Abstract
Objective
Stem cell therapy for angiogenesis and vascular regeneration has been investigated using adult or embryonic stem cells. In the present study, we investigated the potential of endothelial cells (ECs) derived from human induced pluripotent stem cells (hiPSCs) to promote the perfusion of ischemic tissue in a murine model of peripheral arterial disease.
Methods and Results
Endothelial differentiation was initiated by culturing hiPSCs for 14 days in differentiation media supplemented with BMP-4 and VEGF. The hiPSC-ECs exhibited endothelial characteristics by forming capillary-like structures in matrigel and incorporating acetylated-LDL. They stained positively for EC markers such as KDR, CD31, CD144 and eNOS. In vitro exposure of hiPSC-ECs to hypoxia resulted in increased expression of various angiogenic related cytokines and growth factors. hiPSC-ECs were stably transduced with a double fusion construct encoded by the ubiquitin promoter, firefly luciferase for bioluminescence imaging (BLI) and green fluorescence protein (GFP) for fluorescent detection (pUb-Fluc-GFP). The hiPSC-ECs (5×105) were delivered by intramuscular injection into the ischemic hindlimb of SCID mice at day 0 and again on day 7 after femoral artery ligation (n=8). BLI showed that hiPSC-ECs survived in the ischemic limb for at least 2 weeks. In addition, laser Doppler imaging showed that the ratio of blood perfusion was increased by hiPSC-EC treatment by comparison to the saline-treated group (0.58±0.12 vs 0.44±0.04; P=0.005). The total number of capillaries in the ischemic limb of mice receiving hiPSC-EC injections was greater than those in the saline-treated group (1284 ±155 vs. 797±206 capillaries/mm2) (P<0.002).
Conclusion
This study is a first step toward development of a regenerative strategy for peripheral arterial disease based on the use of ECs derived from hiPSCs.
Keywords: induced pluripotent stem cells, differentiation, regeneration, peripheral vascular disease, endothelial cells
Introduction
Stem cell therapy for angiogenesis and vascular regeneration has been investigated previously using diverse cell types, including adult mesenchymal stem cells, endothelial progenitor cells (EPCs), embryonic stem cells (ESCs), and recently with human induced pluripotent stem cells (hiPSCs) (1-5). The hiPSCs share many similar characteristics with human embryonic stem cells (hESCs). In addition to having similar capacity for self-renewal, they are capable of differentiating into cells of all three germ layers. However, unlike hESCs, hiPSCs are derived from reprogramming of somatic cells and therefore represent a source of autologous pluripotent stem cells that may obviate the immunological concerns of hESCs. Furthermore, hiPSCs can be experimentally derived from easily accessible and plentiful human tissue sources such as skin or fat. Consequently, hiPSCs provide a suitable platform for modeling diseases in vitro and also serve as a potential source of cells for regenerative medicine.
Previously, hiPSCs have been shown to differentiate into endothelial cells (ECs) (6). However, the therapeutic potential of hiPSC-derived ECs (hiPSC-ECs) for the treatment of ischemic diseases has not been reported. In this study, we describe the differentiation of hiPSCs into ECs, and characterize their histological and functional properties in vitro. In addition, using molecular imaging and laser Doppler perfusion studies, we observe evidence of cell localization and survival in the ischemic limb in association with improved blood flow in a murine model of peripheral arterial disease (PAD). Immunohistochemical and proteomic studies indicate that hiPSCs increase microvessel density and secrete angiogenic cytokines. Our results demonstrate that fibroblast-derived hiPSCs have the potential to promote vascular regeneration in ischemic tissue.
Methods
(expanded methods section is available in the supplemental files)
Cell lines and in vitro studies
Derivation and differentiation of human induced pluripotential stem cells (hiPSCs)
The hiPSCs were derived from human foreskin fibroblasts using retroviral constructs encoding the Yamanaka factors as previously described (7). Their complete characterization is described elsewhere (Byers B, BS, unpublished data, 2010), but in addition, we performed alkaline phosphatase staining, immunohistochemistry for pluripotency markers, and teratoma assay (see supplemental files). To initiate differentiation, confluent cultures of hiPSCs were transferred to ultra low attachment dishes containing differentiation media for 4 days to form embryoid bodies (EBs). The 4-day EBs were then seeded on 0.2% gelatin-coated dishes and cultured for another 10 days in differentiation media. To purify the hiPSC-ECs, single cell suspensions were incubated with PE-conjugated anti-human CD31 antibody (Ab). Flow cytometry was then performed to obtain purified hiPSC-ECs.
Characterization of hiPSC-ECs
The hiPSC-ECs were stained with Abs against endothelial markers such as PECAM-1, VE-cadherin, endothelial nitric oxide synthase and von Willebrand factor. Uptake of acetylated LDL was assessed by incubating the cells with Dil-labeled ac-LDL. For the tube formation assay, cells were seeded on 24-well plates pre-coated with growth factor-reduced Matrigel and incubated for 24 hours. Human antibody arrays were used to assess the various cytokines secreted by the hiPSC-ECs in normoxic and hypoxic conditions.
In vivo studies
For in vivo Matrigel injection
Matrigel was mixed with bFGF and hiPSC-ECs (5×105). The mixture was subcutaneously injected into SCID mice. After fourteen days, the Matrigel plugs were removed, paraffin-embedded, sectioned and stained with CD31 Ab.
For non invasive tracking in vivo
The hiPSC-ECs and fibroblasts were transduced with a lentiviral vector carrying an ubiquitin promoter driving firefly luciferase and enhanced green fluorescence protein as described previously (9).
The therapeutic effects of hiPSC-ECs were studied in ischemic tissue
Hindlimb ischemia was induced by ligating the femoral artery of male NOD SCID mice (10). The animals were assigned to receive intramuscular (IM) injection into the gastrocnemius muscle of either saline, hiPSC-ECs, or human fibroblasts. On subsequent days, animals were injected with D-luciferin, and bioluminescence imaging(BLI) was performed to assess cell survival and location (11). Perfusion of the ischemic and non-ischemic hindlimb was assessed using laser Doppler spectroscopy. At the end of the study period, the gastrocnemius tissue was harvested, snap frozen in O.C.T. compound for cryosectioning, and stained using a mouse-specific CD31. Capillary density was assessed by counting the number of capillaries in 5 high-powered fields in each of 4 tissue sections and expressing the data as capillaries/mm2 (11). Survival of transplanted cells was visualized by staining with a vWF Ab that reacts with both murine and human capillaries. The hiPSC-ECs were detected by their coexpression of GFP and vWF. The transplanted cells were also detected using a human specific VE-cadherin Ab. All animal studies were approved by our Administrative Panel on Laboratory Animal Care.
