Abstract
Adherens junctions (AJs) and tight junctions (TJs) represent key adhesive structures that regulate the apico-basal polarity and barrier properties of epithelial layers. AJs and TJs readily undergo disassembly and reassembly during normal tissue remodeling and disruption of epithelial barriers in diseases. Such junctional plasticity depends on the orchestrated dynamics of the plasma membrane with its underlying F-actin cytoskeleton, however the interplay between these cellular structures remains poorly understood. Recent studies highlighted the spectrin-adducin-based membrane skeleton as an emerging regulator of AJ and TJ integrity and remodeling. Here we discuss new evidences implicating adducin, spectrin and other membrane skeleton proteins in stabilization of epithelial junctions and regulation of junctional dynamics. Based on the known ability of the membrane skeleton to link cortical actin filaments to the plasma membrane, we hypothesize that the spectrin-adducin network serves as a critical signal and force transducer from the actomyosin cytoskeleton to junctions during remodeling of AJs and TJs.
Key words: adherens junctions, tight junctions, permeability, membrane skeleton, actomyosin, contractility, calcium switch
Introduction
Integrity and barrier properties of epithelial layers depend on the assembly of adhesive contacts between adjacent epithelial cells. These adhesive contacts are composed of multiprotein complexes known as intercellular junctions. In simple polarized epithelia, several junctional complexes span the lateral plasma membrane to interact with opposing complementary junctions in the intercellular space and associate with various cytoskeletal, signaling and trafficking components at the cytosolic face of the membrane.1,2 Among these complexes the most apically-located tight junctions (TJs) and subjacent adherens junctions (AJs) play key roles in regulating epithelial cell adhesion, polarity and differentiation.2–5
TJs and AJs are composed of several types of integral membrane and cytosolic scaffolding proteins. Major transmembrane components of TJs mediating intercellular adhesions include the claudin protein family, occludin and junctional adhesion molecule A.2,3,5 ‘Zonula occludens’ (ZO) proteins are the most abundant molecular constituents of the cytosolic plaque of TJs.2,3,5,6 Adhesive properties of epithelial AJs are determined by E-cadherin and nectins, which are clustered and stabilized at the plasma membrane via interactions with cytosolic scaffolds such as α, β and p120 catenins.2,4,7
Once considered as static, glue-like structures, TJs and AJs are now known to be very dynamic. Such dynamics includes a continuous remodeling (disassembly and reassembly) of junctional complexes in fully-differentiated epithelial layers, as well as large-scale TJ/AJ rearrangements that occur during normal epithelial morphogenesis and disruption of mucosal barriers in many diseases.8–12 Two major mechanisms have been implicated in regulation of junctional dynamics; one is reorganizations of the perijunctional F-actin cytoskeleton and the other is remodeling of the plasma membrane.8–13 The interplay between these mechanisms is well appreciated. Indeed, reorganizations of the perijunctional F-actin belt are coordinated with altered endocytic and exocytic activity of the cell membrane during disassembly and reformation of epithelial contacts.14–17 Furthermore, inhibition of such F-actin reorganizations was shown to suppress membrane dynamics and vice versa.14,15,18,19 These findings suggest that physical attachment of the cortical F-actin cytoskeleton to the plasma membrane play an important role in transducing signals and/or forces that drive remodeling of epithelial junctions.
Several mechanisms can link actin filaments to the cytosolic face of the plasma membrane with the most abundant attachments mediated by the spectrin- based membrane skeleton.20–22 The membrane skeleton is formed by spectrin tetramers composed of two α and β-spectrin heterodimers.20–22 These tetramers represent flexible rods with actin-binding sites at each end. Spectrin rods are linked to the plasma membrane via specialized scaffolding proteins, such as ankyrin and protein 4.1, which have a dual affinity for spectrin and cytoplasmic domains of transmembrane transporters and channels.20–22 Spectrin association with actin filaments is enhanced by other accessory proteins, most notably, by adducin.21,23 Mammalian adducin has three homologous isoforms α, β and γ.21,23 The α and γ isoforms are expressed in various tissues, whereas expression of β-adducin is limited to erythrocytes and the brain.24,25 Adducin readily forms heterodimers and heterotetramers of either α/β or α/γ subunits.24,26 These oligomers are thought to recruit spectrin to actin filaments and to promote assembly of the spectrin lattice at the plasma membrane.26,27 Besides mediating spectrin-F-actin linkage, adducin is also involved in actin filament bundling and capping.28–30 Overall, these data highlight adducin as an important regulator of both the spectrin-based membrane skeleton and the actin cytoskeleton.
