SUMMARY
Hyperactivity of mTORC1, a key mediator of cell growth, leads to stem cell depletion although the underlying mechanisms are poorly defined. Using spermatogonial progenitor cells (SPCs) as a model system, we show that mTORC1 impairs stem cell maintenance by a negative feedback from mTORC1 to receptors required to transduce niche-derived signals. We find that SPCs lacking Plzf, a transcription factor essential for SPC maintenance, have enhanced mTORC1 activity. Aberrant mTORC1 activation in Plzf −/− SPCs inhibits their response to GDNF, a growth factor critical for SPC self-renewal, via negative feedback at the level of the GDNF receptor. Plzf opposes mTORC1 activity by inducing expression of the mTORC1 inhibitor Redd1. Thus, we identify the mTORC1-Plzf functional interaction as a critical rheostat for maintenance of the spermatogonial pool, and propose a model whereby negative feedback from mTORC1 to the GDNF receptor balances SPC growth with self-renewal.
INTRODUCTION
Maintenance of a wide array of adult tissues is dependent on the presence of a resident stem cell pool with self-renewal potential that generates differentiating progeny. Factors regulating the balance between stem cell self-renewal and differentiation ensure tissue homeostasis while disruption of these regulatory mechanisms can lead to tissue degeneration or cancer (Ito et al., 2009). One factor central to stem cell homeostasis is mammalian TOR complex 1 (mTORC1), a signaling complex that promotes protein translation and cell growth by phosphorylating components of the translation machinery (Ma and Blenis, 2009). mTORC1 is regulated in response to diverse stimuli including nutrient availability, energy status, growth factors and cellular stress. Persistent mTORC1 activation in certain tissues leads to increased proliferation but subsequent exhaustion of the stem cell compartment, demonstrating that aberrantly activated mTORC1 is detrimental to stem cell maintenance (Castilho et al., 2009; Gan and DePinho, 2009; Yilmaz et al., 2006). It is proposed that inappropriate mTORC1 activation drives stem cell depletion through aberrant translation of downstream targets and subsequent activation of tumor suppressive/fail-safe mechanisms resulting in cellular senescence or apoptosis (Ito et al., 2009). However, the molecular mechanisms and targets of mTORC1 in this context are currently unknown. Interestingly, inhibition of mTORC1 also extends organism lifespan (Harrison et al., 2009; Schieke and Finkel, 2006), consistent with the notion that declining stem cell potential underlies aging (Rossi et al., 2008).
Undifferentiated germline cells of the testis (spermatogonial progenitor cells; SPCs) are formed from gonocytes during postnatal development of the mouse testis and possess self-renewal potential (de Rooij and Russell, 2000). A major advance in the study of male germline biology was the development of culture systems allowing long-term SPC expansion in vitro while maintaining stem cell potential. Key to this was the observation that mice heterozygous for the glial cell - derived neurotrophic factor (GDNF) cytokine gene had a depletion of SPC activity (Meng et al., 2000). GDNF is produced by Sertoli cells within the testis, and signals via the GFRα1/c-Ret receptor to promote SPC self-renewal and growth through activation of Src family kinases and Akt (Lee et al., 2007b; Oatley et al., 2007). Culture of SPCs with GDNF plus a variety of additional factors (including basic fibroblast growth factor; bFGF) preserves self-renewal capabilities and allows essentially unlimited cell expansion while maintaining in vivo differentiation potential; assessed by the ability to repopulate depleted recipient testis (Kanatsu-Shinohara et al., 2003; Kubota et al., 2004; Seandel et al., 2007). Although some cellular signaling pathways involved in SPC self-renewal have been described, it remains unclear how SPCs integrate signals from general mitogenic stimuli with those required for self-renewal to balance stem cell maintenance and differentiation.
A limited number of cell intrinsic factors have also been implicated in SPC function, foremost amongst which is Promyelocytic Leukemia Zinc Finger (PLZF). PLZF was identified from the translocation breakpoint in t(11;17) acute promyelocytic leukemia (Chen et al., 1993) and encodes a transcription factor belonging to the POZ-Krüppel (POK) family. PLZF binds DNA through carboxy-terminal Krüppel-type Zinc fingers and recruits histone deacetylases (HDACs) via an amino-terminal POZ domain (David et al., 1998). Recruitment of HDACs to target promoters can result in gene repression although PLZF is also able to activate gene expression (Doulatov et al., 2009; Labbaye et al., 2002). Male mice lacking Plzf expression undergo progressive germ cell loss and testis atrophy with age causing infertility (Buaas et al., 2004; Costoya et al., 2004). Plzf is expressed by SPCs and is needed in a cell autonomous fashion for maintenance of the germ lineage. A male patient with biallelic PLZF loss-of-function and gonad hypoplasia has been recently reported (Fischer et al., 2008), emphasizing the role played by PLZF in germ cell biology.
