Abstract
Regular exercise can improve sleep quality, but for whom and by what means this occurs remain unclear. We examined moderators and mediators of objective sleep improvements in a 12-month randomized controlled trial among initially underactive midlife and older adults reporting mild/moderate sleep complaints. Participants (N=66, 67% women, 55–79 years) were randomized to moderate-intensity exercise or health education control. Putative moderators were gender, age, and baseline physical function, self-reported global sleep quality, and physical activity levels. Putative mediators were changes in BMI, depressive symptoms, and physical function at 6 months. Objective sleep outcomes measured by in-home PSG were percent time in Stage 1 sleep, percent time in Stage 2 sleep, and number of awakenings during the first third of sleep at 12 months. Baseline physical function and sleep quality moderated changes in Stage 1 sleep; individuals with higher initial physical function (p=0.01) and poorer sleep quality (p=0.03) had greater improvements. Baseline physical activity level moderated changes in Stage 2 sleep (p=0.04) and number of awakenings (p=0.01); more sedentary individuals had greater improvements. Decreased depressive symptoms (CI:−1.57 to −0.02) mediated change in Stage 1 sleep. Decreased depressive symptoms (CI: −0.75 to −0.01), decreased BMI (CI:−1.08 to −0.06), and increased physical function (CI:0.01 to 0.72) mediated change in number of awakenings. In conclusion, initially less active individuals with higher initial physical function and poorer sleep quality improved the most. Affective, functional, and metabolic mediators specific to different parameters of sleep architecture were suggested. Collectively, the results indicate strategies to more efficiently treat poor sleep through exercise in older adults.
Keywords: objective sleep, exercise, physical activity, multiple mediation, moderation
The majority of Americans over the age of 55 reports mild or more serious sleep complaints (National Sleep Foundation, 2003). Even minor sleep complaints can lead to increased risk of accidents, falls, chronic fatigue, and weight gain (Bloom et al., 2009; Patel, Malhotra, White, Gottlieb, & Hu, 2006), while more significant sleep complaints are associated with cognitive decline, reduced immune function, and depression (Krueger & Friedman, 2009; Merlino et al., 2010). Despite the relevance of sleep across the lifespan, in particular among midlife and older adults, sleep remains one of the least understood health behaviors.
There is growing consensus that regular exercise can improve sleep (Youngstedt, O'Connor, & Dishman, 1997). Cross-sectional studies have consistently found that regular exercise is associated with better sleep (Youngstedt & Kline, 2006) and inversely associated with sleep disorders (Sherrill, Kotchou, & Quan, 1998). Randomized controlled trials (RCTs) of regular exercise that meet national recommendations (Physical Activity Guidelines Advisory Committee, 2008), which is 150 minutes a week of moderate-intensity physical activity, have produced small to moderate improvements in sleep quality in older adults. Self-rated improvements have been reported in global sleep quality, sleep-onset latency, sleep disturbances, and total sleep time (King, Oman, Brassington, Bliwise, & Haskell, 1997; Singh, Clements, & Fiatarone, 1997). Improvements have also been found in objective polysomnographic measures (PSG) of sleep, including decreased percent time in Stage 1 sleep (transitional sleep between wake and sleep), increased percent time in Stage 2 sleep (deeper form of sleep characterized by decreased body temperature, heart rate, and muscle tone), and fewer nighttime awakenings during the first third of sleep (King et al., 2008; a marker of sleep disturbance). This paper explores the potential moderators and mediators of the observed changes in objective sleep originally reported by King et al. (2008).
Why are Moderators and Mediators of the Exercise-Sleep Relationship Important to Study?
Little is known concerning for whom and by what means exercise-induced sleep changes optimally occur. Exploring moderators (consisting of baseline variables) and mediators (changes in variables related to intervention) is critical to identify the groups of individuals who may benefit most from different treatments (moderators) and the mechanisms underlying effective treatments (mediators; Kraemer, Wilson, Fairburn, & Agras, 2002). Knowledge related to moderator variables could be useful in targeting interventions to appropriate subgroups. Exploring mediation could help to maximize treatment gains by enhancing intervention elements that impact key mechanisms and eliminating elements that do not. These improvements in treatment efficiency could result in larger treatment effects or similar effects at lower cost or risk (Kraemer et al., 2002).
Putative Moderators and Mediators of the Exercise-Sleep Relationship
The current evidence linking exercise to sleep may be underestimated due to ceiling or floor effects for some participant subgroups based on certain individual characteristics (Youngstedt, 2003). For instance, individuals with poor initial sleep quality may benefit more from exercise. Youngstedt (2003) reported that, after controlling for initial poorer sleep, the acute effects observed in exercise studies were similar to those in pharmacological treatment studies for poor sleep. Age itself may be an important moderator as normative sleep is known to decline with age, most notably with respect to sleep architecture (Ancoli-Israel & Cooke, 2005) and circadian rhythms (Hood, Bruck, & Kennedy, 2004). Inactive individuals and those with poorer physical function may benefit more from exercise generally (Physical Activity Guidelines Advisory Committee, 2008). Two randomized controlled trials of sedentary and unfit older individuals reported correlated change between cardiorespiratory fitness and exercise-related sleep improvements (King et al., 1997; Tworoger et al., 2003). Other researchers have reported, however, in meta-analytic reviews of primarily small experimental studies of acute exercise, similar exercise-related benefits among fit and unfit individuals (Youngstedt et al., 1997). Finally, an older meta-analysis reported modestly improved sleep response in women relative to men (Kubitz, Landers, Petruzzello, & Han, 1996). While in the last 10 years women have been better represented in exercise and sleep trials, these more recent studies have not, in general, adequately explored gender as a moderator.