Adducin Regulates Remodeling of Apical Junctions in Simple Epithelia
It has been long recognized that α and γ isoforms of adducin are enriched at intercellular junctions in cultured epithelial cell monolayers and simple mucosal epithelia in vivo.24,31 Despite this junctional affiliation, the involvement of adducin in regulation of epithelial AJs and TJs remains poorly understood. Recently, we have examined the role of this membrane skeleton protein in the dynamics of epithelial junctions by using siRNA-mediated knockdown of α and γ adducin isoforms in SK-CO15 human intestinal epithelial cells.32 Remodeling of epithelial junctions was induced by a so called ‘calcium switch'. This model involves removal of extracellular calcium to trigger disassembly of preformed AJs and TJs followed by calcium re-addition to the culture medium (calcium repletion) to induce orchestrated recovery of junctional structure and functions.14–16,33 We observed that knockdown of either α- or γ-adducins in SK-CO15 cells attenuated reassembly of apical junctions and development of the paracellular barrier triggered by calcium repletion. Interestingly, loss of adducin expression delayed reformation of both AJs and TJs, although α- or γ-adducins consistently colocalized with AJ, but not TJ proteins in newly-assembled and mature intercellular contacts.32 Since AJ assembly represents an early step of epithelial differentiation that is required for the subsequent formation of TJs, we believe that adducin depletion directly impairs the establishment of epithelial AJs, which in turn attenuates TJ reassembly. Eventually, epithelial cells were able to assemble morphologically-normal cell-cell contacts even in the absence of adducin, however, such contacts appear to be less stable comparing to those of normal cells. This notion of contact instability is based on the observed collapse of the lateral plasma membrane and the increased long-range intramembrane mobility of E-cadherin in adducin-depleted cells.27
Given our findings that adducin promotes the establishment of epithelial AJs and TJs one can suggest that this membrane skeleton protein should antagonize junctional disassembly. Indeed, we observed such antagonisms while examining the effects of adducin isoforms knockdown on disruption of AJs and TJs in HPAF II human pancreatic epithelial cells exposed to protein kinase C (PKC)-activating phorbol ester. PKC activation is known to potently disrupt cell-cell contacts in several types of epithelia by stimulating remodeling of the peri-junctional F-actin and triggering internalization of AJ/TJ proteins.34,35 On the other hand, PKC phosphorylates adducin at several serine residues (Ser726, Ser712 and Ser660) in their C-terminal MARKS domain.36,37 This phosphorylation has been shown to inhibit adducin functions by decreasing its associations with actin filaments and spectrin.36,37 We found that phorbol ester induced rapid phosphorylation of α- and γ-adducins which was accompanied by their disappearance from the intercellular junctions.32 Loss of adducin from cell-cell contacts appears to be an early event of the phorbol ester signaling that preceded AJ and TJ disassembly. Furthermore, depletion of either α- or γ-adducins significantly accelerated disruption of AJs and TJs induced by PKC activation.32 These results suggest that PKC-dependent phosphorylation of adducin triggers its early release from complexes with spectrin and actin filaments, thereby enhancing remodeling of the cortical cytoskeleton and destabilizing epithelial junctions. Importantly, protein kinase A and Rho-dependent kinase, as well as cytokines such as pleiotropin are known to phosphorylate adducin and alter cellular distribution and activity of this scaffolding protein.37,38 Therefore, adducin can be important down-stream effector of different signaling cascades that regulate stability and remodeling of epithelial junctions.