SPC maintenance is dependent on Plzf plus key growth factors such as GDNF. As mTORC1 is activated in response to growth factor signaling we hypothesized that both Plzf and mTORC1 are involved in the GDNF response of SPCs and that Plzf and mTORC1 may crosstalk. To assess the roles of Plzf and mTORC1 in GDNF-dependent SPC maintenance, we developed systems for isolation and culture of SPCs from wildtype and Plzf −/− prepubertal mice (subsequent to formation of the SPC pool and prior to overt germ cell depletion in the Plzf −/− mouse). We find that Plzf opposes mTORC1 activity and define a cellular signaling network controlling SPC homeostasis whereby mTORC1 integrates mitogenic signals received by SPCs and determines their sensitivity to self-renewal stimuli.
RESULTS
Isolation, culture and comparative analysis of Plzf +/+ and Plzf −/− SPCs
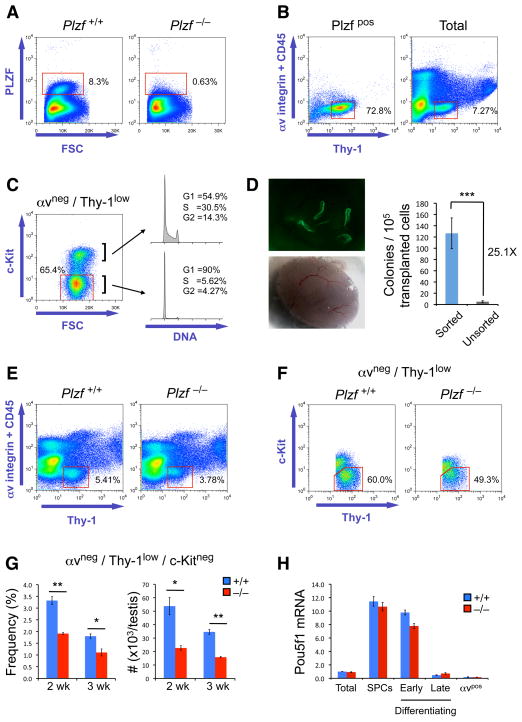
The testis is composed of a heterogeneous mix of mitotic and meiotic germ cells plus multiple types of somatic cells. SPCs are rare within the testis and systems for their purification are poorly developed. We therefore sought to take advantage of the expression of Plzf in SPCs to enrich for this cell type. Plzf-expressing cells were identified from juvenile testis by intracellular staining and flow cytometry using a newly developed monoclonal antibody (Figure 1A). Our aim was to correlate the Plzf-expressing population to sets of cell surface markers that would allow subsequent purification of those live cells. By adapting markers able to enrich for stem cell activity from cryptorchid testis (Kubota et al., 2003; Shinohara et al., 2000), we found that the Plzf-positive population was αv-integrin negative and expressed low levels of Thy-1 (Figure 1B). Negative selection for αv-integrin combined with Thy-1 positive selection allowed isolation of Plzf-positive cells and minimized somatic cell contamination (Figures S1A and S1B) (Virtanen et al., 1986). c-Kit expression is associated with SPC differentiation (Schrans-Stassen et al., 1999) and the αvneg Thy-1low population was composed primarily of c-Kit negative cells with a smaller c-Kit positive fraction (Figure 1C, Left). The c-Kitneg cells were largely in G1/G0 phase of cell cycle while the c-Kitpos population contained more cells in S plus G2 phases (Figure 1C, Right). As SPCs are more quiescent than differentiating spermatogonia (Takubo et al., 2008), the αvneg Thy-1low population contains both SPCs (c-Kitneg, quiescent) and cells undergoing differentiation (c-Kitpos, proliferating). To confirm that the αvneg Thy-1low c-Kitneg fraction was enriched for stem cells, we compared the ability of αvneg Thy-1low c-Kitneg and unfractionated cells to repopulate recipient depleted testis (Ogawa et al., 1997). Donor cells were isolated from transgenic mice expressing GFP from the β-actin promoter, allowing visualization of donor-derived colonies (Figure 1D, Left). Numbers of GFP-positive colonies were scored 2 months post-transplant (Figure 1D, Right), representing numbers of engrafted stem cells. Stem cell activity was enriched ~25-fold in the αvneg Thy-1low c-Kitneg population compared to unfractionated cells. As the αvneg Thy-1low c-Kitneg fraction represents 3–5% of total cells (~1/25th of testis cellularity) from 10–14d postnatal testis (Figures 1B, 1C and 1G), a 25-fold enrichment of stem cell activity indicates that almost all stem cells are contained within this fraction. Given the number of colonies obtained from αvneg Thy-1low c-Kitneg cells (Figure 1D) and an estimated 10% engraftment efficiency (Nagano et al., 1999), there are 1265 stem cells per 105 cells in this fraction (~1 in 80 cells have stem cell potential).
Figure 1. Isolation and analysis of Plzf +/+ and Plzf −/− SPCs.
(A) Detection of Plzf-expressing spermatogonia from juvenile testis (10–14d postnatal) by intracellular staining and flow cytometry using anti-Plzf antibody.
(B) Plzf expressing cells correlate to a discrete αv-integrin negative, Thy-1 low population by flow cytometry. CD45 marker excludes contaminating leukocytes.