The basic mechanisms underlying the exercise-sleep relationship are not fully understood (Buman & King, 2010; Youngstedt, 2005). A number of theories have been posited and three will be discussed and tested here. First, negative affective states, such as depressive symptoms and anxiety, are important contributing factors to poor sleep (Morin et al., 1999). Since exercise is known to have both antidepressant (Dunn, Trivedi, & O'Neal, 2001) and anxiolytic (Stathopoulou, Powers, Berry, Smits, & Otto, 2006) effects, these changes may in turn improve sleep. Second, studies suggest that exercise is an important contributor overall to energy balance and weight change (Shaw, Gennat, O'Rourke, & Del Mar, 2006), and could therefore improve sleep through weight reduction, perhaps even apart from sleep apnea (Fogelholm et al., 2007). Finally, regular exercise could improve sleep by increasing day-to-day activity levels via enhanced functional status, since poor functional abilities are associated with poor sleep in older adults (Ensrud et al., 2009).
One limitation of the extant literature exploring moderators and mediators is its heavy reliance on meta-analytic reviews of small laboratory-based experimental trials that address only acute exercise effects and often have no or inappropriate control condition(s) (Buman & King, 2010). Randomized controlled trials represent the ‘gold standard’ for evaluating whether regular exercise improves sleep; in addition, they can more explicitly reveal moderators and mediators of the exercise-sleep relationship (Kraemer, Kiernan, Essex, & Kupfer, 2008). To date no studies that we are aware of have examined mediators within an RCT context with appropriate temporal sequencing to suggest a causal relationship. Moreover, given the multidimensional nature of both sleep and exercise and their impacts on nearly every system of the human body, it is unlikely that the effect of exercise on sleep is influenced by only a single baseline characteristic or transmitted by a single mechanism; i.e., it is likely that multiple moderators and mediators are operating simultaneously (Buman & King, 2010).
Research Purpose
Using data from a 12-month RCT of moderate-intensity exercise among midlife and older adults with mild to moderate sleep complaints (King et al., 2008), we explored baseline moderators (gender, age, and baseline levels of physical activity, rated sleep quality, and physical function) and mediators (changes in negative affect, overweight, and physical function) of exercise-induced changes in objective sleep parameters measured by in-home PSG.
Method
Design
The study was a 12-month randomized controlled trial of underactive adults aged 55 years or older with chronic mild to moderate sleep complaints who were recruited from the community at large. The study methods are described in detail elsewhere (King et al., 2008) and summarized here. The primary focus of the exercise program was increasing moderate intensity endurance exercise to a level that met or exceeded public health recommendations (Physical Activity Guidelines Advisory Committee, 2008). Exercise intervention participants were instructed to attend exercise classes 2 days/week for 60 minutes (30–45 minutes of which were aimed at moderate-intensity endurance exercise, including brisk walking and aerobic movement) and home-based exercise an additional 3 days/week for 30 minutes throughout the 12-month intervention period. The endurance exercise was targeted at an intensity of 60% to 85% of treadmill-based peak heart rate and took place during the morning or afternoon. Control arm participants received weekly classes similar to health education classes found in many communities throughout the U.S. Both groups received brief printed recommendations for sleep hygiene. The appropriate university institutional review boards approved the study protocol and this trial was registered at clinicaltrials.gov (#NCT00149747).
Participants
The primary eligibility criteria included (a) age 55 years or older; (b) underactive (defined as <60 minutes/week of moderate or more vigorous physical activity over the previous six months); (c) body mass index ≤ 35; (d) free of sleep apnea (objectively verified); and (e) mild to moderate sleep complaints (defined by scores ≥ three on at least two of three items of the Sleep Questionnaire and Assessment of Wakefulness (Miles, 1982). Of 201 persons initially responding to the study promotional announcements, 66 individuals were eligible and randomized (36 intervention, 30 control). Eighty-nine percent completed the 12-month trial (exercise = 32/36, or 89%, control = 27/30, or 90%), with no significant differences in dropout rates by study arm (p > .05). Class attendance was 74% and 80% for the exercise and control groups, respectively. Home-based participation, through regular participant logs, revealed exercise group participants engaged in 2.1 (SD=0.9) home-based sessions/week for 43.3 (SD=19.4) minutes per session. The exercise group reported greater energy expenditure at 6 and 12 months relative to control. Full adherence data are presented elsewhere (King et al., 2008).
Measurements
Figure 1 depicts the potential moderators and mediators being proposed in the current study. All moderator and mediator variables were selected a priori from the existing literature. Demographic information was self-reported at baseline. All standardized questionnaires and measurements were assessed by trained staff blinded to participant study arm assignment. Moderator variables were assessed at baseline prior to study arm assignment. Mediator variables were assessed at baseline and during the midpoint of the intervention (6 months), and outcome variables were assessed at baseline and at posttest (12 months).
Figure 1.
Potential moderators and mediators of exercise-induced sleep outcomes.
Moderators
Gender and age were self-reported. Baseline physical activity was operationalized as minutes of moderate-intensity or more vigorous physical activity (MVPA) and was measured by the CHAMPS physical activity questionnaire (Stewart et al., 2001). This 47-item self-report questionnaire assesses activities across the intensity spectrum during a typical week over the previous month. Moderate and more vigorous activities were included in the calculations, as defined by metabolic equivalent values ≥3.0 from standards in the field (Ainsworth et al., 2000). The CHAMPS has been found to provide valid and reliable estimates of MVPA in older adults (Harada, Chiu, King, & Stewart, 2001; Stewart et al., 2001). Global sleep quality was measured using the Pittsburgh Sleep Quality Index (PSQI; Buysse, Reynolds, Monk, Berman, & Kupfer, 1989). The PSQI, a standard measure, is a self-rated measure composed of seven "component" scores (using a 0–3 scale) summed to form a global sleep quality score (range = 0–21; lower score = better sleep quality). The global sleep quality score was used in the current investigation. Baseline physical function was operationalized as upper-body (number of arm curls performed) and lower-body (number of chair stands) function (Rikli & Jones, 2001). These measures are discussed in more detail as mediator variables below.