Results
Characterization of HUF5 iPSC line
The hiPSC line was generated by retrovirus-mediated transduction of Oct-4, Sox-2, Klf-4 and c-Myc using primary human adult dermal fibroblasts obtained from a healthy 46-year old female. The hiPSC colonies expressed pluripotency markers such as SSEA3/4, TRA-1-81, TRA-I-60, Nanog and alkaline phosphatase (Suppl. Fig Ia) and formed teratomas in vivo (Suppl. Fig Ib).
Isolation and characterization of hiPSC-ECs
The hiPSC-ECs were isolated by FACS after 2 weeks of differentiation and then expanded for further characterization. The typical yield of ECs generated from the HUF5 hiPSC line ranged between 5% to 20% (Figure 1a). The expanded hiPSC-ECs formed a ‘cobblestone’ monolayer, and immunofluorescence staining revealed that these cells were positive for endothelial markers such as CD31, CD144, endothelial nitric oxide synthase and von Willebrand factor (Figure 1b-e). In addition, they were able to incorporate acetylated LDL and form networks of tubular structures after 24 hours of culture on matrigel (Figure 1f-g). Furthermore, when hiPSC-ECs were placed in matrigel, and injected subcutaneously in immunodeficient mice, they formed capillaries (Figure 1h). These capillaries were continuous with the murine systemic circulation as they contained blood cells (Insert, Figure 1h).
Figure 1.
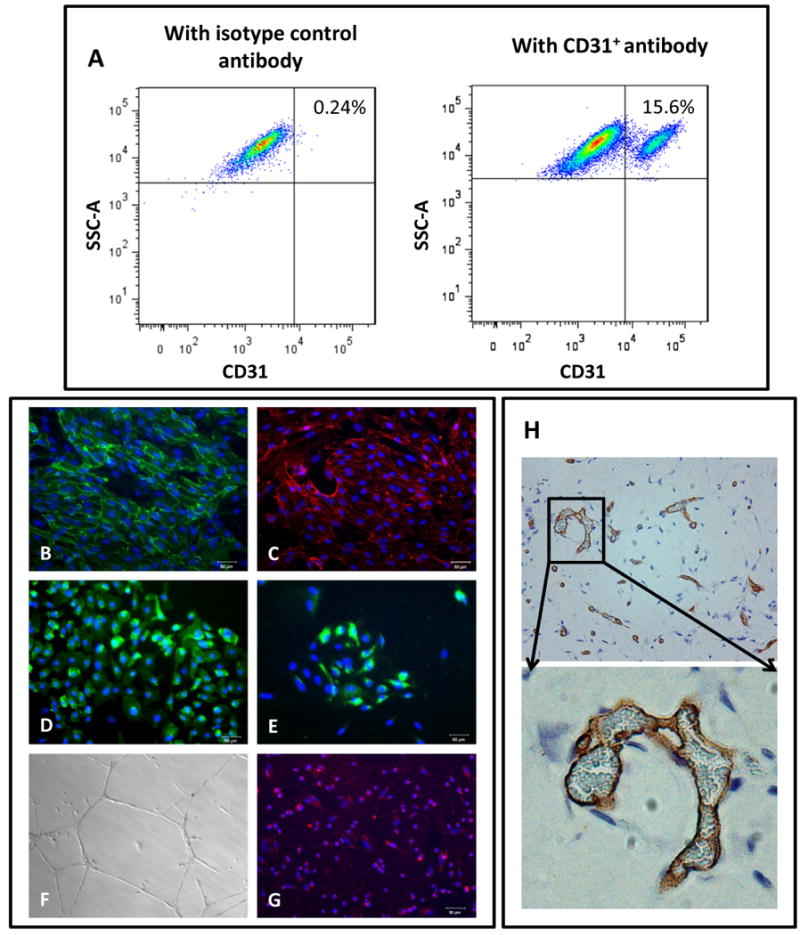
Derivation and characterization of hiPSC-ECs. A) [Left panel] Isotype control. [Right panel] FACS plot of data obtained using CD31+ antibody to quantitate ECs derived from hiPSCs B) to E) Immunofluorescent staining of the hiPSC-ECs for endothelial markers CD31, CD144, eNOS and vWF. F) hiPSC-ECs form a capillary-like network on Matrigel 24 hours after seeding the cells. G) hiPSC-ECs take up acetylated LDL. Scale bar: 50μm. H) in vivo formation of capillary structures in matrigel plugs after subcutaneous transplantation in SCID mice, stained using an Ab specific for human CD31.
Derivation and characterization of transduced hiPSC-ECs
The hiPSC-ECs were stably transduced with the double fusion reporter construct (Suppl. Fig II) and FACS sorted for dual expression of GFP and CD31. Less than 20% of the hiPSC-ECs expressed both GFP and CD31 after transduction, but after several passages, about 75% were GFP and CD31 positive before cell transplantation (Figure 2a). They maintained the cobblestone morphology in culture and manifested mRNA and protein expression of CD31 and CD34 (Figure 2b-d). There was a strong correlation between the Fluc activity and cell number (R2=0.99; Figure 2e).
Figure 2.