Our study also provides an important insight into the mechanisms that mediate the effects of adducin on epithelial junctions. These mechanisms involve organization of the spectrin network and assembly of actin filaments at the intercellular contacts. For example, we found that depletion of adducin isoforms decreased expression of βII-spectrin in intestinal epithelial cells and delayed recruitment of this protein to newly-forming AJs.32 This observation suggests that loss of adducin impairs formation of the highly-ordered spectrin lattice at the plasma membrane of contacting epithelial cells, which is likely to be responsible for the attenuated junctional assembly. Another mechanism that can mediate destabilization of apical junctions in adducin-depleted epithelia involves impaired formation of the perijunctional F-actin belt. Previous biochemical studies described the ability of α-adducin to cap28 and cross-link29,30 actin filaments in cell-free systems. However, we demonstrated for the first time that adducin regulates assembly of the F-actin cytoskeleton in epithelial cells. This conclusion is supported by findings that siRNA-mediated depletion of α and γ adducins increased the G/F actin ratio, which indicates either impaired polymerization or enhanced depolymerization of actin filaments. Furthermore, adducin downregulation attenuated formation of the perijunctional F-actin bundles during reestablishment of epithelial AJs.32 Given the crucial role of the circumferential F-actin belt in supporting structure of epithelial junctions, this defective F-actin assembly should underline the impaired formation of AJs and TJs in adducin-depleted epithelial cells. What type of adducin-F-actin interactions (filament capping or cross-linking) are involved in organization of the perijunctional cytoskeleton and whether spectrin is essential for these events remain to be determined. It is also unclear whether the effects of adducin depletion on the remodeling of actin filaments and spectrin assembly at intercellular junctions represent two distinct mechanisms or if they are mutually dependent. However, based on a classical model of adducin action, it is likely that loss of this scaffolding protein breaks a physical link between spectrin oligomers and actin filaments which is important for proper organization of both cytoskeletal structures at the areas of cell-cell contacts.
Various Molecular Components of the Membrane Skeleton are Essential for the Biogenesis of Epithelial Junctions
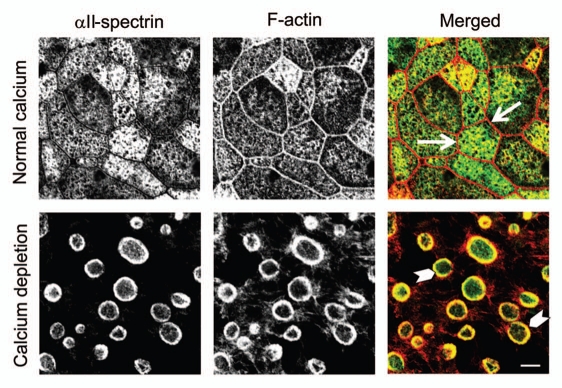
Other molecular constituents of the membrane skeleton have been implicated in regulation of epithelial apical junctions. Spectrin itself appears to be important in invertebrate and mammalian systems. For example, removal of apical spectrin in Drosophila was shown to induce AJ disassembly in follicle epithelium,39 whereas depletion of mammalian βII-spectrin attenuated formation of AJs in human intestinal and bronchial epithelial cells,32,40 and early mouse embryo.41 Two major mechanisms can be responsible for the observed spectrin-dependent regulation of epithelial junctions. The first mechanism involves βII-spectrin binding to the E-cadherin-catenin complex, which can be either direct or mediated by an accessory protein, ankyrin G. Such interactions with βII-spectrin and/or ankyrin are important for E-cadherin trafficking from the Golgi to the plasma membrane.40,42 An alternative mechanism of spectrin actions involves regulation of cortical actin filaments. Thus, a recent study revealed a crucial role for αII-spectrin in organizing perijunctional F-actin bundles in endothelial cells, which stabilized cell-cell contacts and enhanced the endothelial barrier.43 Furthermore, our unpublished data suggest the close interactions between spectrin oligomers and actin filaments during AJ/TJ remodeling. We found that αII-spectrin and the perijunctional F-actin belt are spatially segregated in polarized intestinal epithelial cells with intact junctions (Fig. 1, arrows). By contrast, spectrin became enriched in the apical contractile actin rings that are known to drive AJ/TJ disassembly in calcium-depleted epithelial cells (Fig. 1, arrowheads). Interestingly, adducin also accumulated at these contractile actomyosin rings where it was colocalized with fragments of disassembled AJs (data not shown). This contrasts with adducin behavior during phorbol ester-induced disruption of epithelial junctions and suggests that in some conditions, spectrin-adducin complexes can promote disruption of AJs and TJs by controlling formation and/or contraction of perijunctional actomyosin structures.