Mediators
Negative affect change measures included the 10-item short version of the Center for Epidemiological Studies - Depression Scale (CES-D) to measure depressive symptoms (Andresen, Malmgren, Carter, & Patrick, 1994) and the 40-item State-Trait Anxiety Inventory (STAI) to measure state and trait anxiety (Spielberger, Gorsuch, & Lushene, 1983). Change in overweight was assessed by measuring body weight (kg) and height (meters) using standard procedures to obtain BMI (kg/m2; King, Haskell, Taylor, Kraemer, & DeBusk, 1991). Chair stands and arm curls were used as measures of lower- and upper-body physical function, respectively, as these are standard, field-based measures commonly used for older adults to measure physical function. These tasks were performed following standard procedures (Rikli & Jones, 2001). Finally, cardiorespiratory fitness (VO2peak) data were collected through a standard treadmill-based test. There were notable amounts of missing data (n = 13) in the sample due to participants refusing or clinical staff deciding that it was not safe to initiate and/or complete the test. Preliminary analyses found that data were not missing at random and, therefore, the VO2peak variable was dropped from further analysis.
Objective sleep
Sleep was measured via in-home PSG. Nine-channel PSG was measured for three nights at baseline and two nights at 12 months using the Oxford Medilog MR95 digital recording system (Oxford Instruments, Oxford, U.K.). Polysomnographic data were collected and scored following standard procedures – a full description of these methods are published elsewhere (King et al., 2008). Results indicated that at 12 months, exercisers showed (a) significantly less percent total sleep time in Stage 1 sleep (group difference = 2.3%; effect size = 0.66); (b) significantly greater percent time in Stage 2 sleep (group difference = 3.2%; effect size = 0.41); and (c) significantly fewer awakenings during the first third of the sleep period (group difference = 1.0 awakening; effect size = 0.50) relative to controls. These results informed our decision to explore moderators and mediators of these outcomes.
Statistical Analyses
Three separate hierarchical regression models, one for each objective sleep outcome (percent time in Stage 1, percent time in Stage 2, number of awakenings), were used to examine whether the proposed moderators (see Figure 1) impacted intervention effects. Each baseline objective sleep value and study arm assignment was entered at Step 1. Main effects for all proposed moderators were entered at Step 2. The proposed moderators were mean centered and a group assignment × moderator interaction term was entered for each proposed moderator in Step 3 simultaneously (Aiken & West, 1991). Effect sizes were calculated for moderation effects using Cohen’s d formula: d = 2t/√df (Rosenthal & Rosnow, 1991).
Analysis of covariance (ANCOVA) was used (with baseline values as covariates) to evaluate whether the exercise intervention produced significant changes in the proposed mediators (see Figure 1) at 6 months. Variables that met this criterion were included in the formal tests of mediation. Intent-to-treat principles were used such that baseline values were carried forward when data were missing. Prior to entry in the mediation models, mediator and outcome variables were converted to residualized change scores such that values were centered at 0 and reflected change from baseline (Lance, 1988).
Mediation analyses were performed using methods described by Preacher and Hayes with the accompanying SAS macro (Preacher & Hayes, 2008). This procedure provides total and specific indirect effects (through the proposed mediator[s]) of the predictor (study arm assignment: exercise or control) on outcomes (objective sleep outcomes). Separate statistical models were run for percent time in Stage 1, percent time in Stage 2, and number of awakenings during the first third of sleep. Multiple mediator models have a number of advantages relative to single mediator models. Multiple mediator analyses allow inferences regarding the overall mediational effect of a set of mediators (i.e., total indirect effects) as well as unique effects of each mediator (i.e., specific indirect effects), holding constant the effects of all other mediators in the model. Pair-wise contrasts can also be estimated to assess the relative magnitudes of any two mediators, allowing comparisons to be made regarding competing mechanisms (Preacher & Hayes, 2008). The product-of-coefficients method (Sobel, 1986), most commonly used to assess mediation models,relies upon the assumption that indirect effects are normally distributed; however, this is likely not the case except in very large samples (MacKinnon, Krull, & Lockwood, 2000). Bootstrapping, a nonparametric sampling procedure, has been advocated as a more powerful alternative whereby percentile-based confidence limits are obtained (MacKinnon, Lockwood, & Williams, 2004). We use this bootstrapping procedure here, with 1,000 bootstrap samples with bias-corrected and accelerated intervals (Preacher & Hayes, 2008), to make inferences. We also present product-of-coefficient standard errors and Z for comparison, recognizing their limitations due to our limited sample size.
Results
Moderation Effects
Table 1 displays baseline descriptive statistics for the proposed moderators by group assignment. Both exercise and control study arms were similar in terms of each moderator under study. As reported previously (King et al., 2008), there were also no baseline group differences for other demographic variables, including educational status, race/ethnicity, marital status, employment status, and over-the-counter sleep medication use (p values > 0.10). Global sleep quality results indicated that the sample, on average, was comprised of “poor sleepers” as defined by PSQI global scores >5 (Buysse et al., 1989). For chair stands and arm curls, the sample fell within the normal range (within the middle 50%) for age, according to a national sample (Rikli & Jones, 1999). Chair stands and arm curls were found to be collinear (r = 0.58, p <.0001). The chair stand variable was included in the moderator analyses given the importance of lower-body function in delaying onset of disability in older adults (Pahor et al., 2006).
Table 1.
Descriptive statistics by Study Arm for Proposed Moderators.
| Moderator | Control | Exercise | Total |
|---|---|---|---|
| N | 30 | 36 | 66 |
| Female gender (%) | 66% | 66% | 66% |
| Age, y (SD) | 60.90 (7.19) | 61.86 (6.33) | 61.42 (6.70) |
| MVPA, min·wk−1(SD) | 280.50 (403.0) | 211.70 (204.70) | 242.95 (310.18) |
| +Global sleep quality (SD) | 7.70 (2.84) | 8.47 (3.61) | 8.12 (3.28) |
| Chair stands (SD) | 15.20 (4.79) | 14.56 (3.42) | 14.85 (4.08) |
| Arm curls (SD) | 18.60 (6.61) | 16.92 (4.25) | 17.68 (5.47) |
Notes. +0–21 scale: lower score = better sleep quality.