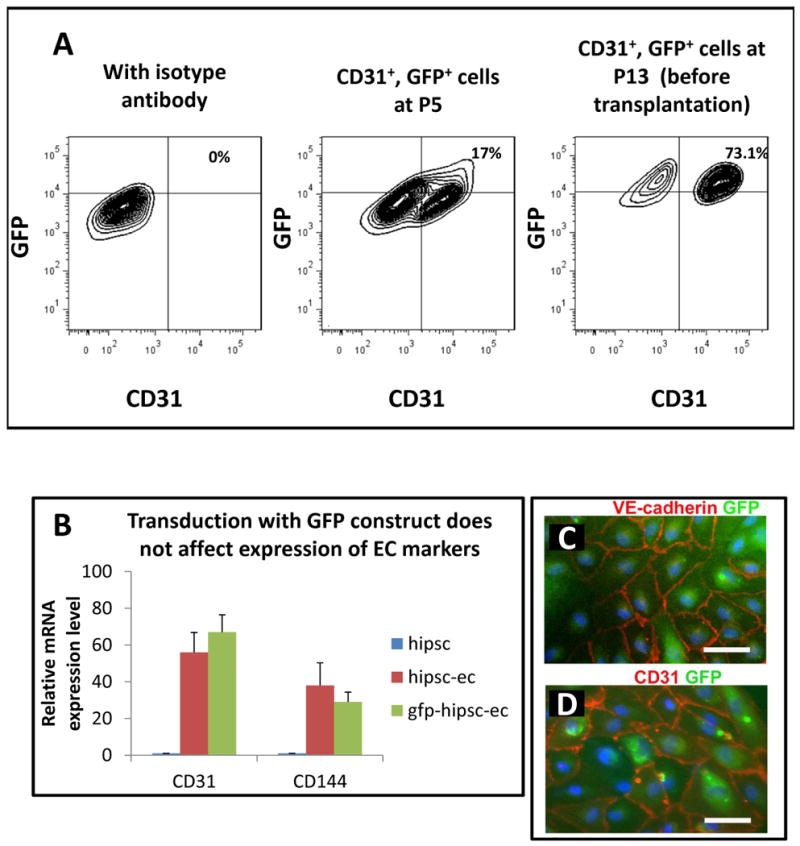
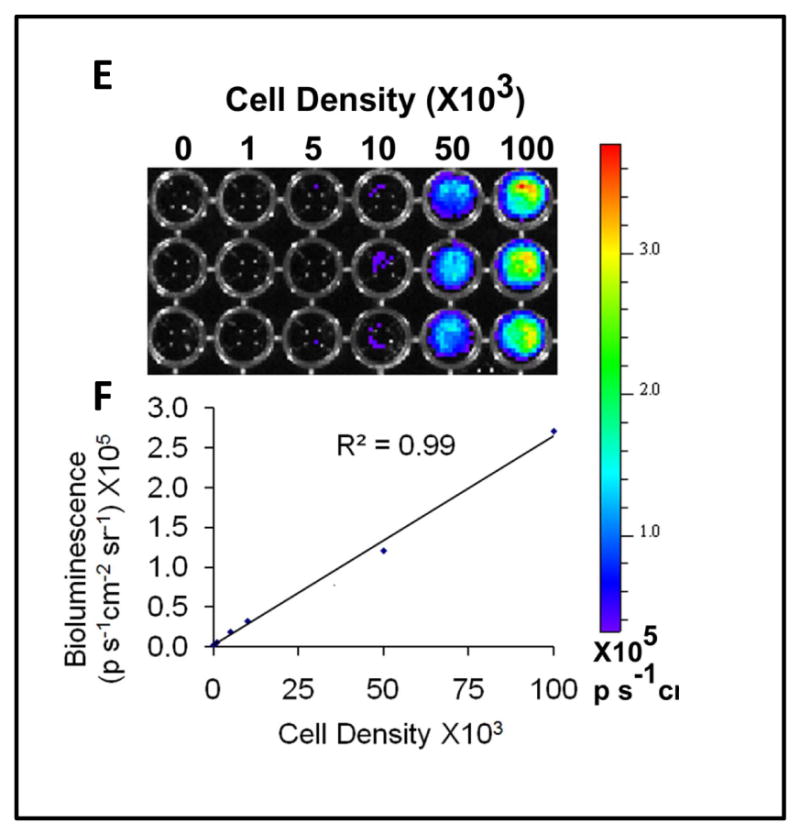
Characterization of hiPSC-ECs transduced with lentiviral construct for bioluminescence imaging and histological detection. A) hiPSCs-ECs previously transduced with a construct encoding luciferase and GFP were sorted by FACS using GFP and CD31 expression at P5, and again at P13 before injection into the experimental animals. B) The transduced hiPSC-ECs (but not the parental hiPSCs) express endothelial genes as confirmed by RT-PCR for CD31 and CD144. C) and D) Immunofluorescence staining of GFP, CD31 and CD144 in the purified transduced hiPSC-ECs. E) and F) Quantification of the correlation between the cell number and the BLI signal (R=0.99). Scale bar: 50μm
Survival of hiPSC-ECs in the ischemic hindlimb
In initial studies, we injected single dosages of 5×105 hiPSC-ECs to the ischemic hindlimb only on day 0 and tracked the survival of the cells over 14 days. We observed a decline in BLI that became undetectable by day 11 (Suppl. Figure IX). For this reason, for the remainder of the studies, we administered cells on day 0 as well as day 7.
Upon transplantation into the ischemic limb, the hiPSC-ECs could be detected in the ischemic limb non-invasively by BLI (Figure 3). After each of the injections on day 0 and on day 7, there was an increase in bioluminescence intensity, followed by a gradual decrease over several days (Figure 3b).
Figure 3.
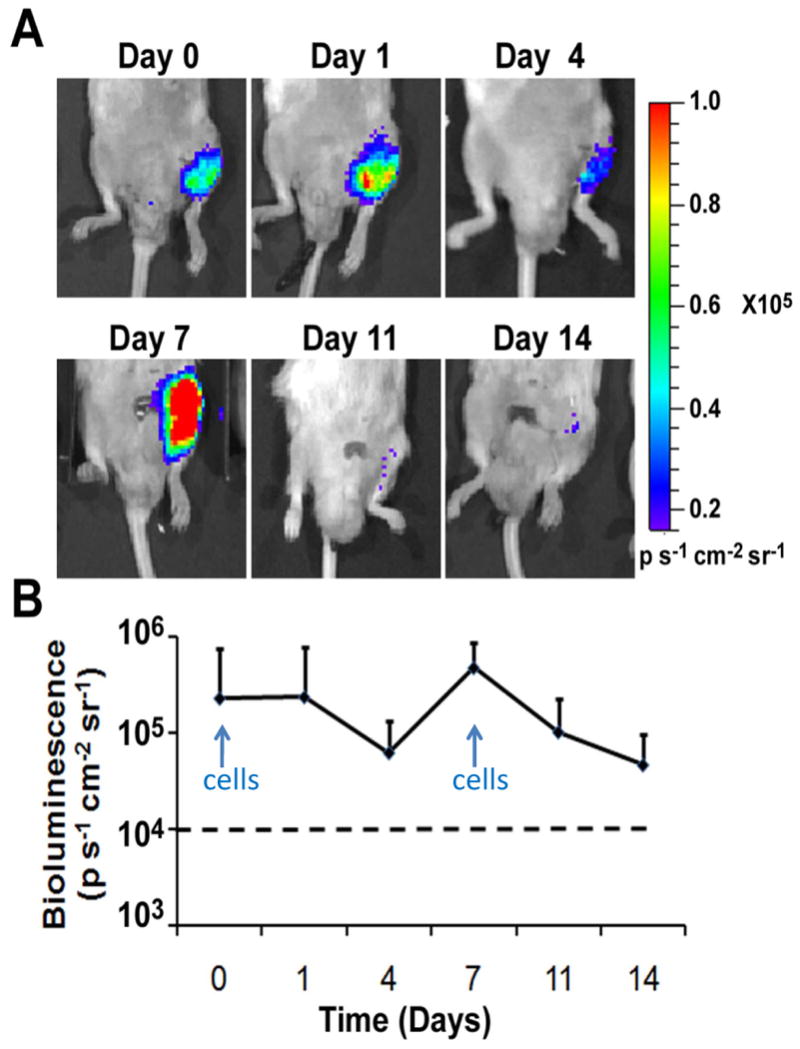
Localization and survival of hiPSC-ECs in the ischemic limb. A) hiPSC-ECs were delivered by IM injection of the ischemic limb and were tracked non-invasively by BLI. B) Quantification of BLI signals in the ischemic limb of mice after cell injection. Dashed line refers to the threshold to discriminate background noise from a positive BLI signal.