Figure 1.
Spectrin is recruited to contractile F-actin rings in calcium-depleted epithelial cells. Confluent T84 human intestinal epithelial cell monolayers and cells subjected to 60 min of calcium depletion were dual immunolabeled for αII -spectrin (green color) and F-actin (red color). Confocal microscopic images show that αII -spectin does not significantly colocalize with the perijunctional F-actin belt (arrows) in control cell monolayers. By contrast, αII-spectrin is enriched in contractile apical F-actin rings assembled in calcium-depleted epithelial cells (arrowheads). Scale bar, 5 µm.
Protein 4.1 is another molecular scaffold in the membrane skeleton that was implicated in regulation of epithelial junctions. Several members of the protein 4.1 family are known to interact with spectrin and actin and to stabilize the spectrin-actin network at the plasma membrane.44,45 In epithelial cells, protein 4.1R isoform was shown to localize at the areas of cell-cell contacts where it was able to associate with β-catenin and ZO-2.46,47 Loss of protein 4.1 expression phenocopied major effects of other membrane skeleton proteins depletion by causing disruption of the E-cadherin-catenin complex, impairing trafficking of junctional proteins to the plasma membrane and attenuating assembly of the cortical F-actin cytoskeleton.47,48
Possible Mechanisms of the Membrane Skeleton-Dependent Regulation of AJ/TJ Structure and Remodeling
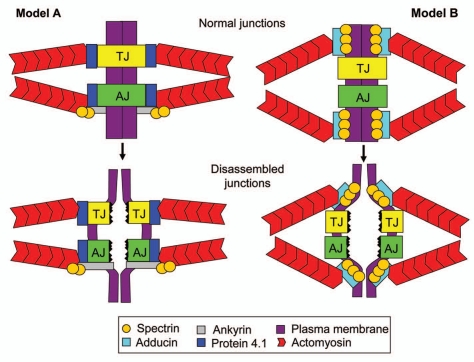
Recent studies that unravel close associations between the spectrin-adducin-based membrane skeleton and epithelial junctions not only add another level of complexity to AJ and TJ regulation but may also help to solve a puzzle about associations of epithelial junctions and the underlying actin filaments. A critical role of F-actin cytoskeleton in regulating structure and barrier properties of apical junctions is well appreciated and is supported by a large amount of morphological, pharmacologic and genetic data.9,10,12,13,49,50 Furthermore, it is known that perijunctional actomyosin bundles regulate junctional structure and remodeling by generating tension or contractile forces.9,10,49,50 The force transduction from actin filaments to epithelial junctions implies that these structures are physically-connected, however molecular organization of the cytoskeletal/junctional interface remains enigmatic. Particularly, it is unclear which cytosolic scaffolds link AJs and TJs to the underlying actin filaments in living cells.51,52 We believe that the spectrin-adducin membrane skeleton can serve as a linker/force transducer between the actin cytoskeleton on epithelial junctions. Figure 2 depicts two hypothetic mechanisms by which membrane skeleton can mediate AJ/TJ disassembly driven by contractile actomyosin structures, as it happens in calcium-depleted epithelial cells. The canonical mechanism presented in Model A implies that membrane skeleton proteins physically link epithelial junctions to underlying actin filaments. For example, protein 4.1 can mediate cytoskeletal attachments of both AJs and TJs due to its known interactions with actin and junctional plaque components ZO-2 and β-catenin.46,47 Additionally, AJs can be linked to actin filaments by a