There were no statistically significant between-arm baseline differences (p values > .10).
SD = standard deviation; MVPA = moderate-vigorous or greater physical activity.
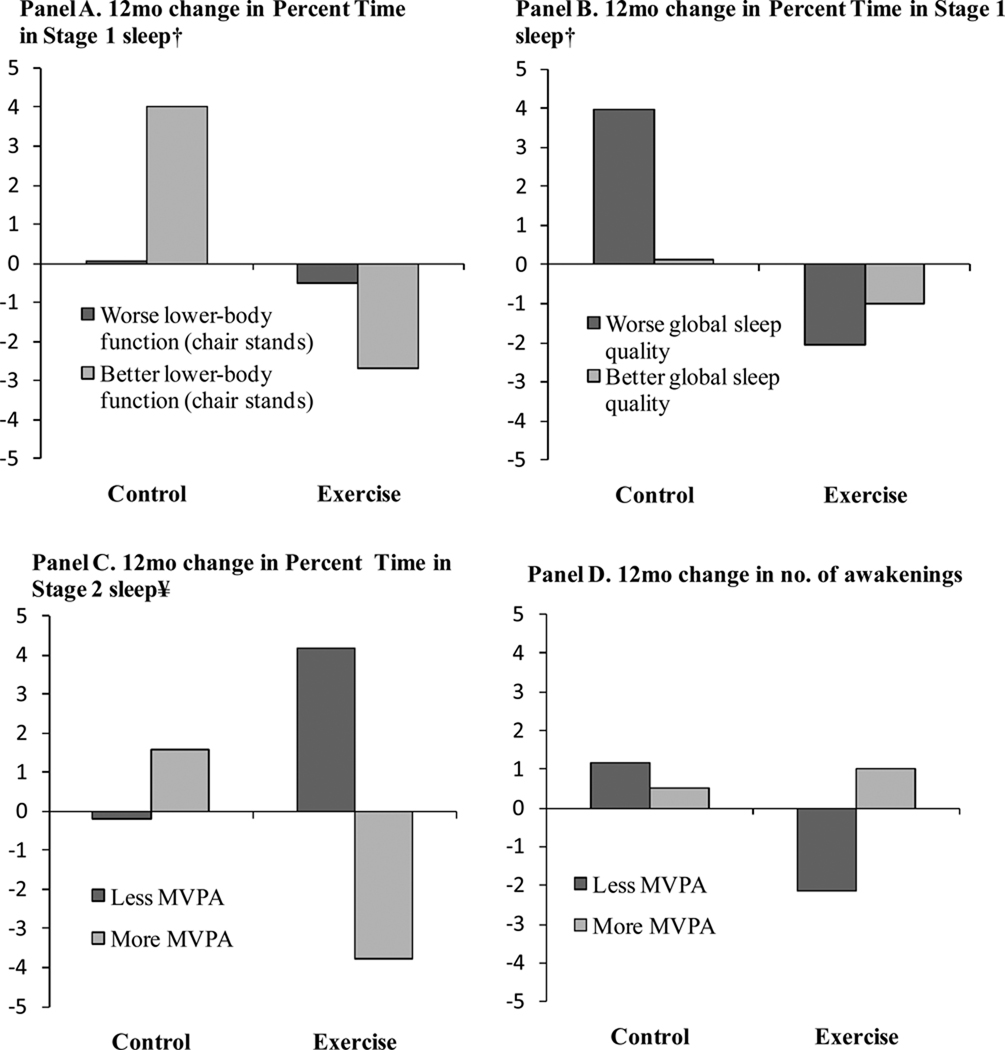
No significant main effects were observed in the moderation models. For percent time in Stage 1, baseline chair stands (t (55) = 2.83, p = .006, β = .33, d = .76) and global sleep quality (t (55) = 3.04, p = .003, β = .37, d = .82) were significant moderators. Figure 2 (Panels A and B for chairs stands and global sleep quality, respectively) displays values graphically, indicating that individuals with better baseline lower-body physical function as measured via the chair stands and worse global sleep quality improved the most (less percent time in Stage 1 sleep = better). For percent time in Stage 2 sleep, baseline MVPA was a significant moderator (t (55) = 2.43, p = .02, β = .35, d = .66). Figure 2 (Panel C) indicates that less active individuals at baseline improved the most (more percent time in Stage 2 sleep = better). For number of awakenings in the first third of sleep, baseline MVPA was a significant moderator (t (55) = 2.61, p = .01, β = .37, d = .70). Figure 2 (Panel D) indicates that less active individuals at baseline improved the most (fewer awakenings = better). Age and gender were not significant moderators.
Figure 2.
Moderator effects of exercise on objective sleep outcomes by baseline lower-body physical function (chair stands), global sleep quality, and moderate-intensity and more vigorous physical activity (MVPA).
Notes: Predicted values derived from the regression equation in which ‘high’ and ‘low’ values for moderators were specified as one standard deviation above and below the mean; Models are adjusted for main effects and interactions for gender, age, MVPA, global sleep quality, and chair stands. † Percent time in Stage 1 sleep: less = better; ¥ Percent time in Stage 2 sleep: more = better.
Mediator Effects
ANOVA tests were conducted to identify which of the proposed mediators significantly improved during the first 6 months of the intervention (Table 2). Depressive symptoms were marginally reduced in the exercise arm relative to the control arm (M diff = −2.16, 95% CI: −4.35 to 0.03, p = .05). Trait and state anxiety were not reduced. Body mass index was significantly reduced in the exercise arm relative to the control arm (M diff = −0.60, 95% CI: −1.02 to −0.17, p = .006). Chair stands were not significantly improved, yet arm curls were (M diff = 1.76, 95% CI: 0.21 to 3.31, p = .03). Changes in depressive symptoms, BMI, and arm curls were therefore included in the mediator models.
Table 2.