Additional experiments were carried out in which ischemic hindlimbs of SCID mice were injected on days 0 and 7 with human fibroblasts that were transduced by the same lentiviral vector for Fluc expression. As shown in Suppl. Figure III, the fibroblast bioluminescence also correlated strongly with cell number. Like hiPSC-ECs, there was a decline in bioluminescence signal during the first 4 days, followed by an increase after the second dose of cell injection on day 7, and then a gradual decrease with time. In contrast to hiPSC-ECs, the fibroblasts were detected for up to 4 weeks in the ischemic limb (Suppl. Figures IV-VI).
Improvement of blood perfusion in ischemic hindlimbs by hiPSC-EC transplantation
Laser Doppler perfusion imaging was performed to determine the effects of localized transplantation of hiPSC-EC on the ischemic hindlimb at days 0, 4, 7 and 14 post-treatment. The hindlimb perfusion ratio (ischemic/control hindlimb) was significantly improved in the hiPSC-EC treated mice by comparison to the saline-treated mice (P=0.005; Figure 4a-b).
Figure 4.
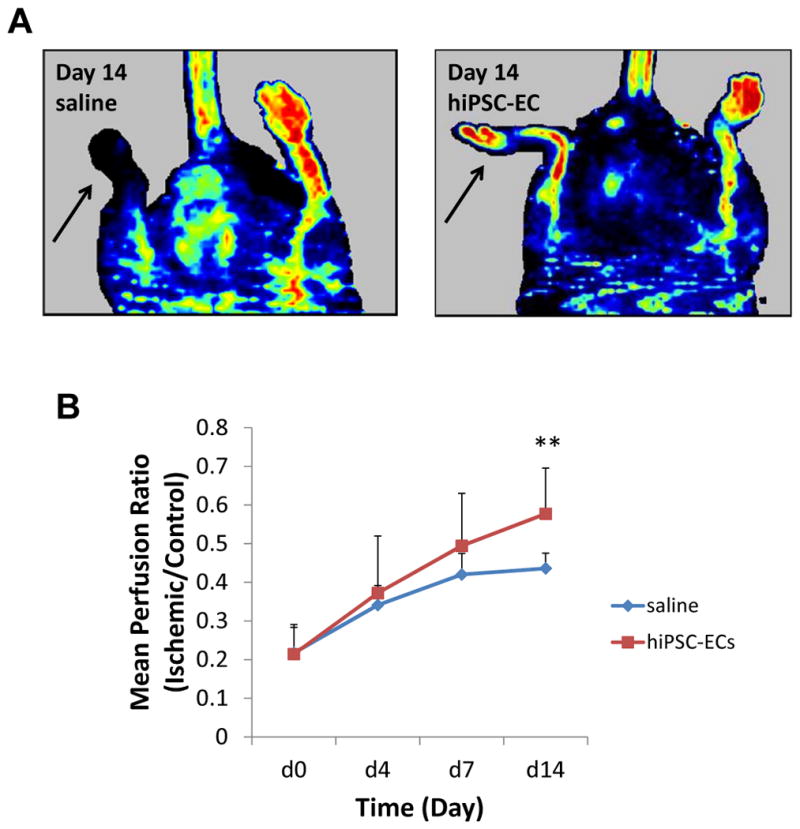
Improvement in blood perfusion in the ischemic hindlimb after hiPSC-EC transplantation. A) Images of laser doppler perfusion imaging at day 14 after treatment. A greater increase in perfusion is observed in the ischemic limb (arrow) of the mouse that received hiPSC-EC transplantation by comparison to the saline-treated animal. B) Perfusion ratio of ischemic limbs at day 14 after treatment. The perfusion ratio (value of the ischemic limb divided by that of the non-ischemic limb) was greater in mice that received hiPSC-EC transplantation compared to those that received saline (n=8 each group, *P<0.05).
To assess the survival and effect of hiPSC-ECs with a longer time course, and in comparison to treatment with fibroblasts, additional experiments were carried out to 4 weeks post-injection. The cells in this experiment were not transduced with Fluc to preclude the possible effects of lentiviral vectors on cell behavior. As shown in Suppl. Figure VI, the mean perfusion ratio of the hiPSC-EC-treated group (0.53±0.10) was significantly greater after 4 weeks, than that of the saline- or fibroblast-treated groups (0.37±0.04 and 0.42±0.07; P=0.009 and P=0.003 respectively). This data suggests that hiPSC-ECs were therapeutic cells that enhanced limb perfusion for up to 4 weeks post-delivery.
Improved blood capillary density upon hiPSC-EC transplantation
To verify the laser Doppler data, we quantified capillary density by immunofluorescence staining. Murine capillary density in the 2-week cell-treated group was greater than that of the saline-treated group (Figure 5a-b). Dual staining for both GFP and vWF demonstrated the persistence of hiPSC-ECs in the ischemic limb at low frequency (Figure 6a). Human-derived VE-cadherin positive cells in capillaries were also occasionally observed in the tissue sections (Suppl. Figure VII). These results suggest that the therapeutic effect of hiPSC-ECs may be related to its ability to incorporate into and/or enhance expansion of the endogenous microvasculature.
Figure 5.