(C) Left panel: Flow cytometric analysis of c-Kit within the Plzf-expressing αv-integrin negative, Thy-1 low population. Right panels: Cell cycle status of c-Kit negative and positive populations plus percentage of cells in G1/S/G2 cell cycle phases from a representative sample.
(D) Left panels: Seminiferous tubules repopulated with donor GFP-positive αv integrin negative, Thy-1 low, c-Kit negative cells in testis transplant assay. Fluorescent image (top) and brightfield image of same recipient testis (bottom) are shown. Right panel: Numbers of GFP positive colonies obtained from αv integrin negative, Thy-1 low, c-Kit negative (sorted) and unsorted testis cells in recipient testes 2 months post-transplant. Data is presented as mean number of colonies per 1×105 donor cells together with standard error of the mean (SEM). Fold enrichment of stem cell activity in sorted populations is indicated (***P<0.0001). Values are averaged from two independent experiments. 13 recipient testes were analyzed for sorted populations and 12 for unsorted.
(E) Representative αv-integrin/Thy-1 flow profiles of Plzf +/+ and Plzf −/− littermate mouse testes at 2 weeks postnatal age. The αv-integrin negative, Thy-1 low gate, containing Plzf-positive cells in WT testis is indicated.
(F) Representative flow cytometry analysis of c-Kit expression within the αv-integrin negative, Thy-1 low testis cell fractions from (E).
(G) Quantification of frequency and absolute numbers of αv integrin negative, Thy-1 low, c-Kit negative testis cells from mice of the indicated postnatal ages and Plzf genotypes (n=3 per genotype and age group, **P<0.002, *P<0.02).
(H) Quantitative RT-PCR analysis of Pou5f1 mRNA expression in testis cell populations from juvenile Plzf +/+ and Plzf −/− littermate mice: Total (unfractionated testis cells), SPCs (αv-integrin negative, Thy-1 low, c-Kit negative), early differentiating spermatogonia (αv-integrin negative, Thy-1 low, c-Kit positive), late differentiating spermatogonia (αv-integrin negative, Thy-1 negative, c-Kit positive) and somatic cells (αv-integrin positive). mRNA levels are normalized to those of β-actin and standard deviations from duplicate reactions are shown.
See also Figure S1.
We next analyzed prepubertal Plzf +/+ and Plzf −/− testis by flow-cytometry to determine if Plzf −/− mice display aberrant SPC activity prior to overt germ cell depletion. While the characteristic αv/Thy-1 flow profile was present in juvenile Plzf −/− testis (2 and 3 weeks postnatal), the αvneg Thy-1low population was depleted relative to the wildtype (Figures 1E and S1C) and the percentage of c-Kitpos cells within the Plzf −/− αvneg Thy-1low fraction was increased (Figures 1F and S1D). The decreased frequency and numbers of αvneg Thy-1low c-Kitneg cells in Plzf −/− testis (Figure 1G) confirms that Plzf loss is detrimental to SPCs while the increase in c-Kitpos cells within the αvneg Thy-1low fraction is suggestive of increased differentiation commitment. As Plzf directly represses c-Kit expression (Filipponi et al., 2007), this can be reflective of a de-repression of the c-Kit locus. However, the Plzf −/− αvneg Thy-1low fraction still contains c-Kitneg cells (Figures 1F and S1D), indicating that aberrant c-Kit expression is unlikely to fully explain the Plzf −/− phenotype. Additionally, both Plzf +/+ and Plzf −/− αvneg Thy-1low c-Kitneg cells show substantial enrichment in expression of the SPC-associated factors Pou5f1/Oct4, Ngn3, Bcl6b and Pou3f1 (Figures 1H and S1E) (Oatley et al., 2006; Ohbo et al., 2003; Wu et al., 2010; Yoshida et al., 2006), confirming the identity of this fraction. We also noted that Plzf −/− αvneg Thy-1low cells had decreased levels of β1 and α6 integrins but equivalent levels of CD9 compared to controls (Figures S1F and S1G) (Kanatsu-Shinohara et al., 2004b; Shinohara et al., 1999). Our data indicate that SPCs from juvenile Plzf −/− mice have an impaired ability to maintain an undifferentiated state, which precedes overt germ cell loss.
We next attempted in vitro culture of isolated Plzf +/+ and Plzf −/− SPCs, adapting previously developed culture systems (Kubota et al., 2004; Seandel et al., 2007). As anticipated, germ cell colony-forming activity under SPC culture conditions was essentially entirely contained within the αvneg Thy-1low fraction (Figure S2A). We successfully derived SPC lines from both Plzf +/+ and Plzf −/− αvneg Thy-1low cells, which grew as discrete colonies on mouse embryonic fibroblast (MEF) feeder cells (Figure 2A) and maintained their cell surface marker identity during exponential growth over 1 year of culture (Figure S2B). Wildtype SPC lines expressed Plzf (Figure 2B) while Plzf −/− SPC lines were more difficult to establish (not shown) consistent with a role for Plzf in SPC function. Early passages of Plzf −/− SPCs had a slower growth rate than wildtype cells (Figures S2C and S2D) but could be maintained long-term and growth became more comparable to wildtype cells at later passages (Figure 5C). The ability to culture Plzf −/− SPCs seemed counterintuitive, but the generation of these cultures became critical for further dissection of Plzf function and to the solution of this apparent contradiction.
