Abstract
Metastasis involves tumor cells moving through tissues and crossing tissue boundaries, which requires cell migration, remodeling of cell-to-cell contacts and interactions with the extracellular matrix. Individual tumor cells move in three-dimensional environments with either a rounded “ameboid” or an elongated “mesenchymal” morphology. These two modes of movement are tightly regulated by Rho family GTPases: elongated movement requires activation of Rac1, whereas rounded movement engages specific Cdc42 and Rho signaling pathways. It has been known for some time that events unfolding downstream of Ras GTPases are also involved in regulating multiple aspects of cell migration and invasion. More recently, RasGRF2—a Ras activator—has been identified as a suppressor of rounded movement, by inhibiting the activation of Cdc42, independently of its capacity to activate Ras. Here, we discuss how Rho and Ras signals can either cooperate or oppose each other in the regulation of cell migration and invasion.
Key words: Ras GTPases, Rho GTPases, RasGRF, cell migration and invasion, metastasis
Types of Individual Cell Movement
Abnormal cell migration is an essential component of metastasis, the main clinical problem in cancer. While collective cell movement permits entry into the lymphatic system, individual cell movement is necessary for tumor cells to cross basement membranes and enter blood vessels to enable dissemination to distant organs.1 Individual tumor cells have two different modes of movement: an elongated “mesenchymal-like” mode, characterized by a marked cell polarity, actin-dependent protrusions, requirement for high extracellular proteolysis2 and low actomyosin contractility.3–5 The second type is a rounded “amoeboid-like” mode, driven by high actomyosin contractility and a blebbing surface where polarity is generated at the rear of the cell.6 Rounded movement occurs when the composition of the ECM allows the contractile force of the cell to deform the matrix.3,7 It has become clear that these two modes of tumor cell movement are inter-convertible, depending on environmental conditions.4,7,8 This capacity of switching migration strategies is what we have previously described as tumor cell plasticity.4,9
Therapies directed to blocking metastatic dissemination will have to inhibit both rounded and elongated types of movement, since efficient invasion may need both elongated movement for rigid tissues requiring extracellular proteolysis and rounded movement for rapid migration in deformable environments. In addition, high actomyosin contractility in rounded movement may provide mechanical strength to resist shear forces after entry into the bloodstream.4,10,11
Rho GTPases Signaling in Individual Cell Movement
Rho family GTPases are key regulators of cell movement through their effects on actin assembly, actomyosin contractility and microtubules.12 Expression of Rho family proteins is deregulated in many tumors and correlates with progression of disease.12 Most Rho GTPases switch between active, GTP-bound and inactive, GDP-bound, forms. Cycling between these two states is orchestrated by three sets of regulatory proteins: guanine nucleotide exchange factors (GEFs), which function as activators; and GTPase activating proteins (GAPs) plus guanine nucleotide dissociation inhibitors (GDIs) that act as negative regulators.12
The rounded/amoeboid form of movement is driven by high actomyosin contractility evoked by Rho and Cdc42 activation,4,13 while Rac signaling is required for actin assembly in elongated-protrusive movement.4,5 A major Rho effector pathway in rounded movement is mediated by the Rho-kinases, ROCK I and II that generate actomyosin contractility by phosphorylating and inactivating MYPT1, the regulatory subunit of Myosin phosphatase.5 Actomyosin contractility can also be generated via Cdc42 signaling through the kinases MRCK5 or Pak2.13
Regarding the regulation of Rho GTPases in these two types of movement, it has been observed that Rac1 is activated by the GEF, DOCK3 in order to trigger elongated movement4 while another member of the same GEF family, DOCK10, activates Cdc42 in order to promote rounded motility.11,13 On the other hand, Rho inhibits Rac1 through activation of the Rac1 specific GAP, ARHGAP22.4 Furthermore, downregulation of SMURF1 a ubiquitin ligase that targets RhoA for localized destruction at Rac-dependent protrusions results in conversion from elongated to rounded movement.14 In addition, low levels of the cell cycle inhibitor p27Kip1 promotes the rounded form of movement and it is known that p27Kip1 can bind RhoA in the cytoplasm and prevent it from being activated (reviewed in ref. 9). These inter-regulatory connections between different Rho GTPases highlight the importance of their signals in the regulation of cellular plasticity.