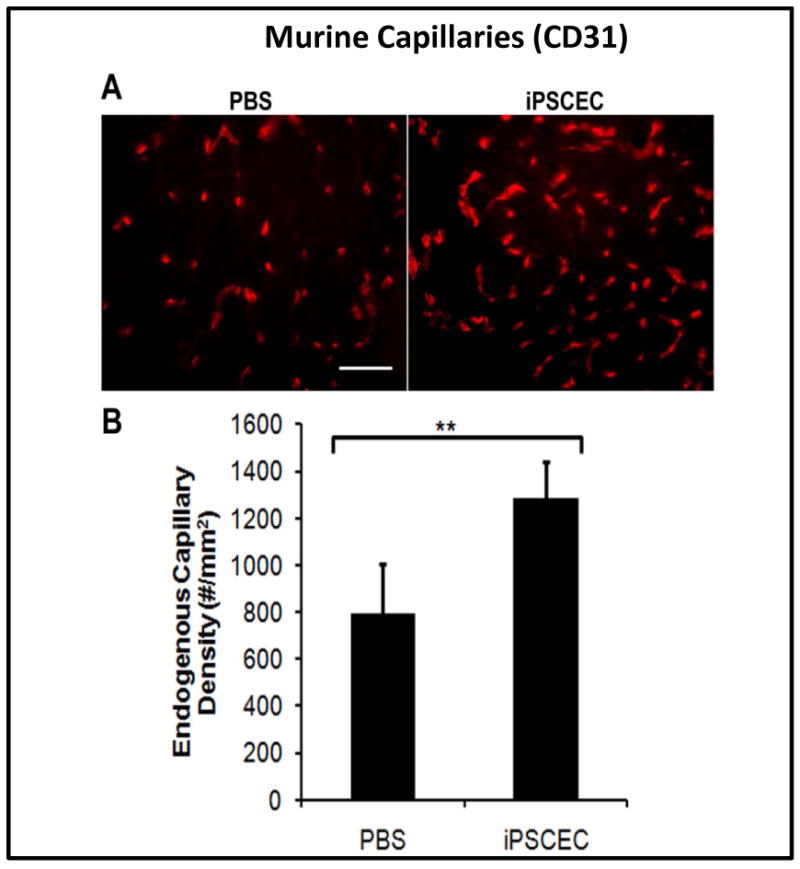
Improved neovascularization in the ischemic limbs of mice receiving hiPSC-ECs after 14 days. A) Immunofluorescent CD31 staining of ischemic tissues from mice treated with saline or hiPSC-ECs. B) Quantification of total capillary density in the ischemic limbs. Capillary formation was significantly enhanced by injection of hiPSC-ECs by comparison to saline (**P<0.05).
Figure 6.
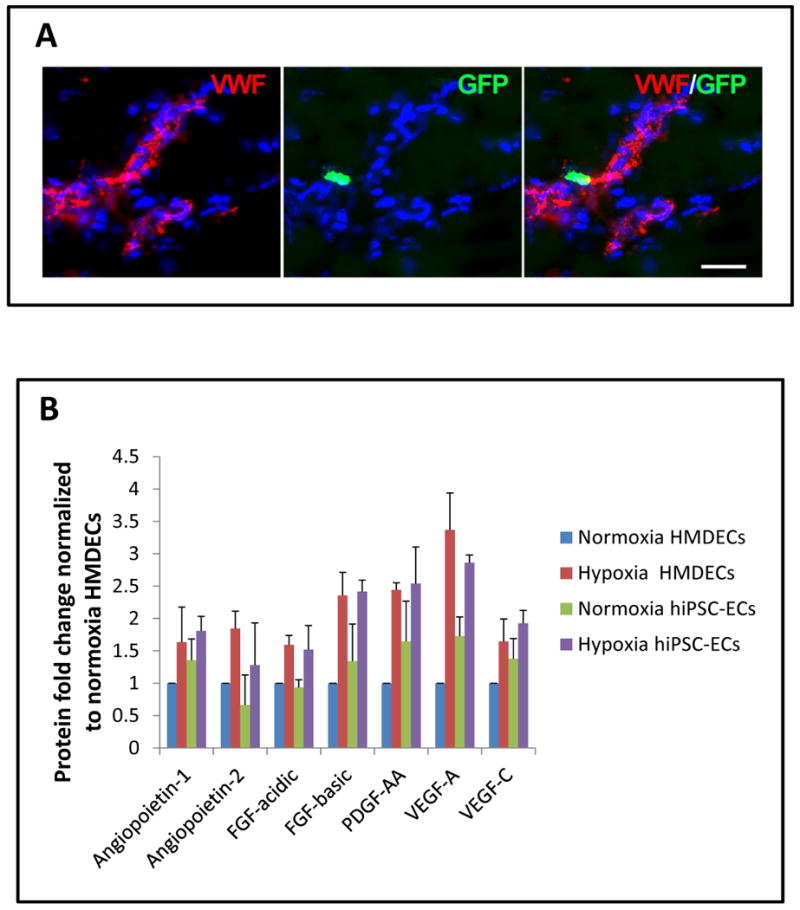
hiPSC-ECs may also have paracrine effects on microvasculature A) Immunofluorescent staining endothelial marker, vWF(red), GFP (green) and Hoechst nuclear dye (blue) showing GFP+hiPSC-ECs in close proximity to capillaries in the ischemic hindlimb. B) Growth factors and angiogenic cytokines are produced by hiPSC-ECs, and are significantly upregulated with hypoxia. By comparison to human endothelial cells, the growth factor expression of hiPSC-ECs is similar.
Angiogenic cytokines secreted by the transplanted hiPSC-ECs in the ischemic hindlimbs
To further understand the mechanism by which hiPSC-ECs enhanced angiogenesis in the ischemic limbs, the cells were assessed in vitro for the expression of angiogenic cytokines under hypoxic and normoxic conditions. Indeed, hiPSC-ECs expressed a majority of the angiogenic cytokines, in some cases at higher levels of expression than primary HMDECs. These cytokines include Angiopoietin-1, VEGF-A, VEGF-C and PDGF-AA (Figure 6b). The results for the entire angiogenic protein array of 55 cytokines and growth factors are shown in Suppl. Figure VIII. This upregulation of angiogenic cytokines by the hiPSC-ECs in hypoxia is consistent with the enhanced blood perfusion and capillary density in the ischemic limbs that received hiPSC-ECs.
Discussion
Somatic cells may be reprogrammed into iPSCs using induced expression of transcriptional factors involved in maintaining pluripotency (7,12). Expression of Oct3/4, Sox2, Klf4 and c-Myc in somatic cells, reprograms these cells as evidenced by the relengthening of the telomeres (13,14) and the acquisition of pluripotency similar to that of embryonic stem cells (15). Thus, hiPSCs represent a promising cell source for regenerative medicine. These cells are particularly attractive since they can be used to generate patient specific pluripotent cells that do not face an immunological barrier. However, the therapeutic potential of hiPSCs remains largely untested. Recently, hiPSC-derived neurons, cardiomyocytes, and mesenchymal stem cells have shown therapeutic promise in pre-clinical studies(16,17,18). The present study is the first to assess the potential of hiPSC-derived endothelial cells for ischemic vascular disease. We find that hiPSCs can be differentiated into ECs as indicated by typical cobblestone monolayer morphology; expression of EC surface markers and cytoplasmic factors; and manifestation of characteristic endothelial functions (acetylated LDL uptake and capillary-like network formation in matrigel).