Mean (SD) of Proposed Mediators at Baseline and Six Months by Study Arm (N = 66).
| Variable | Control (n = 30) |
Exercise (n = 36) |
Cohen's d |
|---|---|---|---|
| Depressive symptoms | |||
| Baseline | 7.03 (4.44) | 8.17 (5.06) | |
| 6 months | 8.30 (5.58) | 7.06 (6.04)* | .50 |
| Trait Anxiety† | |||
| Baseline | 35.69 (9.09) | 35.94 (12.19) | |
| 6 months | 37.07 (10.44) | 34.94 (11.71) | .43 |
| State Anxiety† | |||
| Baseline | 33.76 (9.91) | 33.66 (12.98) | |
| 6 months | 36.62 (11.30) | 35.43 (14.02) | .16 |
| Body mass index | |||
| Baseline | 27.18 (3.92) | 27.86 (4.06) | |
| 6 months | 27.49 (3.78) | 27.55 (4.03)** | .71 |
| Chair stands | |||
| Baseline | 15.20 (4.79) | 14.56 (3.42) | |
| 6 months | 16.60 (5.47) | 16.78 (4.50) | .22 |
| Arm curls | |||
| Baseline | 18.60 (6.61) | 16.92 (4.25) | |
| 6 months | 19.27 (6.32) | 19.53 (5.20)* | .57 |
Notes.
Between-arm difference (ANCOVA), p ≤.05, two-tailed.
Between-arm difference (ANCOVA), p < .01, two-tailed.
Two individuals (1 exercise, 1 control) did not have complete baseline scores.
SD = standard deviation.
Mediation results are displayed in Table 3. For percent time in Stage 1 sleep, the total indirect effect of the variables in combination did not mediate the effects of the intervention. Specific indirect effects indicated that decreased depressive symptoms mediated changes in percent time in Stage 1 sleep. For percent time in Stage 2 sleep, total and specific indirect effects were not significant. Finally, for number of awakenings, the total indirect effect was not significant. However, specific indirect effects indicated that decreased depressive symptoms, decreased BMI, and increased arm curls mediated changes in number of awakenings. Pair-wise contrasts revealed that among the significant mediators for number of awakenings, BMI change was the best mediator, following by changes in depressive symptoms and arm curls, respectively.
Table 3.
Mediation of the Effect of Exercise on 12-Month Polysomnographic Sleep Outcomes through Depressive Symptoms, BMI, and Physical Function.
| Variable | Point Estimate |
Product-of- coefficents method |
Bootstrapped BCa 95% CI |
||
|---|---|---|---|---|---|
| SE | Z | Lower | Upper | ||
| Percent time in Stage 1 Sleep | |||||
| TOTAL | −0.30 | 0.48 | −0.63 | −1.58 | 0.49 |
| Depressive symptoms | −0.41* | 0.28 | −1.45 | −1.57 | −0.02 |
| BMI | 0.28 | 0.29 | 0.98 | −0.22 | 0.98 |
| Arm curls | −0.18 | 0.23 | −0.79 | −0.88 | 0.17 |
| Percent time in Stage 2 Sleep | |||||
| TOTAL | 0.29 | 1.05 | 0.27 | −2.46 | 2.44 |
| Depressive symptoms | 0.18 | 0.48 | 0.36 | −0.60 | 1.56 |
| BMI | 0.59 | 0.71 | 0.83 | −0.58 | 2.47 |
| Arm curls | −0.48 | 0.58 | −0.83 | −2.47 | 0.55 |
| Number of awakenings: 1st third of sleep period† | |||||
| TOTAL | 0.41 | 0.35 | 1.16 | −0.33 | 1.22 |
| Depressive symptoms | −0.26* | 0.18 | −1.44 | −0.75 | −0.01 |
| BMI | −0.41* | 0.23 | −1.78 | −1.08 | −0.06 |
| Arm curls | 0.36* | 0.18 | 1.44 | 0.01 | 0.72 |
Notes.
denotes CI does not include zero, point estimate represents unstandardized beta coefficient.
Bca = bias corrected and accelerated, 1,000 bootstrap samples;
Significant contrasts: BMI > Depressive symptoms, Depressive symptoms > Arm curls.
Discussion
Understanding for whom and by what means exercise impacts sleep is critical to optimize exercise interventions for midlife and older adults. Moderator results suggested that initially less active individuals with higher initial physical function and poorer sleep quality had the greatest objective sleep improvements. Mediation results suggest that the observed exercise-induced improvements in sleep at 12 months were at least partially due to decreased depressive symptoms, decreased weight, and improvements in physical function at six months. Results also indicated that moderators and mediators differed for each parameter of sleep architecture.
Moderator results
Initial level of physical activity was the strongest and most consistent moderator of sleep. This was observed despite excluding individuals reporting >60 minutes of moderate-intensity exercise in a typical week at baseline, suggesting that those almost completely inactive were likely to benefit more from the intervention relative to those reporting greater, yet still insufficient, amounts of exercise. It should also be noted that these effects were observed for a sleep parameter that reflects more consolidated sleep patterns (number of awakenings). This is an important finding given shifts in circadian rhythms with age that may lead to more fragmented sleep (Ancoli-Israel & Cooke, 2005; Hood et al., 2004). Individuals with poorer self-rated global sleep quality at baseline had greater improvements in percent time in Stage 1 sleep. This result was present despite study eligibility criteria targeting only participants reporting mild to moderate sleep complaints (participants with diagnosed sleep disorders and those without sleep complaints were excluded). At least for percent time in Stage 1 sleep, this indicates that exercise is beneficial for the large number of midlife and older adults in the mild to moderate sleep complaint range, with individuals at the more serious end of this range appearing to benefit most.
We also observed that individuals with higher levels of lower-body physical function had greater improvements in percent time in Stage 1 sleep. This suggests that higher functioning participants may have had greater overall exercise volume during the intervention. The exercise intensity prescription given was based on relative intensity (60–85% peak heart rate), and, therefore, individuals with higher physical function were likely able to exercise at higher levels of absolute intensity relative to lower functioning individuals. This would result in an increased overall volume of exercise, suggesting a dose-response effect for exercise. A dose-response effect has previously been reported in an exercise RCT (Singh et al., 2005). Additionally, higher functioning individuals in our sample may have participated in more resistance training activities as part of the intervention. Unfortunately, our adherence measures did not assess physical activity modality at this level of detail to formally explore this hypothesis.