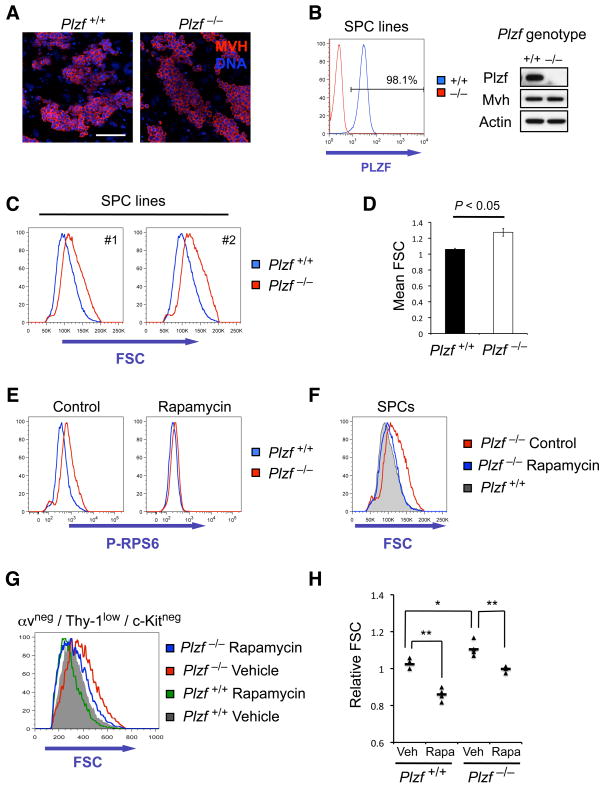
Figure 2. Increased mTORC1 pathway activity in Plzf −/− SPCs.
(A) SPC lines established from culture of αv-integrin negative, Thy-1 low cells from juvenile Plzf +/+ and Plzf −/− littermate mice immunostained for the germ cell marker Mvh and counterstained with DAPI to detect DNA. Scale bar is 100μm.
(B) Cultured SPCs of the indicated genotype analyzed for Plzf expression by intracellular staining and flow cytometry (left panel) and by Western blot (right panel). The germ cell marker Mvh plus actin are used as loading controls.
(C) Analysis of SPC size by flow cytometry. Forward-scatter (FSC) profiles of two independently derived sets of littermate Plzf +/+ and Plzf −/− SPC lines (#1 and #2) are shown.
(D) Quantification of flow cytometry profiles from (C) showing averaged mean FSC with standard deviation and P value.
(E) Control (DMSO) and Rapamycin-treated Plzf +/+ and Plzf −/− SPCs were fixed and permeabilized 48 hours post-treatment and levels of Phospho-RPS6 analyzed by flow cytometry.
(F) Normalization of increased Plzf −/− SPC size by mTORC1 inhibition. FSC profiles of Plzf −/− SPCs treated with DMSO (control) or Rapamycin for 48 hours compared to untreated Plzf +/+ SPCs.
(G) Overlay of representative αv-integrin negative, Thy-1 low, c-Kit negative testis cell fraction FSC profiles from Plzf +/+ and Plzf −/− littermate mice treated from 10 days postnatal age for a period of 1 week with Vehicle or Rapamycin.
(H) Quantification of relative FSC values of αv-integrin negative, Thy-1 low, c-Kit negative testis cells of mice treated as in (G) (n=4 per genotype and treatment group, *P<0.05, **P<0.01).
See also Figure S2.
Figure 5. A negative feedback loop from mTORC1 to the GDNF receptor is activated in Plzf −/− SPCs.
(A) Plzf +/+ and Plzf −/− SPCs starved overnight in basal SPC medium supplemented with DMSO (vehicle control) or Rapamycin were stimulated with 10ng/ml GDNF for 20 minutes prior to harvesting for Western blot analysis.
(B) Quantification of relative levels of phospho-Akt and phospho-RPS6 (corrected to total levels of respective proteins) from (A).
(C) Plzf +/+ and Plzf −/− SPCs were plated in medium containing decreasing concentrations of GDNF, harvested one week later and counted to determine fold cell recovery as an indicator of growth. The concentration of GDNF was varied from 4ng/ml (left bars) to 1ng/ml (right bars). Standard deviations from duplicate wells are indicated (**P<0.01, *P<0.02).
(D) Quantitative RT-PCR analysis of GDNF receptor components in Plzf +/+ and Plzf −/− SPCs treated with DMSO (vehicle control) or Rapamycin for 48 hours. mRNA levels are normalized to those of β-actin and standard deviations from duplicate reactions are shown.
(E) Flow cytometry analysis of Gfr 1 levels from SPC lines treated as in (D).