scaffolding complex composed of ankyrin G, spectrin and adducin. Such membrane skeleton mediated linkage between apical junctions and actin filaments will ensure disassembly of AJs and TJs upon synchronized contraction of perijunctional actin bundles in two contacting epithelial cells (Fig. 2). However, the membrane skeleton can transduce actomyosin-generated forces to epithelial junctions even without direct attachments of AJs and/or TJs to actin filaments. This may explain recent live cell imaging data that demonstrated different turnover rates of junctional proteins and cortical actin thereby arguing against their direct physical associations.51,52 Model B depicted in Figure 2 illustrates how this indirect mechanism can mediate junctional disassembly. Spectrin-adducin complexes are known to attach actin filaments to the cytosolic domains of integral plasma membrane proteins such as Na/K ATPase or ammonium transporter.20,21,44 Because of this attachment, synchronized contraction of perimembrane actomyosin bundles should result in pulling apart two opposing plasma membranes of contacting epithelial cells. If such membrane retraction occurs in a close vicinity to apical junctions, it will create sufficient pulling forces to disrupt trans-interactions between adhesive AJ/TJ proteins thereby triggering junctional disassembly. Similar mechanism can contribute to junctional reassembly and to the organization of mature AJ and TJ in polarized epithelial cells. In the last scenario, spectrin-adducin-mediated membrane attachments of the perijunctional F-actin belt may stabilize junctional structures by corralling AJ/TJ proteins at the cell apex and limiting their diffusion within the plasma membrane.
Figure 2.
Hypothetical mechanisms by which the membrane skeleton can mediate F-actin dependent disassembly of epithelial junctions. The scheme depicts two different models proposed to explain how the spectrin-adducin membrane skeleton can mediate epithelial AJ/TJ disassembly driven by perijunctional actomyosin contractility. Model A implies that the membrane skeleton physically links actin filaments to cytosolic plaques of AJs and TJs, whereas Model B proposes indirect force transduction via adducin-spectrin-mediated actin attachment to the plasma membrane in a close vicinity of apical junctions. See detailed explanation in the text.
In summary, several recent studies shed a new light on a long forgotten association between epithelial junctions and the membrane skeleton by demonstrating crucial involvement of adducin and spectrin in regulating AJ/TJ dynamics. We hope that future works will unravel important molecular mechanisms that underlie membrane skeleton-dependent remodeling of apical junctions during normal epithelial morphogenesis and/or disruption of mucosal barriers in different diseases.
Acknowledgments
The authors thank Drs. Somesh Baranwal, Ann Hopkins and Alan Fanning for critical reading and Gianni Harris for editing this manuscript. This work was supported by National Institute of Health RO1 grants DK084953 and DK083968 to A.I.I.
Perspective on: Naydenov NG, Ivanov AI. Adducins regulate remodeling of apical junctions in human epithelial cells. Mol Biol Cell. 2010;21:3506–3517. doi: 10.1091/mbc.E10-03-0259.