It should be noted that the significant percent time in Stage 1 sleep moderator results for chair stands (Figure 2, Panel A) and global sleep quality (Figure 2, Panel B) were likely partially driven by increases in percent time in Stage 1 sleep in control group participants with better lower-body function and worse global sleep quality. While these were not expected effects, this may reflect (a) individuals with better physical function may require at least modest levels of physical activity to maintain sleep quality; and (b) poorer sleepers, in the absence of physical activity and with the expectation that their sleep would improve, may have adopted maladaptive strategies to enhance sleep (i.e., spending extra time in bed to achieve sleep) that had the opposite effect. Additionally, we also acknowledge because these findings were not found consistently across all sleep outcomes and our sample size was small, it may be that these findings are not replicable and should be interpreted with caution.
Gender and age were not significant moderators in any models. For gender, our results are in contrast with conclusions from an older meta-analysis that indicated women improved more in response to exercise than men (Kubitz et al., 1996). Women generally were underrepresented in the studies used in this meta-analysis. Our sample was more evenly distributed (66% women) and, therefore, may reflect a more accurate assessment of this effect. Age also was not a significant moderator, which was not surprising given that we only sampled older adults (55–79 years of age). Age-related variations were likely related to differences in rated sleep quality and therefore were likely more accurately captured in the rated sleep quality moderator variable discussed above. Exercise-induced sleep changes may still be amplified or attenuated among younger (or older) age cohorts not included in our sample.
Mediator results
Depressive symptoms, as measured by a screening tool for depression, were found to mediate both percent time in Stage 1 sleep and number of awakenings. This result is in line with the Singh et al.(1997) study, where improvements in depressive symptoms co-occured with improvements in subjective sleep-onset latency and sleep disturbances following 10 weeks of high intensity progressive resistance training in depressed older adults. Our results extend these results in three ways: (a) our sample as a whole did not appear to be clinically depressed, as indicated by baseline levels on the CESD measure, suggesting reasonably modest improvements in a non-clinical sample may be large enough to lead to improved sleep; (b) our sleep results were based on objective as opposed to subjective measures; and (c) the temporal sequence of our measures (depressive symptoms at 6 months preceded sleep outcome at 12 months) suggest that shorter-term improvements in depressive symptoms drive longer-term sleep improvements, although the effects may be bi-directional. There are a number of plausible mechanisms by which depressive symptoms may mediate sleep improvements, including reductions in general hyper-arousal (Morin et al., 1999) and improved autonomic control (Youngstedt, 2005).
Despite excluding participants with substantial levels of sleep apnea (King et al., 2008), the small, yet significant reductions in body weight mediated number of awakenings during the first third of sleep. It is possible that the reduction of such awakenings might have reflected small changes in whatever residual levels of sleep disordered breathing that persisted in our sample, despite careful screening. Unfortunately, our PSG montage did not include recordings of sleep disordered breathing as outcomes. Additionally, evidence is now accruing that body weight and sleep quality could be associated, even apart from sleep apnea (Fogelholm et al., 2007), which leaves open yet another path of mediation. Such an effect would be compatible with the known link between sleep integrity and successful thermoregulation (Shaw, 2005), a process that may be optimized by even modest levels of physical activity (Levine, Eberhardt, & Jensen, 1999).
Poor sleep is associated with greater risk for falls and functional disability in older adults (Ensrud et al., 2009), along with a number of other health-related quality of life outcomes (e.g., cognitive function, chronic pain). While researchers primarily have discussed how sleep problems lead to these impairments, our results suggest that improved physical function may also lead to improved sleep, specifically number of awakenings during the first third of sleep. Changes in upper-body physical function, as measured in the current study through arm curls (a commonly used measure among older adults), may reflect overall improvements in functional capacity (Rikli & Jones, 2001). These results are similar to studies that have observed correlated change between fitness measures and sleep improvements (King et al., 1997; Tworoger et al., 2003). Our results strengthen these conclusions by establishing temporal order of these effects.
Two important caveats are worth noting in regard to the mediation outcomes. First, none of the proposed mediators explained changes in percent time in Stage 2 sleep. This was likely due to the relatively small exercise-induced effect that was observed for Stage 2 sleep. We likely did not have sufficient statistical power to account for such a small effect via our mediators. Second, the mediators tested in this study represent only three potential mechanisms through which exercise may impact sleep. Other mechanisms are also plausible that were not measured in this study, most notably thermoregulatory effects (Van Someren, 2000; Youngstedt, 2005), pro-inflammatory cytokines such as IL-1, IL-6 and TNF-α (Santos, Tufik, & De Mello, 2007), and brain neuropeptides and neurotransmitters (Buman & King, 2010). This is one likely explanation for why the sleep outcomes were only partially mediated by the set of proposed mediators. Future RCT studies should explore these additional mechanisms.
Strengths and Limitations
The primary strengths of this study are the testing of multiple moderators and mediators within a single RCT, the appropriate temporal order of treatment, mediator, and outcome variables, and the use of objective parameters of sleep architecture. Past conclusions regarding moderators and mediators of exercise-induced sleep improvements have been limited primarily to meta-analytic results of experimental studies addressing acute exercise effects that rely on cross-sectional associations to establish moderation and mediation evidence. We chose potential moderators and mediators, guided by this limited evidence, to formally test moderation and mediation within an RCT and within recommended temporal conditions (Kraemer et al., 2008).
Our findings are also limited by an inability to control for light exposure. This is a shared limitation with virtually all other RCTs exploring exercise effects on sleep. Our intervention included two weekly indoor group-based exercise sessions supplemented with several home-based exercise bouts—with many participants choosing to walk outdoors. We viewed this exercise prescription as a necessary ‘trade-off’ to maintain high levels of adherence, a sufficient volume of exercise to optimize health effects, and strong external validity of our program.