(F) Quantitative RT-PCR analysis of GDNF receptor components in αv-integrin negative, Thy-1 low, c-Kit negative testis cell fractions pooled from 2 weeks-old Plzf +/+ and Plzf −/− mice. mRNA levels are normalized to those of β-actin and standard deviations from duplicate reactions are shown.
See also Figure S5.
An additional indicator of SPC potential is the ability to derive embryonic stem-like cells from the cultured lines (multipotent adult spermatogonial-derived stem cells; MASCs) (Kanatsu-Shinohara et al., 2004a; Seandel et al., 2007). We observed spontaneous formation of MASC colonies from 2 out of 7 wildtype lines (Figure S2E). MASC formation was not observed in 4 Plzf −/− SPC lines, possibly indicating a defective capability to generate MASCs. The multipotent capabilities of MASC lines were verified by teratoma formation assay (Figure S2F). Upon SPC to MASC conversion, expression of the pluripotency-associated factors Pou5f1/Oct4, Sox2 and Nanog were substantially increased while Plzf expression was lost (Figure S2G and data not shown) (Seandel et al., 2007). Formation of MASCs indicates that SPC potential is maintained in our culture system, while the apparent inability of Plzf −/− SPCs to form MASCs can be consistent with their defective function.
Plzf −/− SPCs show enhanced mTORC1 activity
From analysis of cultured SPC lines, we noticed that Plzf −/− cells were physically larger than wildtype SPCs, measured by the FSC parameter of flow cytometry (Figures 2C and 2D). Alterations in cell size are associated with changes in activity of mTORC1 acting through its downstream targets S6Kinase1 (S6K1), which phosphorylates ribosomal S6 protein (RPS6), and 4EBP1 (Fingar et al., 2002; Ruvinsky et al., 2005). Given the role of mTORC1 in hematopoietic stem cells (Gan and DePinho, 2009), we considered that aberrant mTORC1 activity in Plzf −/− SPCs could contribute to their defective maintenance. Importantly, Plzf −/− SPCs had elevated levels of phosphorylated RPS6 compared to wildtype cells, confirming increased mTORC1 activity (Figures 2E and 3C). Inhibition of mTORC1 with Rapamycin decreased the size of Plzf −/− SPCs to that of wildtype cells suggesting that elevated mTORC1 activity was responsible for the size increase (Figure 2F). Rapamycin was confirmed to inhibit RPS6 phosphorylation in Plzf −/− SPCs (Figure 2E). Increased mTORC1 activity in Plzf −/− SPCs was associated with elevated levels of cellular protein (Figure S2H), consistent with the role of mTORC1 in regulating protein translation. Importantly, freshly isolated Plzf −/− SPCs were also physically larger (Figures 2G and 2H) and Rapamycin treatment of Plzf −/− mice normalized SPC size (Figures 2G and 2H). Together, our data indicates that Plzf inhibits mTORC1 activation in SPCs.
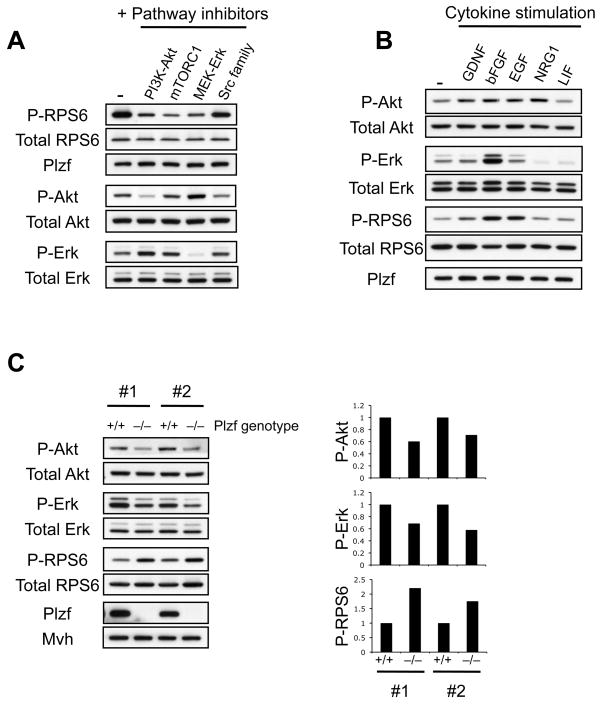
Figure 3. Upstream pathways and growth factors regulating mTORC1 in SPCs.
(A) WT SPCs fed with complete SPC medium containing inhibitors to the indicated signaling pathways were harvested 5 hours after treatment and analyzed by Western blot for the indicated proteins and phospho (P)-proteins.
(B) WT SPCs were starved overnight in basal SPC medium lacking cytokine supplements. The indicated cytokines were then added and SPCs harvested 20 minutes later then analyzed by Western blot. Cytokines were used at the concentrations present in complete SPC medium. Neuregulin1 (NRG1) is not a standard supplement for SPC medium although SPCs are responsive to it (see text).