Proliferation and Invasion Driven by Rho/Ras Signaling
Traditionally, Rho GTPases have been involved in cell migration/invasion while Ras GTPases have been linked to proliferation and survival. However, it is becoming evident that both families cooperate in promoting these hallmarks of cancer, as defined by Hanahan and Weinberg.15 While there are studies supporting the notion that proliferative and invasive states are mutually exclusive,16 there is also considerable experimental evidence implicating the ERK pathway, one of the main Ras effector cascades, in regulating not only cell proliferation, but also cell motility. For example, B-raf signaling augments the levels of fibronectin and promotes the expression of integrin beta 3. Other Ras effector pathways, like those mediated by PI3K and Ral-GDS, can also contribute to cell migration and invasion in multiple ways. For example, PI3K can activate Rac GEFs (e.g., Sos, Vav) to promote activation of Rac.17 So, it could be argued that Ras signals, can, in certain cases, exert both pro-proliferative and pro-invasive functions. Other inter-regulatory mechanisms between both pathways involve interactions between Rho and Ras regulatory proteins. For example, phosphorylation of p190RhoGap by Brk promotes its interaction with p120RasGAP, thereby stimulating p190 and attenuating p120 functions, leading to RhoA inactivation and Ras activation, respectively to promote breast carcinoma growth, migration and invasion.18 On the other hand, there are multiple evidences of Rho GTPases contributing to cell proliferation.19 Some Rho GTPases stimulate cell cycle progression and regulate gene transcription, something that could partly explain their pro-oncogenic properties, for example in promoting Ras-induced transformation.20 Furthermore, some Rho GEFs have been directly implicated in cell proliferation; such is the case of Tiam1 Dbl and Vav among others.21
“Epithelial-mesenchymal transition” (EMT) has become prominently implicated in cancer cell invasion as a means by which transformed epithelial cells can acquire the abilities to invade, resist apoptosis and disseminate.15 TGFβ has been described as a key regulator of this transition. TGFβ is a tumor suppressor that becomes a tumor promoter. Tumor suppressive functions include inhibition of cell proliferation, induction of apoptosis and regulation of autophagy. As tumors develop, they switch their response to TGFβ and utilize this factor as a potent promoter of cell motility, invasion, metastasis and tumor stem cell maintenance.22 Ras signaling is essentially involved in the TGFβ switch from tumor-suppressive to tumor-promoting functions, leading to enhanced growth and metastatic dissemination of primary tumors.22 On the other hand, there is also evidence showing that TGF-induced EMT requires cytoskeletal rearrangements undertaken by Rho23 while Rac inactivation needs to be tightly regulated in this process.24 This suggests that the cooperation between Rho and Ras pathways can promote invasiveness in later stages of cancer progression.
Interplay between RasGRF and Cdc42
RasGRF is a Ras family GEF cloned by virtue of its homology with the Saccharomyces cerevisiae CDC25 gene product that stimulates nucleotide exchange on S. cerevisiae RAS.25,26 The RasGRF GEFs family includes RasGRF1 and RasGRF2, that exhibit an 80% of overall homology (revised in ref. 27). Both contain a number of functional motifs involved in diverse signaling control mechanisms and protein-protein interactions. The carboxyl-terminal Cdc25 domain is sufficient to catalyze nucleotide exchange on Ras and to induce cellular transformation in fibroblasts.26 In its amino terminus, RasGRF GEFs contain a Dbl homology domain (DH) which is generally present in GEFs for the Rho family of small G proteins. The DH domain is flanked by two Pleckstrin homology domains (PH) also present in Rho family GEFs and other unrelated proteins. The presence of regulatory domains for Rho and Ras GTPases make of RasGRF a confluence point in the control of the signals flowing through both pathways. Even though the rasgrf genes are preferentially expressed in the central nervous system, both RasGRF proteins can also be found in several other tissues, whilst their functional roles at those locations remain less defined and require further studies (reviewed in ref. 27).
Most interestingly, RasGRF functions can be inhibited by its interaction with Cdc42 in its inactive GDP bound form. As such, Cdc42-GDP negatively regulates the activation of the Ras/ERK cascade and of TC21 as induced by RasGRF.28,29 Reciprocally, we have recently demonstrated that the effects of Cdc42 on cytoskeletal dynamics are inhibited by RasGRF1/2, independently of their functions as Ras activators, by outcompeting bona fide Cdc42 exchange factors. In this respect, RasGRF GEFs are unique because they can behave as Rho GTPase inhibitors. Remarkably, an important consequence of Cdc42 inhibition by RasGRF overexpression is decreased actomyosin contractility.11
In Ras GRF1/2, the DH domain is responsible for binding to Cdc42, thereby regulating the switch between rounded and elongated invasion strategies. On the other hand, the DH domain is also required for RasGRF translocation to the membrane.29 As such, it is possible that the cross-talk between Cdc42 and RasGRF could be spatially restricted. As a precedent, RasGRF has been described to activate Ras and TC21 in particular sub-cellular locations.28,30 Thus, it is possible that the control of actomyosin contractility may take place at a particular sub-cellular location. This level of complexity in the interplay between RasGRF and Cdc42 remains to be investigated.