References
- 1.Green KJ, Getsios S, Troyanovsky S, Godsel LM. Intercellular junction assembly, dynamics and homeostasis. Cold Spring Harb Perspect Biol. 2010;2:125. doi: 10.1101/cshperspect.a000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta. 2008;1778:660–669. doi: 10.1016/j.bbamem.2007.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harbor Perspect Biol. 2009;1:2584. doi: 10.1101/cshperspect.a002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pokutta S, Weis WI. Structure and mechanism of cadherins and catenins in cell-cell contacts. Annu Rev Cell Dev Biol. 2007;23:237–261. doi: 10.1146/annurev.cellbio.22.010305.104241. [DOI] [PubMed] [Google Scholar]
- 5.Tsukita S, Furuse M, Itoh M. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol. 2001;2:285–293. doi: 10.1038/35067088. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Mariscal L, Tapia R, Chamorro D. Crosstalk of tight junction components with signaling pathways. Biochim Biophys Acta. 2008;1778:729–756. doi: 10.1016/j.bbamem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- 7.Takai Y, Nakanishi H. Nectin and afadin: novel organizers of intercellular junctions. J Cell Sci. 2003;116:17–27. doi: 10.1242/jcs.00167. [DOI] [PubMed] [Google Scholar]
- 8.Bryant DM, Stow JL. The ins and outs of E-cadherin trafficking. Trends Cell Biol. 2004;14:427–434. doi: 10.1016/j.tcb.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 9.Cavey M, Lecuit T. Molecular bases of cell-cell junctions stability and dynamics. Cold Spring Harbor Perspect Biol. 2009;1:2998. doi: 10.1101/cshperspect.a002998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivanov AI. Actin motors that drive formation and disassembly of epithelial apical junctions. Front Biosci. 2008;13:6662–6681. doi: 10.2741/3180. [DOI] [PubMed] [Google Scholar]
- 11.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of the apical junctional complex: mechanisms and possible roles in regulation of epithelial barriers. Bioessays. 2005;27:356–365. doi: 10.1002/bies.20203. [DOI] [PubMed] [Google Scholar]
- 12.Turner JR. Intestinal mucosal barrier function in health and disease. Nat Rev Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Moreno M, Jamora C, Fuchs E. Sticky business: orchestrating cellular signals at adherens junctions. Cell. 2003;112:535–548. doi: 10.1016/s0092-8674(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 14.Ivanov AI, Hunt D, Utech M, Nusrat A, Parkos CA. Differential roles for actin polymerization and a myosin II motor in assembly of the epithelial apical junctional complex. Mol Biol Cell. 2005;16:2636–2650. doi: 10.1091/mbc.E05-01-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivanov AI, Nusrat A, Parkos CA. Endocytosis of epithelial apical junctional proteins by a clathrin-mediated pathway into a unique storage compartment. Mol Biol Cell. 2004;15:176–188. doi: 10.1091/mbc.E03-05-0319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pitelka DR, Taggart BN, Hamamoto ST. Effects of extracellular calcium depletion on membrane topography and occluding junctions of mammary epithelial cells in culture. J Cell Biol. 1983;96:613–624. doi: 10.1083/jcb.96.3.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Volberg T, Geiger B, Kartenbeck J, Franke WW. Changes in membrane-microfilament interaction in intercellular adherens junctions upon removal of extracellular Ca2+ ions. J Cell Biol. 1986;102:1832–1842. doi: 10.1083/jcb.102.5.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma TY, Tran D, Hoa N, Nguyen D, Merryfield M, Tarnawski A. Mechanism of extracellular calcium regulation of intestinal epithelial tight junction permeability: role of cytoskeletal involvement. Microsc Res Tech. 2000;51:156–168. doi: 10.1002/1097-0029(20001015)51:2<156::AID-JEMT7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 19.Nejsum LN, Nelson WJ. A molecular mechanism directly linking E-cadherin adhesion to initiation of epithelial cell surface polarity. J Cell Biol. 2007;178:323–335. doi: 10.1083/jcb.200705094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baines AJ. Evolution of spectrin function in cytoskeletal and membrane networks. Biochem Soc Trans. 2009;37:796–803. doi: 10.1042/BST0370796. [DOI] [PubMed] [Google Scholar]