When these cells were delivered to the ischemic limb of the mouse by intramuscular injection, they increased increased capillary density and limb perfusion. In contrast, injection of fibroblasts did not improve limb perfusion by comparison to vehicle. We observed some hiPSC-ECs that appeared to incorporate into the microvasculature and others that were in close proximity. However, the observed increase in capillary density could not be accounted for simply by the incorporation of hiPSC-EC into the existing vasculature. Instead, it is likely that the injected cells had a paracrine effect. Indeed, we find that the hiPSC-ECs secrete angiogenic cytokines and growth factors in the presence of hypoxia, as well or better than primary human endothelial cells.
One concern in using iPSC-derived cells is the possibility of contamination of the therapeutic cells with parental iPSCs, which could lead to teratoma formation. We did not observe any tumor formation in any of our animals receiving hiPSC-ECs. However, longer term studies with greater numbers of mice, and/or more sensitive probes to detect undifferentiated cells, are necessary to provide greater assurance that the differentiation process is complete. Also, further study is needed to resolve concerns regarding the possible adverse effects of abnormal imprinting, copy number variation or other somatic mutations arising from the reprogramming or differentiation process.
Using molecular imaging, we observed a reduction in cell number over time in the ischemic limb. Bioluminescence imaging is a sensitive and accurate method for tracking cells in vivo with as few as 500 cells (9). We found that the number of transplanted cells began to decrease within 24 hours post-transplantation. In order to improve the efficacy of our hiPSC-EC transplantation, we did a second injection at day 7 post-surgery. The transplanted cells face the serious challenge of an unfriendly environment characterized by reduced oxygen and nutrient supply and the presence of various cytotoxic and inflammatory products in the ischemic limb. In addition, although we are using an immunodeficient mouse model, the human cells may stimulate a mild immune reaction and/or may not be receiving appropriate signaling required for their efficient integration into the mouse vasculature.
Adult stem cells (such as those derived from the bone marrow, or circulating mononuclear cells, or mesenchymal stem cells) have been employed in small clinical trials of patients with myocardial ischemia or peripheral arterial disease (19-24). Only a few of these trials have been randomized, with large enough numbers of subjects, followed for sufficient period of time, to draw conclusions. These early data have shown proof of concept that cell therapy can provide some benefit in the setting of ischemic syndromes. However, adult stem cells such as endothelial progenitor cells (EPCs) have limited replicative capacity, and are few in number and dysfunctional, in elderly patients with cardiovascular risk factors (25-27). By contrast hESCs have unlimited capacity to replicate, can be differentiated into ECs, and in pre-clinical studies have shown beneficial effects in the setting of myocardial or limb ischemia (28-34). Previously we have observed that ESC-derived ECs can home to sites of peripheral ischemia, incorporate into the microvasculature, increase capillary density and improve limb perfusion. However, there is an immunogenic barrier for ESC-derivatives and this can pose a challenge in clinical application.
By contrast, hiPSCs can be used to generate autologous therapies, minimizing or eliminating the need for immune suppression after cell transplantation. However, before hiPSCs can be considered for clinical applications, a number of technical issues need to be addressed. Currently most hiPSCs are generated using integrating viral vectors (7,12). However, integration of foreign DNA could inadvertently silence indispensable genes, or generate an oncogenic phenotype. Thus, clinical grade therapeutic cells might be derived using nonintegrating episomal vector systems (35), cell permeant peptides (36,37), RNA (38) and/or small molecules (39), or RNA-based approaches (40). In addition, the efficiency and speed of hiPSC generation should be increased, and the fidelity of reprogramming (as assessed by epigenetic, genomic, proteomic and functional studies) must be assured. In this regard, we and others note heterogeneity between hiPSC clones in differentiation potential. These differences are more exaggerated in hiPSC as compared to hESC clones (41). The reasons for the reduced tendency to differentiate down some lineages in some hiPSC lines are not fully known, but may include factors such as persistent ‘epigenetic memory’ as recently described (42,43). Differentiation to functional lineages must be assured, and the risk reduced of transplanting pluripotential or suboptimally differentiated cells. Finally, it is unclear how iPSC-ECs compare in therapeutic efficacy to other clinically relevant cell types such as bone marrow mononuclear cells, endothelial progenitor cells, and mesenchymal stem cells, which each enhance blood flow recovery and improve angiogenesis in the murine hindlimb ischemia model (44-47; Suppl. Table I).
In conclusion, this is the first study to demonstrate that functional human ECs may be differentiated from hiPSCs, as evidenced by in vitro characterization and in vivo application in a murine model of peripheral arterial disease. This is another step forward toward developing hiPSC-derived cell therapy for vascular regeneration.
Supplementary Material
Acknowledgments
This study was supported by grants to J.P.C. from National Institutes of Health (U01HL100397, RC2HL103400,1K12HL087746), the California Tobacco Related Disease Research Program of the University of California (18XT0098), and the American Heart Association (0970036N). R.A.J. was supported by the National University of Singapore Overseas Postdoctoral Fellowship. N.F.H. was supported by a grant from the National Institutes of Health (HL098688).
Footnotes
Disclosures: None.
