 1995;32:90–94. doi: 10.1002/cm.970320203. [DOI] [PubMed] [Google Scholar]
- Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- Schlaeppi K, Deflorin J, Seebeck T. The major component of the paraflagellar rod of Trypanosoma brucei is a helical protein that is encoded by two identical, tandemly linked genes. J Cell Biol. 1989;109:1695–1709. doi: 10.1083/jcb.109.4.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma R, Peacock L, Gluenz E, Gull K, Gibson W, Carrington M. Asymmetric cell division as a route to reduction in cell length and change in cell morphology in trypanosomes. Protist. 2008;159:137–151. doi: 10.1016/j.protis.2007.07.004. [DOI] [PubMed] [Google Scholar]
- Sherwin T, Gull K. The cell division cycle of Trypanosoma brucei brucei: timing of event markers and cytoskeletal modulations. Philos Trans R Soc Lond Ser B, Biol Sci. 1989;323:573–588. doi: 10.1098/rstb.1989.0037. [DOI] [PubMed] [Google Scholar]
- Simpson L. Behavior of the kinetoplast of Leishmania tarentolae upon cell rupture. J Protozool. 1968;15:132–136. doi: 10.1111/j.1550-7408.1968.tb02097.x. [DOI] [PubMed] [Google Scholar]
- Smith EF, Yang P. The radial spokes and central apparatus: mechano-chemical transducers that regulate flagellar motility. Cell Motil Cytoskeleton. 2004;57:8–17. doi: 10.1002/cm.10155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan A, Vaughan S, Shaw MK, Gull K, McKean PG. An essential quality control mechanism at the eukaryotic basal body prior to intraflagellar transport. Traffic. 2007;8:1323–1330. doi: 10.1111/j.1600-0854.2007.00611.x. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Delgado-Escueta AV, Aguan K, Alonso ME, Shi J, Hara Y, Nishida M, Numata T, Medina MT, Takeuchi T, Morita R, Bai D, Ganesh S, Sugimoto Y, Inazawa J, Bailey JN, Ochoa A, Jara-Prado A, Rasmussen A, Ramos-Peek J, Cordova S, Rubio-Donnadieu F, Inoue Y, Osawa M, Kaneko S, Oguni H, Mori Y, Yamakawa K. Mutations in EFHC1 cause juvenile myoclonic epilepsy. Nat Genet. 2004;36:842–849. doi: 10.1038/ng1393. [DOI] [PubMed] [Google Scholar]
- Tetley L, Vickerman K. Differentiation in Trypanosoma brucei: host-parasite cell junctions and their persistence during acquisition of the variable antigen coat. J Cell Sci. 1985;74:1–19. doi: 10.1242/jcs.74.1.1. [DOI] [PubMed] [Google Scholar]
- Tetley L, Turner CM, Barry JD, Crowe JS, Vickerman K. Onset of expression of the variant surface glycoproteins of Trypanosoma brucei in the tsetse fly studied using immunoelectron microscopy. J Cell Sci. 1987;87 ( Pt 2):363–372. doi: 10.1242/jcs.87.2.363. [DOI] [PubMed] [Google Scholar]
- Tu X, Wang CC. The involvement of two cdc2-related kinases (CRKs) in Trypanosoma brucei cell cycle regulation and the distinctive stage-specific phenotypes caused by CRK3 depletion. J Biol Chem. 2004;279:20519–20528. doi: 10.1074/jbc.M312862200. [DOI] [PubMed] [Google Scholar]
- Tyler KM, Matthews KR, Gull K. Anisomorphic cell division by African trypanosomes. Protist. 2001;152:367–378. doi: 10.1078/1434-4610-00074. [DOI] [PubMed] [Google Scholar]
- Van Den Abbeele J, Claes Y, van Bockstaele D, Le Ray D, Coosemans M. Trypanosoma brucei spp. development in the tsetse fly: characterization of the post-mesocyclic stages in the foregut and proboscis. Parasitology. 1999;118:469–478. doi: 10.1017/s0031182099004217. [DOI] [PubMed] [Google Scholar]
- Vaughan S, Kohl L, Ngai I, Wheeler RJ, Gull K. A Repetitive Protein Essential for the Flagellum Attachment Zone Filament Structure and Function in Trypanosoma brucei. Protist. 2008;159:127–136. doi: 10.1016/j.protis.2007.08.005. [DOI] [PubMed] [Google Scholar]