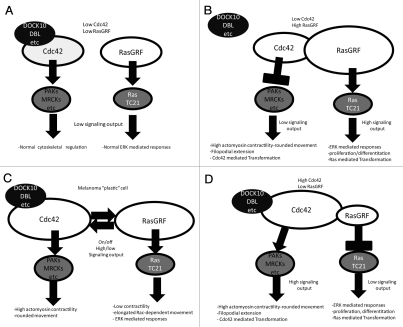
By controlling the balance between Ras and Rho signal output the interaction between RasGRF1/2 and Cdc42 could ultimately determine cell fate. For example, it could be hypothesized that, under normal circumstances, cells with moderate to low levels of both RasGRF1/2 and Cdc42 will exhibit low levels of Ras/TC21 and Cdc42 signals, evoking normal/physiological responses that depending on the cell context would result in normal proliferative and migratory states (Fig. 1A).
Figure 1.
Schematic representation of the balance between Cdc42 and RasGRF pathways. (A) The balance between both signaling pathways is in equilibrium and both are active to a similar extent. (B) RasGRF expression is higher and there is more signaling through the ERK pathway while Cdc42 signaling is low. (C) Tumor cell plasticity allows that both pathways signal on and off depending on environmental conditions. (D) RasGRF expression is lower and there is less signaling through the ER K pathway while Cdc42 signaling is high.
On the other hand, high levels of RasGRF1/2 would result in high flux through TC21 and Ras pathways and, at the same time, in the inhibition of Cdc42 and its associated actomyosin contractility (Fig. 1B). The latter would allow cell elongation and protrusive activity. In line with this notion, it has been shown that RasGRF favors neuronal differentiation under physiological conditions.27 In agreement, neurite outgrowth requires downregulation of actomyosin contractility.31 Thus, it is tempting to speculate that in neurons, RasGRF could contribute to neurite outgrowth by downregulating Cdc42-mediated acto-myosin contractility. A similar mechanism could take place in certain cancer contexts and at the onset of tumor progression. Before local invasion and metastatic dissemination process takes place, RasGRF could promote Ras signals (Fig. 1B) while inhibiting Cdc42 mediated processes. This would result in an initial promotion of cancer cell proliferation via Ras-ERK signaling while preventing invasion.
All the melanoma cell lines used in our study11 harbor either BRAF or N-Ras mutations, which render constitutive activation of the ERK pathway.32 In such a scenario, one would imagine that a Ras activator—RasGRF—would have less of a role in activating the ERK pathway. Surprisingly, all the lines displayed detectable levels of RasGRF2.11 Metastatic melanoma cells express genes associated with a diverse range of cell lineages. Something that may partly explain the diverse modes of motility that melanoma cells can exhibit.33 So, the question that arises is why would a melanoma cell retain RasGRF expression?
Our work11 supports a prominent role for RasGRF2 in suppressing the invasive “highly contractile” rounded phenotype11 by inhibiting the activation of Cdc42. An attractive possibility is that RasGRF would serve a key role in the switch between rapid, highly contractile and proteolytic/low contractile phenotypes (Fig. 1C). Indeed, a system for providing agile inter-convertibility between these forms of migration would facilitate tumor cells to metastasize. When measured in vivo, rounded movement can be 10–100 times faster than protrusive movement. But, while rounded movement can be much faster, elongated movement would still be required whenever progress through a rigid extra-cellular matrix requiring proteolysis is necessary. So the ability to convert to this form of movement is essential wherever tumor cells meet such barriers (reviewed in ref. 9). Taking this into consideration, melanoma cells would exploit RasGRF1/2 functions in order to finely tune the transition from rounded to elongated types of movement—whenever dictated by environmental conditions. Melanoma cells could maintain RasGRF expression in order to regulate this phenotypic switch, independently of its role as a regulator of the Ras/ERK pathway. The main metastatic sites in melanoma are lung, liver, brain and bone and noticeably we have shown how depletion of RasGRF2 favors initial stages of melanoma lung colonization. It would be very interesting to assess the effects of re-expressing RasGRF2, once melanoma cells have invaded the lung, in order to promote proliferation in this newly colonized site via the activation of the Ras-ERK pathway.