References
- 1.Jujo K, Ii M, Losordo DW. Endothelial progenitor cells in neovascularization of infarcted myocardium. J Mol Cell Cardiol. 2008;45:530–544. doi: 10.1016/j.yjmcc.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ikenaga S, Hamano K, Nishida M, Kobayashi T, Li TS, Kobayashi S, Matsuzaki M, Zempo N, Esato K. Autologous bone marrow implantation induced angiogenesis and improved deteriorated exercise capacity in a rat ischemic hindlimb model. J Surg Res. 2001;96:277–283. doi: 10.1006/jsre.2000.6080. [DOI] [PubMed] [Google Scholar]
- 3.Kamihata H, Matsubara H, Nishiue T, Fujiyama S, Tsutsumi Y, Ozono R, Masaki H, Mori Y, Iba O, Tateishi E, Kosaki A, Shintani S, Murohara T, Imaizumi T, Iwasaka T. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation. 2001;104:1046–1052. doi: 10.1161/hc3501.093817. [DOI] [PubMed] [Google Scholar]
- 4.Yamahara K, Sone M, Itoh H, Yamashita J, Yurugi-K T, Homma K, Chao TH, Miyashita K, Park K, Oyamada N, Sawada N, Taura D, Fukunaga Y, Tamura N, Nakao K. Augmentation of Neovascularization in hindlimb ischemia by combined transplantation of human embryonic stem cells-derived endothelial and mural cells. PLOS one. 2008;3:e1666. doi: 10.1371/journal.pone.0001666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cho SW, Moon SH, Lee SH, Kang SW, Kim J, Lim JM, Kim HS, Kim BS, Chung HM. Improvement of Postnatal Neovascularization by Human Embryonic Stem Cell–Derived Endothelial-Like Cell Transplantation in a Mouse Model of Hindlimb Ischemia. Circulation. 2007;116:2409–2419. doi: 10.1161/CIRCULATIONAHA.106.687038. [DOI] [PubMed] [Google Scholar]
- 6.Daisuke T, Masakatsu S, Koichiro H, Naoumi O, Kazutoshi T, Naohisa T, Shinya Y, Kazuwa N. Induction and isolation of vascular cells from human induced pluripotent stem cells- brief report. Arterioscler Thromb Vasc Biol. 2009;29:1100–1103. doi: 10.1161/ATVBAHA.108.182162. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 8.Byrne JA, Nguyen HN, Reijo Pera RA. Enhanced generation of induced pluripotent stem cells from a subpopulation of human fibroblasts. PLOS one. 2009;4:e7118. doi: 10.1371/journal.pone.0007118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao F, van der Bogt KE, Sadrzadeh A, Xie X, Sheikh AY, Wang H, Connolly AJ, Robbins RC, Wu JC. Spatial and temporal kinetics of teratoma formation from murine embryonic stem cell transplantation. Stem Cells Dev. 2007;6:883–91. doi: 10.1089/scd.2007.0160. [DOI] [PubMed] [Google Scholar]
- 10.Niiyama H, Huang NF, Rollins MD, Cooke JP. Murine model of hindlimb ischemia. J Vis Exp. 2009;23:1035. doi: 10.3791/1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang NF, Niiyama H, Peter C, De A, Natkunam Y, Fleissner F, Li Z, Rollins MD, Wu JC, Gambhir SS, Cooke JP. Embryonic stem cell-derived endothelial cells engraft into the ischemic hindlimb and restore perfusion. Arterioscler Thromb Vasc Biol. 2010;30:984–91. doi: 10.1161/ATVBAHA.110.202796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu JY, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 13.Marion RM, Strati K, Li H, Tejera A, Schoeftner S, Ortega S, Serrano M, Blasco MA. Telomeres acquire embryonic stem cell characteristics in induced pluripotent stem cells. Cell Stem Cell. 2009;4:141–54. doi: 10.1016/j.stem.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 14.Suhr ST, Chang EA, Rodriguez RM, Wang K, Ross PJ, Beyhan Z, Murthy S, Cibelli JB. Telomere dynamics in human cells reprogrammed to pluripotency. PLoS One. 2009;4:e8124. doi: 10.1371/journal.pone.0008124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva J, Barrandon O, Nichols J, Kawaguchi J, Theunissen TW, Smith A. Promotion of reprogramming to ground state pluripotency by signal inhibition. PLoS Biol. 2008;6:e253. doi: 10.1371/journal.pbio.0060253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wernig M, Zhao JP, Pruszak J, Hedlund E, Fu D, Soldner F, Broccoli V, Constantine-Paton M, Isacson O, Jaenisch R. Neurons derived from reprogrammed fibroblasts functionally integrate into the fetal brain and improve symptoms of rats with Parkinson's disease. Proc Natl Acad Sci U S A. 2008;105:5856–5861. doi: 10.1073/pnas.0801677105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson TJ, Martinez-Fernandez A, Yamada S, Perez-Terzic C, Ikeda Y, Terzic A. Repair of acute myocardial infarction by human stemness factors induced pluripotent stem cells. Circulation. 2009;120:408–16. doi: 10.1161/CIRCULATIONAHA.109.865154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Zhang Y, Lam FF, Kang S, Xia JC, Lai WH, Au KW, Chow YY, Siu CW, Lee CN, Tse HF. Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 2010;121:1113–23. doi: 10.1161/CIRCULATIONAHA.109.898312. [DOI] [PubMed] [Google Scholar]
- 19.Strauer BE, Brehm M, Zeus T, Kostering M, Hernandez A, Sorg RV, Kogler G, Wernet P. Repair of infarcted myocardium by autologous intracoronary mononuclear bone marrow cell transplantation in humans. Circulation. 2002;106:1913–1918. doi: 10.1161/01.cir.0000034046.87607.1c. [DOI] [PubMed] [Google Scholar]
- 20.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone marrow cell transfer after myocardial infarction: the BOOST randomized controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 21.Schachinger V, Erbs S, Elsasser A, Haberbosch W, Hambrecht R, Holschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Suselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 22.Erbs S, Linke A, Schachinger V, Assmus B, Thiele H, Diederich KW, Hoffmann C, Dimmeler S, Tonn T, Hambrecht R, Zeiher AM, Schuler G. Restoration of microvascular function in the infarct-related artery by intracoronary transplantation of bone marrow progenitor cells in patients with acute myocardial infarction: the Doppler Substudy of the Reinfusion of Enriched Progenitor Cells and Infarct Remodeling in Acute Myocardial Infarction (REPAIR-AMI) trial. Circulation. 2007;116:366–374. doi: 10.1161/CIRCULATIONAHA.106.671545. [DOI] [PubMed] [Google Scholar]
- 23.Tateishi-Yuyama E, Matsubara H, Murohara T, Ikeda U, Shintani S, Masaki H, Amano K, Kishimoto Y, Yoshimoto K, Akashi H, Shimada K, Iwasaka T, Imaizumi T. Therapeutic angiogenesis for patients with limb ischaemia by autologous transplantation of bone-marrow cells: a pilot study and a randomised controlled trial. Lancet. 2002;360:427–435. doi: 10.1016/S0140-6736(02)09670-8. [DOI] [PubMed] [Google Scholar]
- 24.Higashi Y, Kimura M, Hara K, Noma K, Jitsuiki D, Nakagawa K, Oshima T, Chayama K, Sueda T, Goto C, Matsubara H, Murohara T, Yoshizumi M. Autologous bone-marrow mononuclear cell implantation improves endothelium-dependent vasodilation in patients with limb ischemia. Circulation. 2004;109:1215–1218. doi: 10.1161/01.CIR.0000121427.53291.78. [DOI] [PubMed] [Google Scholar]