(C) Western blot analysis of two independently derived sets of littermate Plzf +/+ and Plzf −/− SPC lines (#1 and #2) under steady state conditions is shown to the left. Quantification of the relative levels of phospho-Akt, Erk and RPS6 (corrected to the total levels of respective proteins) are shown in panels on the right.
See also Figure S3.
Growth factor-mediated regulation of mTORC1 in SPCs
Given the possibility that mTORC1 activity can regulate SPC function, we next defined key regulatory inputs of mTORC1 in wildtype SPCs. Growth factor signaling represents a major activating input of mTORC1 (Ma and Blenis, 2009; Shaw and Cantley, 2006) and mTORC1 inhibition interferes with mitogenic stimuli causing cell cycle arrest (Brown et al., 1994). Indeed, Rapamycin suppressed SPC colony growth and caused an accumulation of cells in G1 phase of the cell cycle (Figure S3A and S3B).
Growth factor receptors activate mTORC1 by signaling through phosphoinositide 3-kinase (PI3K)/Akt and Ras/Erk MAPK, which inhibit the TSC1/TSC2 complex (Huang and Manning, 2008); TSC1/TSC2 negatively regulates mTORC1 via its GTPase-activating protein activity towards the small G-protein Rheb. Treatment of wildtype SPCs with PI3K/Akt or Erk MAPK pathway inhibitors significantly reduced levels of phosphorylated RPS6 (Figure 3A), indicating that efficient mTORC1 activation in SPCs requires both PI3K/Akt and Erk MAPK. Inhibition of Src family kinases, implicated in the GDNF response of SPCs (Oatley et al., 2007), had modest effects on phospho-RPS6 (Figure 3A). Therefore, despite the ability of Src family kinases to activate PI3K/Akt downstream GDNF (Encinas et al., 2001; Oatley et al., 2007), this SPC self-renewal pathway couples inefficiently to mTORC1.
SPCs are maintained with a cocktail of growth factors (Kanatsu-Shinohara et al., 2003; Seandel et al., 2007) and are responsive to others (Hamra et al., 2007); thus we next assessed contributions of these factors to mTORC1 activation in wildtype SPCs. We found that GDNF triggered a modest phosphorylation of RPS6 while other mitogens (including bFGF) induced much higher levels of phospho-RPS6 (Figure 3B). The relative abilities of GDNF and bFGF to activate mTORC1 correlated to their efficiency of Akt and Erk activation (Figure 3B). However, this correlation did not hold true for epidermal growth factor (EGF) stimulation, possibly reflecting additional pathways by which EGF activates mTORC1 (Fan et al., 2009). In summary, the self-renewal signal GDNF poorly activates mTORC1 when compared to more general mitogens for SPCs (bFGF, EGF), in agreement with the Src kinase inhibition data (see above). However, we note that while GDNF is considered the key SPC self-renewal signal, SPC expansion and self-renewal in vitro requires a combination of GDNF plus other factors (e.g. bFGF) that stimulate mTORC1 more efficiently (Lee et al., 2007b).
Plzf uncouples mTORC1 activity from growth factor signaling in SPCs through Redd1 modulation
Given that mTORC1 activity in SPCs is regulated by growth factors present in the culture medium and resultant PI3K/Akt and Erk-MAPK activation, we next assessed whether the elevated mTORC1 activity of Plzf −/− SPCs correlated with an enhanced growth factor response. However, in comparison to wildtype cells, the Akt and Erk activities of steady state Plzf −/− SPCs were decreased while phospho-RPS6 was increased (Figure 3C), indicating that the elevated mTORC1 activity is not due to increased activity of growth factor pathways.
We next considered alternative mechanisms by which mTORC1 activity would be increased in Plzf −/− SPCs. Cellular stress-response pathways provide additional regulatory inputs resulting in mTORC1 inhibition when conditions such as low energy availability or hypoxia require temporary arrest of cell growth (Huang and Manning, 2008). We hypothesized that mTORC1 hyperactivity in Plzf −/− SPCs could be due to reduced activity of stress response pathways. Accordingly, we first assessed levels of upstream regulatory proteins as stress pathways converge on mTORC1 at the level of TSC1/TSC2. However, we did not find significant differences in levels of Tsc1, Tsc2, Rheb or the mTOR kinase itself in Plzf −/− SPCs compared to wildtype cells (Figure 4A).
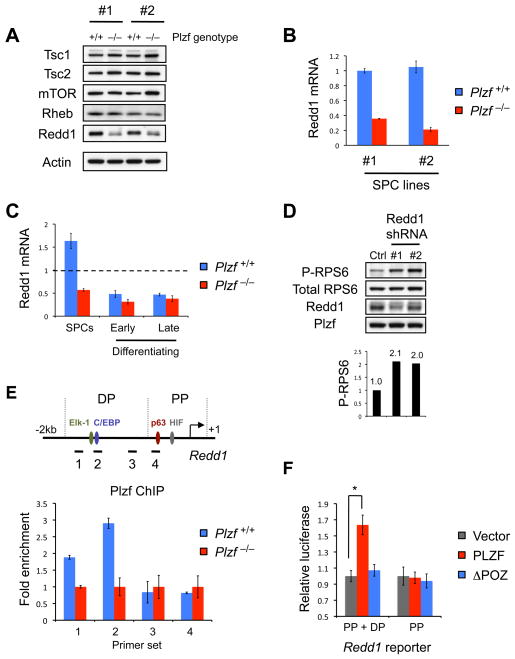
Figure 4. Plzf regulates expression of the mTORC1 pathway inhibitor Redd1.