- 21.Bennett V, Baines AJ. Spectrin and ankyrin-based pathways: metazoan inventions for integrating cells into tissues. Physiol Rev. 2001;81:1353–1392. doi: 10.1152/physrev.2001.81.3.1353. [DOI] [PubMed] [Google Scholar]
- 22.Thomas GH. Spectrin: the ghost in the machine. Bioessays. 2001;23:152–160. doi: 10.1002/1521-1878(200102)23:2<152::AID-BIES1022>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 23.Matsuoka Y, Li X, Bennett V. Adducin: structure, function and regulation. Cell Mol Life Sci. 2000;57:884–895. doi: 10.1007/PL00000731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong L, Chapline C, Mousseau B, Fowler L, Ramsay K, Stevens JL, Jaken S. 35H, a sequence isolated as a protein kinase C binding protein, is a novel member of the adducin family. J Biol Chem. 1995;270:25534–25540. doi: 10.1074/jbc.270.43.25534. [DOI] [PubMed] [Google Scholar]
- 25.Joshi R, Gilligan DM, Otto E, McLaughlin T, Bennett V. Primary structure and domain organization of human alpha and beta adducin. J Cell Biol. 1991;115:665–675. doi: 10.1083/jcb.115.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes CA, Bennett V. Adducin: a physical model with implications for function in assembly of spectrin-actin complexes. J Biol Chem. 1995;270:18990–18996. doi: 10.1074/jbc.270.32.18990. [DOI] [PubMed] [Google Scholar]
- 27.Abdi KM, Bennett V. Adducin promotes micrometerscale organization of β2-spectrin in lateral membranes of bronchial epithelial cells. Mol Biol Cell. 2008;19:536–545. doi: 10.1091/mbc.E07-08-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuhlman PA, Hughes CA, Bennett V, Fowler VM. A new function for adducin. Calcium/calmodulin-regulated capping of the barbed ends of actin filaments. J Biol Chem. 1996;271:7986–7991. doi: 10.1074/jbc.271.14.7986. [DOI] [PubMed] [Google Scholar]
- 29.Mische SM, Mooseker MS, Morrow JS. Erythrocyte adducin: a calmodulin-regulated actin-bundling protein that stimulates spectrin-actin binding. J Cell Biol. 1987;105:2837–2845. doi: 10.1083/jcb.105.6.2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taylor KA, Taylor DW. Formation of two-dimensional complexes of F-actin and crosslinking proteins on lipid monolayers: demonstration of unipolar alpha-actinin-F-actin crosslinking. Biophys J. 1994;67:1976–1983. doi: 10.1016/S0006-3495(94)80680-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaiser HW, O'Keefe E, Bennett V. Adducin: Ca++-dependent association with sites of cell-cell contact. J Cell Biol. 1989;109:557–569. doi: 10.1083/jcb.109.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naydenov NG, Ivanov AI. Adducins regulate remodeling of apical junctions in human epithelial cells. Mol Biol Cell. 2010;21:3506–3517. doi: 10.1091/mbc.E10-03-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cereijido M, Robbins ES, Dolan WJ, Rotunno CA, Sabatini DD. Polarized monolayers formed by epithelial cells on a permeable and translucent support. J Cell Biol. 1978;77:853–880. doi: 10.1083/jcb.77.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ivanov AI, Samarin SN, Bachar M, Parkos CA, Nusrat A. Protein kinase C activation disrupts epithelial apical junctions via ROCK-II dependent stimulation of actomyosin contractility. BMC Cell Biol. 2009;10:36. doi: 10.1186/1471-2121-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamei T, Matozaki T, Sakisaka T, Kodama A, Yokoyama S, Peng YF, et al. Coendocytosis of cadherin and c-Met coupled to disruption of cell-cell adhesion in MDCK cells—regulation by Rho, Rac and Rab small G proteins. Oncogene. 1999;18:6776–6784. doi: 10.1038/sj.onc.1203114. [DOI] [PubMed] [Google Scholar]