RasGRF could still contribute to ERK mediated functions at later stages of melanoma. In support of this notion, constitutive activation of the RAS/ERK pathway through a mechanism involving RasGRF1 has been described to promote secretion of the protease MMP934 that could help in metastasis.
In contrast to melanoma, a number of reports have described reduced levels of RasGrf2 expression in human and rodent tumors of pancreatic, mammary, colon and lung origin, frequently in association with aberrant methylation of the RasGrf2 genomic locus (reviewed in ref. 27). In these cases, RasGRF function has been lost and the situation would be most likely as shown in Figure 1D. As such, RasGRF2 deficiency favors the development and dissemination of lymphomas in mouse models.35 Lymphocytes have been described to rely on amoeboid shape to traffic through interstitial tissues.36 In this particular scenario of tumor cells relying mainly on amoeboid strategies, depletion of RasGRF would be favorable as a mechanism to sustain efficient amoeboid highly contractile phenotype. Alternatively, other negative regulators of acto-myosin contractility could have a role once RasGRF2 is lost. Regarding this possibility and quite intriguingly, RasGRF1 has been identified in a signature for B-chronic lymphocytic leukemia (B-CLL).37 Even though both RasGRF1 and RasGRF2 can activate Ras27 and inhibit Cdc42,11 a thorough comparative analysis of their capabilities in these processes, in physiologically relevant contexts, has not been performed. Thus, the possibility exists that the presence of one or the other isoform could tilt the threshold either towards Ras activation or Cdc42 inhibition.
Future Perspectives
In conclusion, further work is needed to ascertain the potential role for RasGRF as a tumor/metastasis suppressor in those tumors where its expression is reduced or blocked as a selective mechanism to promote tumor progression and metastatic spread. Moreover it would be necessary to study such tumors in comparison with those in which RasGRF expression is retained. In such RasGRF-expressing tumors, it would be very interesting to assess whether RasGRF2 could be a biomarker for elongated cell movement in clinical samples and search for any correlations with progression of the disease. Furthermore, it will be important to distinguish between RasGRF1 and RasGRF2 functions as they seem to be differentially expressed in human malignancies.
We are starting to understand the complexity behind intracellular pathways regulating the plasticity of tumor cell migration. More efforts will have to be made now to further understand the extracellular signals governing this plasticity. On that note, RasGRF regulation and interactions with Cdc42 are extracellular stimuli-dependent.11 Different microenvironments within the tumor could provide distinct signals into tumor cells in order to switch on and off the interplay between Cdc42 and RasGRF, and that would add the last level of complexity in balancing the output signals emanating from these two signalling pathways.
Acknowledgments
V.S.M. is a CRUK Career Development Fellow. P.C. lab is supported by grants BFU2008-01728 from the Spanish Ministry of Education; GROWTHSTOP (LSHC CT-2006-037731) project from the EUVI Framework Programme and Red Tematica de Investigacion Cooperativa en Cancer (RTICC) (RD06/0020/0105), Spanish Ministry of Health. We thank Prof. Chris Marshall at ICR (London) and Dr. Erik Sahai at LRI (London) for their contributions to the work discussed here. We apologize for all those whose work we cannot cite due to space restrictions.