- 25.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, Finkel T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 26.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 27.Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, Martin H, Zeiher AM, Dimmeler S. Profoundly reduced neovascularization capacity of bone marrow mononuclear cells derived from patients with chronic ischemic heart disease. Circulation. 2004;109:1615–22. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 28.Nourse MB, Halpin DE, Scatena M, Mortisen DJ, Tulloch NL, Hauch KD, Torok-Storb B, Ratner BD, Pabon L, Murry CE. VEGF induces differentiation of functional endothelium from human embryonic stem cells: implications for tissue engineering. Arterioscler Thromb Vasc Biol. 2010;30:80–9. doi: 10.1161/ATVBAHA.109.194233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kane NM, Meloni M, Spencer HL, Craig MA, Strehl R, Milligan G, Houslay MD, Mountford JC, Emanueli C, Baker AH. Derivation of endothelial cells from human embryonic stem cells by directed differentiation: analysis of microRNA and angiogenesis in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2010;30:1389–97. doi: 10.1161/ATVBAHA.110.204800. [DOI] [PubMed] [Google Scholar]
- 30.Sone M, Itoh H, Yamahara K, Yamashita JK, Yurugi-Kobayashi T, Nonoguchi A, Suzuki Y, Chao TH, Sawada N, Fukunaga Y, Miyashita K, Park K, Oyamada N, Sawada N, Taura D, Tamura N, Kondo Y, Nito S, Suemori H, Nakatsuji N, Nishikawa S, Nakao K. Pathway for differentiation of human embryonic stem cells to vascular cell components and their potential for vascular regeneration. Arterioscler Thromb Vasc Biol. 2007;27:2127–34. doi: 10.1161/ATVBAHA.107.143149. [DOI] [PubMed] [Google Scholar]
- 31.James D, Nam HS, Seandel M, Nolan D, Janovitz T, Tomishima M, Studer L, Lee G, Lyden D, Benezra R, Zaninovic N, Rosenwaks Z, Rabbany SY, Rafii S. Expansion and maintenance of human embryonic stem cell–derived endothelial cells by TGFb inhibition is Id1 dependent. Nat Biotechnol. 2010;28:161–6. doi: 10.1038/nbt.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:4391–6. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rufaihah AJ, Haider HK, Heng BC, Ye L, Tan RS, Toh WS, Tian XF, Sim EK, Cao T. Therapeutic angiogenesis by transplantation of human embryonic stem cell-derived CD133+ endothelial progenitor cells for cardiac repair. Regen Med. 2010;5:231–44. doi: 10.2217/rme.09.83. [DOI] [PubMed] [Google Scholar]
- 34.Li Z, Wilson KD, Smith B, Kraft DL, Jia F, Huang M, Xie X, Robbins RC, Gambhir SS, Weissman IL, Wu JC. Functional and transcriptional characterization of human embryonic stem cell-derived endothelial cells for treatment of myocardial infarction. PLoS One. 2009;4:e8443. doi: 10.1371/journal.pone.0008443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Okita K, Nakagawa M, Hyenjong H, Ichisaka T, Yamanaka S. Generation of mouse induced pluripotent stem cells without viral vectors. Science. 2008;322:949–53. doi: 10.1126/science.1164270. [DOI] [PubMed] [Google Scholar]
- 36.Zhou H, Wu S, Joo JY, Zhu S, Han DW, Lin T, Trauger S, Bien G, Yao S, Zhu Y, Siuzdak G, Schöler HR, Duan L, Ding S. Generation of induced pluripotent stem cells using recombinant proteins. Cell Stem Cell. 2009;4:381–4. doi: 10.1016/j.stem.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim D, Kim CH, Moon JI, Chung YG, Chang MY, Han BS, Ko S, Yang E, Cha KY, Lanza R, Kim KS. Generation of human induced pluripotent stem cells by direct delivery of reprogramming proteins. Cell Stem Cell. 2009;4:472–6. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, Daley GQ, Brack AS, Collins JJ, Cowan C, Schlaeger TM, Rossi DJ. Highly Efficient Reprogramming to Pluripotency and Directed Differentiation of Human Cells with Synthetic Modified mRNA. Cell Stem Cell. 2010 doi: 10.1016/j.stem.2010.08.012. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi Y, Desponts C, Do JT, Hahm HS, Schöler HR, Ding S. Induction of pluripotent stem cells from mouse embryonic fibroblasts by Oct4 and Klf4 with small-molecule compounds. Cell Stem Cell. 2008;3:568–74. doi: 10.1016/j.stem.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Yakubov E, Rechavi G, Rozenblatt S, Givol D. Reprogramming of human fibroblasts to pluripotent stem cells using mRNA of four transcription factors. Biochem Biophys Res Commun. 2010 Mar 26;394(1):189–93. doi: 10.1016/j.bbrc.2010.02.150. Epub 2010 Feb 25. [DOI] [PubMed] [Google Scholar]
- 41.Belmonte JC, Ellis J, Hochedlinger K, Yamanaka S. Induced pluripotent stem cells and reprogramming: seeing the science through the hype. Nat Rev Genet. 2009;10:878–83. doi: 10.1038/nrg2700. [DOI] [PubMed] [Google Scholar]
- 42.Marchetto MC, Yeo GW, Kainohana O, Marsala M, Gage FH, Muotri AR. Transcriptional signature and memory retention of human-induced pluripotent stem cells. PLoS One. 2009;4:e7076. doi: 10.1371/journal.pone.0007076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P, Kim J, Aryee MJ, Ji H, Ehrlich LI, Yabuuchi A, Takeuchi A, Cunniff KC, Hongguang H, McKinney-Freeman S, Naveiras O, Yoon TJ, Irizarry RA, Jung N, Seita J, Hanna J, Murakami P, Jaenisch R, Weissleder R, Orkin SH, Weissman IL, Feinberg AP, Daley GQ. Epigenetic memory in induced pluripotent stem cells. Nature. 2010;467:285–90. doi: 10.1038/nature09342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ikenaga S, Hamano K, Nishida M, Kobayashi T, Li TS, Kobayashi S, Matsuzaki M, Zempo N, Esato K. Autologous bone marrow implantation induced angiogenesis and improved deteriorated exercise capacity in a rat ischemic hindlimb model. J Surg Res. 2001;96:277–283. doi: 10.1006/jsre.2000.6080. [DOI] [PubMed] [Google Scholar]
- 45.Kalka C, Masuda H, Takahashi T, Kalka-Moll W, Silver M, Kearney M, Li T, Isner J, Asahara T. Transplantation of ex-vivo expanded endothelial progenitor cells for therapeutic neovascularization. Proc Natl Acad Sci USA. 2000;97:3422–3427. doi: 10.1073/pnas.070046397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner J, Asahara T. Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 47.Suuronen EJ, Veinot JP, Wong S, Kapila V, Price J, Griffith M, Mesana TG, Ruel M. Tissue-engineered injectable collagen-based matrices for improved cell delivery and vascularization of ischemic tissue using CD133+ progenitors expanded from the peripheral blood. Circulation. 2006;114(1 Suppl):I138–44. doi: 10.1161/CIRCULATIONAHA.105.001081. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