(A) Western blot analysis for components of the mTORC1 pathway in two independently derived sets of littermate Plzf +/+ and Plzf −/− SPC lines (#1 and #2) under steady state conditions.
(B) Quantitative RT-PCR analysis of Redd1 mRNA expression in independently derived Plzf +/+ and Plzf −/− SPC lines. mRNA levels are normalized to those of β-actin and standard deviations from duplicate reactions are shown.
(C) Quantitative RT-PCR analysis of Redd1 mRNA expression in spermatogonial fractions from juvenile Plzf +/+ and Plzf −/− littermate mice: SPCs (αv-integrin negative, Thy-1 low, c-Kit negative), early differentiating spermatogonia (αv-integrin negative, Thy-1 low, c-Kit positive), late differentiating spermatogonia (αv-integrin negative, Thy-1 negative, c-Kit positive). mRNA levels are normalized to those of β-actin and standard deviations from duplicate reactions are shown. Dashed line indicates Redd1 expression levels in unfractionated testis cells.
(D) WT SPCs infected with control shRNA (against GFP) or two independent shRNA constructs against Redd1 were analyzed by Western blot for the indicated proteins. Quantification of relative levels of phospho-RPS6 (corrected to total RPS6 levels) is shown under respective lanes. Cells were under steady-state culturing conditions (see also Figure S4).
(E) Top panel: Redd1 promoter (from translation start site at +1 to 2kb upstream) with location of previously described transcription factor binding sites. The promoter is divided into proximal and distal regions (PP and DP respectively). Positions of ChIP amplicons are indicated. Bottom panel: ChIP assay. Quantitative PCR for Redd1 promoter regions from WT SPC chromatin pulled down with Plzf antibody. Fold enrichment is shown relative to background of chromatin pulled down from Plzf −/− SPCs with the same antibody.
(F) Luciferase reporter assay with constructs containing the PP+DP Redd1 promoter regions or PP alone. 293HEK cells were transfected with the appropriate luciferase reporter together with PLZF constructs or vector control as indicated. Reporter activities were normalized to that of TK-Renilla and empty vector luciferase reporter controls (*P<0.001).
By contrast, we noticed that levels of Redd1 (also known as Ddit4, Rtp801 and Dig2) were substantially lower in Plzf −/− SPCs compared to controls, at both protein and mRNA levels (Figure 4A and 4B). Redd1 is induced by multiple types of cell stress (e.g. hypoxia, DNA damage) and by developmental signals and inhibits mTORC1 through regulation of TSC1/TSC2 (Brugarolas et al., 2004; Corradetti et al., 2005; Ellisen et al., 2002). As a role for Redd1 in SPCs is not described, we analyzed the distribution of Redd1 expression in isolated spermatogonial fractions and found a relative enrichment of Redd1 mRNA in SPCs (Figure 4C). We also confirmed that freshly isolated Plzf −/− SPCs had lower Redd1 expression compared to controls (Figure 4C). Furthermore, shRNA knockdown of Redd1 in wildtype SPCs increased mTORC1 activity (Figure 4D and S4). Together, our data suggest that Plzf opposes mTORC1 by maintaining Redd1 expression and that Redd1 is a key mTORC1 regulator in SPCs.
Plzf is a transcriptional activator of the mTORC1 inhibitor Redd1 in SPCs
Redd1 expression was decreased at the mRNA level in Plzf −/− SPCs. We therefore tested whether Plzf could directly induce Redd1 expression. We first performed a chromatin immunoprecipitation (ChIP) assay to assess whether Plzf was bound to the Redd1 promoter in SPCs. We scanned a 2kb region upstream Redd1 that contains binding sites for multiple transcription factors known to regulate Redd1 expression (Figure 4E) (Ellisen et al., 2002; Lin et al., 2005; Shoshani et al., 2002). Importantly, we could detect Plzf association with distal promoter (DP) regions but not proximal promoter (PP) regions (Figure 4E), indicating that Plzf regulates Redd1 expression through recruitment to the DP. To confirm this we performed luciferase assays with REDD1 promoter constructs containing both DP+PP elements and the PP element alone (Figure 4F). In agreement with our ChIP results, PLZF activated the DP+PP construct but not the PP construct. Further, deletion of the PLZF POZ domain prevented PP+DP reporter activation, indicating a requirement for this protein-protein interaction domain. We conclude that Plzf directly activates Redd1 through the DP in order to inhibit mTORC1 in SPCs.