References
- 1.Giampieri S, Manning C, Hooper S, Jones L, Hill CS, Sahai E. Localized and reversible TGFbeta signalling switches breast cancer cells from cohesive to single cell motility. Nat Cell Biol. 2009;11:1287–1296. doi: 10.1038/ncb1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sabeh F, Shimizu-Hirota R, Weiss SJ. Protease-dependent versus -independent cancer cell invasion programs: three-dimensional amoeboid movement revisited. J Cell Biol. 2009;185:11–19. doi: 10.1083/jcb.200807195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wyckoff JB, Pinner SE, Gschmeissner S, Condeelis JS, Sahai E. ROCK- and myosin-dependent matrix deformation enables protease-independent tumor-cell invasion in vivo. Curr Biol. 2006;16:1515–1523. doi: 10.1016/j.cub.2006.05.065. [DOI] [PubMed] [Google Scholar]
- 4.Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, et al. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–523. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson S, Paterson HF, Marshall CJ. Cdc42-MRCK and Rho-ROCK signalling cooperate in myosin phosphorylation and cell invasion. Nat Cell Biol. 2005;7:255–261. doi: 10.1038/ncb1230. [DOI] [PubMed] [Google Scholar]
- 6.Lorentzen A, Bamber J, Sadok A, Elson-Schwab I, Marshall CJ. An ezrin-rich, rigid uropod-like structure directs movement of amoeboid blebbing cells. J Cell Sci. 2011;124:1256–1267. doi: 10.1242/jcs.074849. [DOI] [PubMed] [Google Scholar]
- 7.Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. doi: 10.1038/nrc1075. [DOI] [PubMed] [Google Scholar]
- 8.Wolf K, Mazo I, Leung H, Engelke K, von Andrian UH, Deryugina EI, et al. Compensation mechanism in tumor cell migration: mesenchymal-amoeboid transition after blocking of pericellular proteolysis. J Cell Biol. 2003;160:267–277. doi: 10.1083/jcb.200209006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanz-Moreno V, Marshall CJ. The plasticity of cytoskeletal dynamics underlying neoplastic cell migration. Curr Opin Cell Biol. 2010;22:690–696. doi: 10.1016/j.ceb.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 10.Pinner S, Sahai E. PDK1 regulates cancer cell motility by antagonising inhibition of ROCK1 by RhoE. Nat Cell Biol. 2008;10:127–137. doi: 10.1038/ncb1675. [DOI] [PubMed] [Google Scholar]
- 11.Calvo F, Sanz-Moreno V, Agudo-Ibanez L, Wallberg F, Sahai E, Marshall CJ, et al. RasGRF suppresses Cdc42-mediated tumour cell movement, cytoskeletal dynamics and transformation. Nat Cell Biol. 2011;13:819–826. doi: 10.1038/ncb2271. [DOI] [PubMed] [Google Scholar]
- 12.Vega FM, Ridley AJ. Rho GTPases in cancer cell biology. FEBS Lett. 2008;582:2093–2101. doi: 10.1016/j.febslet.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 13.Gadea G, Sanz-Moreno V, Self A, Godi A, Marshall CJ. DOCK10-mediated Cdc42 activation is necessary for amoeboid invasion of melanoma cells. Curr Biol. 2008;18:1456–1465. doi: 10.1016/j.cub.2008.08.053. [DOI] [PubMed] [Google Scholar]
- 14.Sahai E, Garcia-Medina R, Pouyssegur J, Vial E. Smurf1 regulates tumor cell plasticity and motility through degradation of RhoA leading to localized inhibition of contractility. J Cell Biol. 2007;176:35–42. doi: 10.1083/jcb.200605135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 16.Hoek KS, Eichhoff OM, Schlegel NC, Dobbeling U, Kobert N, Schaerer L, et al. In vivo switching of human melanoma cells between proliferative and invasive states. Cancer Res. 2008;68:650–656. doi: 10.1158/0008-5472.CAN-07-2491. [DOI] [PubMed] [Google Scholar]
- 17.Campbell PM, Der CJ. Oncogenic Ras and its role in tumor cell invasion and metastasis. Semin Cancer Biol. 2004;14:105–114. doi: 10.1016/j.semcancer.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Shen CH, Chen HY, Lin MS, Li FY, Chang CC, Kuo ML, et al. Breast tumor kinase phosphorylates p190RhoGAP to regulate rho and ras and promote breast carcinoma growth, migration and invasion. Cancer Res. 2008;68:7779–7787. doi: 10.1158/0008-5472.CAN-08-0997. [DOI] [PubMed] [Google Scholar]
- 19.Sahai E, Olson MF, Marshall CJ. Cross-talk between Ras and Rho signalling pathways in transformation favours proliferation and increased motility. EMBO J. 2001;20:755–766. doi: 10.1093/emboj/20.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Olson MF, Paterson HF, Marshall CJ. Signals from Ras and Rho GTPases interact to regulate expression of p21Waf1/Cip1. Nature. 1998;394:295–299. doi: 10.1038/28425. [DOI] [PubMed] [Google Scholar]