Conclusion
Our results suggest older adults with poor initial sleep quality who are physically inactive and are relatively higher functioning are most likely to receive sleep benefits from moderate-intensity physical activity. Interventions should target these subgroups for maximal benefit. It also appears that these sleep-related benefits may have been conferred through reductions in depressive symptoms as measured by a screening tool for depression, decreased weight, and improved physical function. Intervention strategies aimed at optimally impacting these specific outcomes are recommended to improve treatment efficiency.
Acknowledgments
This study was supported by U.S. Public Health Service Grant R01MH58853 from the National Institute of Mental Health (Dr. King). Drs. Buman and Hekler were supported by U.S. Public Health Service Grant 5T32HL007034 from the National Heart, Lung, and Blood Institute.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/hea.
Contributor Information
Matthew P. Buman, Stanford Prevention Research Center, Stanford University School of Medicine
Eric B. Hekler, Stanford Prevention Research Center, Stanford University School of Medicine
Donald L. Bliwise, Department of Neurology, Emory University School of Medicine
Abby C. King, Department of Health Research and Policy and Stanford Prevention Research Center, Department of Medicine, Stanford University School of Medicine
References
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Newbury Park, CA: Sage; 1991. [Google Scholar]
- Ainsworth B, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: An update on activity codes and MET intensities. Medicine & Science in Sports & Exercise. 2000;32(9):S498–S516. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- Ancoli-Israel S, Cooke JR. Prevalence and comorbidity of insomnia and effect on functioning in elderly populations. Journal of the American Geriatrics Society. 2005;53(7):S264–S271. doi: 10.1111/j.1532-5415.2005.53392.x. [DOI] [PubMed] [Google Scholar]
- Andresen EM, Malmgren JA, Carter WB, Patrick DL. Screening for depression in well older adults - Evaluation of a short-form of the CES-D. American Journal of Preventive Medicine. 1994;10(2):77–84. [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research - Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Bloom HG, Ahmed I, Alessi CA, Ancoli-Israel S, Buysse DJ, Kryger MH, et al. Evidence-based recommendations for the assessment and management of sleep disorders in older persons. Journal of the American Geriatrics Society. 2009;57(5):761–789. doi: 10.1111/j.1532-5415.2009.02220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buman MP, King AC. Exercise as treatment to enhance sleep. American Journal of Lifestyle Medicine. (in press) [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Dunn AL, Trivedi MH, O'Neal HA. Physical activity dose-response effects on outcomes of depression and anxiety. Medicine & Science in Sports & Exercise. 2001;33(6):S587–S597. doi: 10.1097/00005768-200106001-00027. [DOI] [PubMed] [Google Scholar]
- Ensrud KE, Blackwell TL, Redline S, Ancoli-Israel S, Paudel ML, Cawthon PM, et al. Sleep disturbances and frailty status in older community-dwelling men. Journal of the American Geriatrics Society. 2009;57(11):2085–2093. doi: 10.1111/j.1532-5415.2009.02490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelholm M, Kronholm E, Kukkonen-Harjula K, Partonen T, Partinen M, Harma M. Sleep-related disturbances and physical inactivity are independently associated with obesity in adults. International Journal of Obesity. 2007;31(11):1713–1721. doi: 10.1038/sj.ijo.0803663. [DOI] [PubMed] [Google Scholar]
- Harada ND, Chiu V, King AC, Stewart AL. An evaluation of three self-report physical activity instruments for older adults. Medicine & Science in Sports & Exercise. 2001;33(6):962–970. doi: 10.1097/00005768-200106000-00016. [DOI] [PubMed] [Google Scholar]
- Hood B, Bruck D, Kennedy G. Determinants of sleep quality in the healthy aged: The role of physical, psychological, circadian and naturalistic light variables. Age and Ageing. 2004;33(2):159–165. doi: 10.1093/ageing/afh051. [DOI] [PubMed] [Google Scholar]
- King AC, Haskell WL, Taylor CB, Kraemer HC, DeBusk RF. Group- vs. home-based exercise training in healthy older men and women. A community-based clinical trial. Journal of the American Medical Association. 1991;266(11):1535–1542. [PubMed] [Google Scholar]
- King AC, Oman RF, Brassington GS, Bliwise DL, Haskell WL. Moderate-intensity exercise and self-rated quality of sleep in older adults. A randomized controlled trial. Journal of the American Medical Association. 1997;277(1):32–37. [PubMed] [Google Scholar]
- King AC, Pruitt LA, Woo S, Castro CM, Ahn DK, Vitiello MV, et al. Effects of moderate-intensity exercise on polysomnographic and subjective sleep quality in older adults with mild to moderate sleep complaints. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2008;63(9):997–1004. doi: 10.1093/gerona/63.9.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer H, Kiernan M, Essex M, Kupfer DJ. How and why criteria defining moderators and mediators differ between the Baron & Kenny and Macarthur approaches. Health Psychology. 2008;27(2) Suppl:S101–S108. doi: 10.1037/0278-6133.27.2(Suppl.).S101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Archives of General Psychiatry. 2002;59(10):877–883. doi: 10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- Krueger PM, Friedman EM. Sleep duration in the United States: A cross-sectional population-based study. American Journal of Epidemiology. 2009;169(9):1052–1063. doi: 10.1093/aje/kwp023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitz KA, Landers DM, Petruzzello SJ, Han NW. The effects of acute and chronic exercise on sleep - A meta-analytic review. Sports Medicine. 1996;21(4):277–291. doi: 10.2165/00007256-199621040-00004. [DOI] [PubMed] [Google Scholar]
- Lance CE. Residual centering, exploratory and confirmatory moderator analysis, and decomposition of effects in path models containing interactions. Applied Psychological Measurement. 1988;12(2):163–175. [Google Scholar]
- Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283(5399):212–214. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding and suppression effect. Prevention Science. 2000;1(4):173–181. doi: 10.1023/a:1026595011371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behavioral Research. 2004;39(1):99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlino G, Piani A, Gigli GL, Cancelli I, Rinaldi A, Baroselli A, et al. Daytime sleepiness is associated with dementia and cognitive decline in older Italian adults: A population-based study. Sleep Medicine. 2010;11(4):372–377. doi: 10.1016/j.sleep.2009.07.018. [DOI] [PubMed] [Google Scholar]
- Miles LE. A sleep questionnaire. In: Guilleminault C, editor. Sleeping and waking disorders: Indications and techniques. Menlo Park, CA: Addison-Wesley; 1982. [Google Scholar]
- Morin CM, Hauri PJ, Espie CA, Spielman AJ, Buysse DJ, Bootzin RR. Nonpharmacologic treatment of chronic insomnia. Sleep. 1999;22(8):1134–1156. doi: 10.1093/sleep/22.8.1134. [DOI] [PubMed] [Google Scholar]
- National Sleep Foundation. 2003 Sleep in America Poll. Washington, DC: National Sleep Foundation; 2003. [Google Scholar]
- Pahor M, Blair SN, Espeland M, Fielding R, Gill TM, Guralnik JM, et al. Effects of a physical activity intervention on measures of physical performance: Results of the lifestyle interventions and independence for elders pilot (LIFE-P) study. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2006;61(11):1157–1165. doi: 10.1093/gerona/61.11.1157. [DOI] [PubMed] [Google Scholar]
- Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. American Journal of Epidemiology. 2006;164(10):947–954. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Physical Activity Guidelines Advisory Committee. Physical activity guidelines advisory committee report. Washington, DC: U.S. Department of Health and Human Services; 2008. [DOI] [PubMed] [Google Scholar]
- Preacher K, Hayes A. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods. 2008;40(3):879–891. doi: 10.3758/brm.40.3.879. [DOI] [PubMed] [Google Scholar]
- Rikli RE, Jones CJ. Functional fitness normative scores for community-residing older adults, ages 60–94. Journal of Aging and Physical Activity. 1999;7(2):162–181. [Google Scholar]
- Rikli RE, Jones CJ. Senior fitness test manual. Champaign, IL: Human Kinetics; 2001. [Google Scholar]
- Rosenthal R, Rosnow RL. Essentials of behavioral research: Methods and data analysis. 2nd ed. New York: McGraw-Hill; 1991. [Google Scholar]
- Santos RVT, Tufik S, De Mello MT. Exercise, sleep and cytokines: Is there a relation? Sleep Medicine Reviews. 2007;11(3):231–239. doi: 10.1016/j.smrv.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Shaw K, Gennat H, O'Rourke P, Del Mar C. Exercise for overweight or obesity. Cochrane Database of Systematic Reviews. 2006;4:1–104. doi: 10.1002/14651858.CD003817.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw PJ. Thermoregulatory changes. In: Kushida CA, editor. Sleep deprivation: Basic science, physiology, and behavior. New York: Marcel Dekker; 2005. pp. 319–338. [Google Scholar]
- Sherrill DL, Kotchou K, Quan SF. Association of physical activity and human sleep disorders. Archives of Internal Medicine. 1998;158(17):1894–1898. doi: 10.1001/archinte.158.17.1894. [DOI] [PubMed] [Google Scholar]
- Singh NA, Clements KM, Fiatarone MA. Sleep, sleep deprivation, and daytime activities - A randomized controlled trial of the effect of exercise on sleep. Sleep. 1997;20(2):95–101. doi: 10.1093/sleep/20.2.95. [DOI] [PubMed] [Google Scholar]
- Singh NA, Stavrinos TM, Scarbek Y, Galambos G, Liber C, Fiatarone Singh MA. A randomized controlled trial of high versus low intensity weight training versus general practitioner care for clinical depression in older adults. Journals of Gerontology Series A-Biological Sciences & Medical Sciences. 2005;60(6):768–776. doi: 10.1093/gerona/60.6.768. [DOI] [PubMed] [Google Scholar]
- Sobel ME. Some new results on indirect effects and their standard errors in covariance structure models. In: Leinhart S, editor. Sociological Methodology 1982. San Francisco: Jossey-Bass; 1986. pp. 290–312. [Google Scholar]
- Spielberger C, Gorsuch R, Lushene R. The State Trait Anxiety Inventory (STAI) test manual. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- Stathopoulou G, Powers MB, Berry AC, Smits JAJ, Otto MW. Exercise interventions for mental health: A quantitative and qualitative review. Clinical Psychology-Science and Practice. 2006;13(2):179–193. [Google Scholar]
- Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: Outcomes for interventions. Medicine & Science in Sports & Exercise. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- Tworoger SS, Yasui Y, Vitiello MV, Schwartz RS, Ulrich CM, Aiello EJ, et al. Effects of a yearlong moderate-intensity exercise and a stretching intervention on sleep quality in postmenopausal women. Sleep. 2003;26(7):830–836. doi: 10.1093/sleep/26.7.830. [DOI] [PubMed] [Google Scholar]
- Van Someren EJW. More than a marker: Interaction between the circadian regulation of temperature and sleep, age-related changes, and treatment possibilities. Chronobiology International. 2000;17(3):313–354. doi: 10.1081/cbi-100101050. [DOI] [PubMed] [Google Scholar]
- Youngstedt SD. Ceiling and floor effects in sleep research. Sleep Medicine Reviews. 2003;7(4):351–365. doi: 10.1053/smrv.2001.0239. [DOI] [PubMed] [Google Scholar]
- Youngstedt SD. Effects of exercise on sleep. Clinics in Sports Medicine. 2005;24(2):355–365. doi: 10.1016/j.csm.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Youngstedt SD, Kline CE. Epidemiology of exercise and sleep. Sleep & Biological Rhythms. 2006;4:215–221. doi: 10.1111/j.1479-8425.2006.00235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngstedt SD, O'Connor PJ, Dishman RK. The effects of acute exercise on sleep: A quantitative synthesis. Sleep. 1997;20(3):203–214. doi: 10.1093/sleep/20.3.203. [DOI] [PubMed] [Google Scholar]