- Vickerman K. Patterns of cellular organisation in Limax amoebae. An electron microscope study. Exp Cell Res. 1962a;26:497–519. doi: 10.1016/0014-4827(62)90155-6. [DOI] [PubMed] [Google Scholar]
- Vickerman K. The mechanism of cyclical development in trypanosomes of the Trypanosoma brucei sub-group: an hypothesis based on ultrastructural observations. Trans R Soc Trop Med Hyg. 1962b;56:487–495. doi: 10.1016/0035-9203(62)90072-x. [DOI] [PubMed] [Google Scholar]
- Vickerman K. On the surface coat and flagellar adhesion in trypanosomes. J Cell Sci. 1969;5:163–193. doi: 10.1242/jcs.5.1.163. [DOI] [PubMed] [Google Scholar]
- Vickerman K, Preston TM. Comparative cell biology of the Kinetoplastid flagellates. In: Lumsden WHR, Evans DA, editors. Biology of the Kinetoplastida. Vol. 1. Academic Press; London, England: 1976. pp. 35–130. [Google Scholar]
- Vickerman K. Developmental cycles and biology of pathogenic trypanosomes. Br Med Bull. 1985;41:105–114. doi: 10.1093/oxfordjournals.bmb.a072036. [DOI] [PubMed] [Google Scholar]
- Vickerman K, Tetley L, Hendry KA, Turner CM. Biology of African trypanosomes in the tsetse fly. Biol Cell. 1988;64:109–119. doi: 10.1016/0248-4900(88)90070-6. [DOI] [PubMed] [Google Scholar]
- Vlachou D, Schlegelmilch T, Runn E, Mendes A, Kafatos FC. The developmental migration of Plasmodium in mosquitoes. Curr Opin Genet Dev. 2006;16:384–391. doi: 10.1016/j.gde.2006.06.012. [DOI] [PubMed] [Google Scholar]
- Walker PJ. Organization of function in trypanosome flagella. Nature. 1961;189:1017–1018. doi: 10.1038/1891017a0. [DOI] [PubMed] [Google Scholar]
- Walker PJ, Walker JC. Movement of trypanosome flagella. J Protozool. 1963;10(suppl, 32) [Google Scholar]
- Wargo MJ, Smith EF. Asymmetry of the central apparatus defines the location of active microtubule sliding in Chlamydomonas flagella. Proc Natl Acad Sci USA. 2003;100:137–142. doi: 10.1073/pnas.0135800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner FD. Ciliary inter-microtubule bridges. J Cell Sci. 1976;20:101–114. doi: 10.1242/jcs.20.1.101. [DOI] [PubMed] [Google Scholar]
- Webster P, Russell DG. The flagellar pocket of trypanosomatids. Parasitol Today. 1993;9:201–206. doi: 10.1016/0169-4758(93)90008-4. [DOI] [PubMed] [Google Scholar]
- Welburn SC, Odiit M. Recent developments in human African trypanosomiasis. Curr Opin Infect Dis. 2002;15:477–484. doi: 10.1097/00001432-200210000-00004. [DOI] [PubMed] [Google Scholar]
- Woodward R, Carden MJ, Gull K. Molecular characterisation of a novel, repetitive protein of the paraflagellar rod in Trypanosoma brucei. Mol Biochem Parasitol. 1994;67:31–39. doi: 10.1016/0166-6851(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Yamamoto R, Yanagisawa HA, Yagi T, Kamiya R. A novel subunit of axonemal dynein conserved among lower and higher eukaryotes. FEBS Lett. 2006;580:6357–6360. doi: 10.1016/j.febslet.2006.10.047. [DOI] [PubMed] [Google Scholar]
- Zhang H, Mitchell DR. Cpc1, a Chlamydomonas central pair protein with an adenylate kinase domain. J Cell Sci. 2004;117:4179–4188. doi: 10.1242/jcs.01297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoraghi R, Seebeck T. The cAMP-specific phosphodiesterase TbPDE2C is an essential enzyme in bloodstream form Trypanosoma brucei. Proc Natl Acad Sci U S A. 2002;99:4343–4348. doi: 10.1073/pnas.062716599. [DOI] [PMC free article] [PubMed] [Google Scholar]