- 21.Lazer G, Katzav S. Guanine nucleotide exchange factors for RhoGTPases: good therapeutic targets for cancer therapy? Cell Signal. 23:969–979. doi: 10.1016/j.cellsig.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 22.Inman GJ. Switching TGFbeta from a tumor suppressor to a tumor promoter. Curr Opin Genet Dev. 2011;21:93–99. doi: 10.1016/j.gde.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Das S, Becker BN, Hoffmann FM, Mertz JE. Complete reversal of epithelial to mesenchymal transition requires inhibition of both ZEB expression and the Rho pathway. BMC Cell Biol. 2009;10:94. doi: 10.1186/1471-2121-10-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodcock SA, Rooney C, Liontos M, Connolly Y, Zoumpourlis V, Whetton AD, et al. SRC-induced disassembly of adherens junctions requires localized phosphorylation and degradation of the rac activator tiam1. Mol Cell. 2009;33:639–653. doi: 10.1016/j.molcel.2009.02.012. [DOI] [PubMed] [Google Scholar]
- 25.Martegani E, Vanoni M, Zippel R, Coccetti P, Brambilla R, Ferrari C, et al. Cloning by functional complementation of a mouse cDNA encoding a homologue of CDC25, a Saccharomyces cerevisiae RAS activator. EMBO J. 1992;11:2151–2157. doi: 10.1002/j.1460-2075.1992.tb05274.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cen H, Papageorge AG, Vass WC, Zhang KE, Lowy DR. Regulated and constitutive activity by CDC25Mm (GRF), a Ras-specific exchange factor. Mol Cell Biol. 1993;13:7718–7724. doi: 10.1128/mcb.13.12.7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fernandez-Medarde A, Santos E. The RasGrf family of mammalian guanine nucleotide exchange factors. Biochim Biophys Acta. 2011;1815:170–188. doi: 10.1016/j.bbcan.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 28.Calvo F, Crespo P. Structural and spatial determinants regulating TC21 activation by RasGRF family nucleotide exchange factors. Mol Biol Cell. 2009;20:4289–4302. doi: 10.1091/mbc.E09-03-0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arozarena I, Matallanas D, Crespo P. Maintenance of CDC42 GDP-bound state by Rho-GDI inhibits MAP kinase activation by the exchange factor Ras-GRF. evidence for Ras-GRF function being inhibited by Cdc42-GDP but unaffected by CDC42-GTP. J Biol Chem. 2001;276:21878–21884. doi: 10.1074/jbc.M011383200. [DOI] [PubMed] [Google Scholar]
- 30.Arozarena I, Matallanas D, Berciano MT, Sanz-Moreno V, Calvo F, Munoz MT, et al. Activation of H-Ras in the endoplasmic reticulum by the RasGRF family guanine nucleotide exchange factors. Mol Cell Biol. 2004;24:1516–1530. doi: 10.1128/MCB.24.4.1516-1530.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosner H, Moller W, Wassermann T, Mihatsch J, Blum M. Attenuation of actinomyosinII contractile activity in growth cones accelerates filopodia-guided and microtubule-based neurite elongation. Brain Res. 2007;1176:1–10. doi: 10.1016/j.brainres.2007.07.081. [DOI] [PubMed] [Google Scholar]
- 32.Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- 33.Hendrix MJ, Seftor EA, Meltzer PS, Gardner LM, Hess AR, Kirschmann DA, et al. Expression and functional significance of VE-cadherin in aggressive human melanoma cells: role in vasculogenic mimicry. Proc Natl Acad Sci USA. 2001;98:8018–8023. doi: 10.1073/pnas.131209798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhu TN, He HJ, Kole S, D'Souza T, Agarwal R, Morin PJ, et al. Filamin A-mediated downregulation of the exchange factor Ras-GRF1 correlates with decreased matrix metalloproteinase-9 expression in human melanoma cells. J Biol Chem. 2007;282:14816–14826. doi: 10.1074/jbc.M611430200. [DOI] [PubMed] [Google Scholar]
- 35.Ruiz S, Santos E, Bustelo XR. The use of knockout mice reveals a synergistic role of the Vav1 and Rasgrf2 gene deficiencies in lymphomagenesis and metastasis. PLoS One. 2009;4:8229. doi: 10.1371/journal.pone.0008229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolf K, Muller R, Borgmann S, Brocker EB, Friedl P. Amoeboid shape change and contact guidance: T-lymphocyte crawling through fibrillar collagen is independent of matrix remodeling by MMPs and other proteases. Blood. 2003;102:3262–3269. doi: 10.1182/blood-2002-12-3791. [DOI] [PubMed] [Google Scholar]
- 37.Jelinek DF, Tschumper RC, Stolovitzky GA, Iturria SJ, Tu Y, Lepre J, et al. Identification of a global gene expression signature of B-chronic lymphocytic leukemia. Mol Cancer Res. 2003;1:346–361. [PubMed] [Google Scholar]