- 36.Fowler L, Dong L, Bowes RC, 3rd, van de Water B, Stevens JL, Jaken S. Transformation-sensitive changes in expression, localization and phosphorylation of adducins in renal proximal tubule epithelial cells. Cell Growth Differ. 1998;9:177–184. [PubMed] [Google Scholar]
- 37.Matsuoka Y, Li X, Bennett V. Adducin is an in vivo substrate for protein kinase C: phosphorylation in the MARCKS-related domain inhibits activity in promoting spectrin-actin complexes and occurs in many cells, including dendritic spines of neurons. J Cell Biol. 1998;142:485–497. doi: 10.1083/jcb.142.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kimura K, Fukata Y, Matsuoka Y, Bennett V, Matsuura Y, Okawa K, et al. Regulation of the association of adducin with actin filaments by Rho-associated kinase (Rho-kinase) and myosin phosphatase. J Biol Chem. 1998;273:5542–5548. doi: 10.1074/jbc.273.10.5542. [DOI] [PubMed] [Google Scholar]
- 39.Zarnescu DC, Thomas GH. Apical spectrin is essential for epithelial morphogenesis but not apicobasal polarity in Drosophila. J Cell Biol. 1999;146:1075–1086. doi: 10.1083/jcb.146.5.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kizhatil K, Davis JQ, Davis L, Hoffman J, Hogan BL, Bennett V. Ankyrin-G is a molecular partner of E-cadherin in epithelial cells and early embryos. J Biol Chem. 2007;282:26552–26561. doi: 10.1074/jbc.M703158200. [DOI] [PubMed] [Google Scholar]
- 41.Kizhatil K, Yoon W, Mohler PJ, Davis LH, Hoffman JA, Bennett V. Ankyrin-G and β2-spectrin collaborate in biogenesis of lateral membrane of human bronchial epithelial cells. J Biol Chem. 2007;282:2029–2037. doi: 10.1074/jbc.M608921200. [DOI] [PubMed] [Google Scholar]
- 42.Kizhatil K, Bennett V. Lateral membrane biogenesis in human bronchial epithelial cells requires 190-kDa ankyrin-G. J Biol Chem. 2004;279:16706–16714. doi: 10.1074/jbc.M314296200. [DOI] [PubMed] [Google Scholar]
- 43.Benz PM, Blume C, Moebius J, Oschatz C, Schuh K, Sickmann A, et al. Cytoskeleton assembly at endothelial cell-cell contacts is regulated by αII-spectrin-VASP complexes. J Cell Biol. 2008;180:205–219. doi: 10.1083/jcb.200709181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baines AJ, Bennett PM, Carter EW, Terracciano C. Protein 4.1 and the control of ion channels. Blood Cells Mol Dis. 2009;42:211–215. doi: 10.1016/j.bcmd.2009.01.016. [DOI] [PubMed] [Google Scholar]
- 45.Calinisan V, Gravem D, Chen RP, Brittin S, Mohandas N, Lecomte MC, Gascard P. New insights into potential functions for the protein 4.1 superfamily of proteins in kidney epithelium. Front Biosci. 2006;11:1646–1666. doi: 10.2741/1911. [DOI] [PubMed] [Google Scholar]
- 46.Mattagajasingh SN, Huang SC, Hartenstein JS, Benz EJ., Jr Characterization of the interaction between protein 4.1R and ZO-2. A possible link between the tight junction and the actin cytoskeleton. J Biol Chem. 2000;275:30573–30585. doi: 10.1074/jbc.M004578200. [DOI] [PubMed] [Google Scholar]
- 47.Yang S, Guo X, Debnath G, Mohandas N, An X. Protein 4.1R links E-cadherin/beta-catenin complex to the cytoskeleton through its direct interaction with beta-catenin and modulates adherens junction integrity. Biochim Biophys Acta. 2009;1788:1458–1465. doi: 10.1016/j.bbamem.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kang Q, Wang T, Zhang H, Mohandas N, An X. A Golgi-associated protein 4.1B variant is required for assimilation of proteins in the membrane. J Cell Sci. 2009;122:1091–1099. doi: 10.1242/jcs.039644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maddugoda MP, Crampton MS, Shewan AM, Yap AS. Myosin VI and vinculin cooperate during the morphogenesis of cadherin cell cell contacts in mammalian epithelial cells. J Cell Biol. 2007;178:529–540. doi: 10.1083/jcb.200612042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smutny M, Cox HL, Leerberg JM, Kovacs EM, Conti MA, Ferguson C, et al. Myosin II isoforms identify distinct functional modules that support integrity of the epithelial zonula adherens. Nat Cell Biol. 2010;12:696–702. doi: 10.1038/ncb2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]