Active mTORC1 inhibits SPC self-renewal pathways via a negative feedback loop
As Plzf −/− SPCs show increased mTORC1 but decreased Akt activities (Figure 3C), we considered that negative feedback from mTORC1 could prevent Plzf −/− cells from activating Akt in response to growth factors. In cells lacking TSC1/TSC2, increased mTORC1-signaling induces a negative feedback loop from the mTORC1 downstream target S6K to upstream signaling components causing inhibition of PI3K/Akt (Harrington et al., 2004; Shah et al., 2004). Therefore, we tested whether aberrant mTORC1 activity inhibited the response of Plzf −/− SPCs to GDNF, a growth factor required for SPC self-renewal, which could explain the maintenance defect of Plzf −/− SPCs. We compared the response of starved Plzf +/+ and Plzf −/− SPCs to GDNF in the absence or presence of Rapamycin to vary mTORC1 activity (Figures 5A and 5B). Consistent with previous data, Plzf −/− SPCs showed lower levels of basal and GDNF-stimulated Akt activity when compared to wildtype SPCs, indicating a substantially reduced ability of Plzf −/− cells to activate PI3K/Akt. However, upon Rapamycin treatment, the ability of Plzf −/− SPCs to activate Akt in response to GDNF was significantly increased and became comparable to that of wildtype cells. This rescue of GDNF responsiveness by Rapamycin indicates that a negative feedback response from aberrantly activated mTORC1 suppresses the response of Plzf −/− SPCs to GDNF. Indeed, if GDNF levels in the media were reduced, Plzf −/− SPC growth was inhibited more substantially than that of the wildtype cells (Figure 5C). A decreased sensitivity to GDNF due to negative feedback from mTORC1 can explain the defect in Plzf −/− SPC maintenance in vivo when levels of GDNF are limiting (Meng et al., 2000) and how Plzf −/− SPCs can be cultured in vitro when GDNF is supplied to excess. Importantly, GDNF expression in Plzf −/− testis was equivalent to that of the wildtype (Figure S5), ruling out non-cell autonomous defects in production of niche factors.
Activated mTORC1 suppresses expression of GDNF receptor components
We next sought to determine the mechanism by which activated mTORC1 inhibits response of Plzf −/− SPCs to GDNF. Negative feedback from mTORC1 to PI3K/Akt was originally characterized in the context of insulin responsiveness and signaling through IRS1/2 proteins (Harrington et al., 2005). Although IRS proteins have been implicated in activation of PI3K by the GDNF receptor component c-Ret (Hennige et al., 2000), we noticed that expression of the GDNF receptor components GFRα1 and c-Ret in SPCs were responsive to Rapamycin (Figure 5D and 5E). Cultured Plzf −/− SPCs expressed lower levels of GFRα1 and c-Ret compared to wildtype cells (correlating with reduced GDNF-responsiveness) but upon mTORC1 inhibition, expression of these GDNF receptor components was increased back to wildtype levels. Expression of GFRα1 and c-Ret were also lower in freshly isolated Plzf −/− SPCs compared to wildtype SPCs (Figure 5F), confirming that Plzf loss disrupts GDNF receptor expression in vivo. Our data suggest that aberrantly activated mTORC1 inhibits the response of Plzf −/− SPCs to GDNF by opposing expression of the receptor. Interestingly, mTORC1 inhibits upstream signaling events in MEFs through transcriptional inhibition of the PDGF receptor (Zhang et al., 2007). In SPCs, negative feedback between mTORC1 and the GDNF receptor will lead to loss of self-renewal when mTORC1 is hyperactivated, such as occurs upon loss of Plzf expression (Figure S6A). This feedback loop would inversely couple cell growth to self-renewal, so that upon expansion of the stem cell pool by mitogenic stimulation and resultant mTORC1 activation, response of the cells to self-renewal signals is inhibited, restricting stem cell numbers. Interestingly, GDNF activates mTORC1 weakly in SPCs when compared to other mitogens (e.g. bFGF) (Figure 3B), suggesting that GDNF would trigger a limited activation of the negative feedback response while bFGF could cause more extensive inhibition of GDNF signaling via mTORC1.
Rapamycin treatment attenuates the SPC maintenance defect of Plzf −/− mice and increases SPCs in WT control mice
As inhibition of aberrant mTORC1 activity in Plzf −/− SPCs in vitro rescues their response to GDNF, we next asked whether mTORC1 inhibition in vivo could rescue the Plzf −/− SPC maintenance defect. We initiated Rapamycin treatment of Plzf −/− and wildtype control mice during early stages of postnatal development (10d postnatal) and continued daily treatment for 1 week, a regimen able to normalize the increased size of Plzf −/− SPCs (Figure 2G and 2H). Subsequent analysis of SPC compartment status demonstrated that Rapamycin increased both frequency and numbers of vneg Thy-1low c-Kitneg cells in Plzf −/− testis, approaching those values found in vehicle-treated wildtype controls (Figures 6A and 6B). Rapamycin treatment of Plzf −/− mice increased both the frequency of αvneg Thy-1low cells and normalized the increased percentage of c-Kitpos cells within this fraction compared to controls (Figure 6A). We conclude that aberrant mTORC1 activation can explain, at least in part, the SPC maintenance defect of Plzf −/− mice.
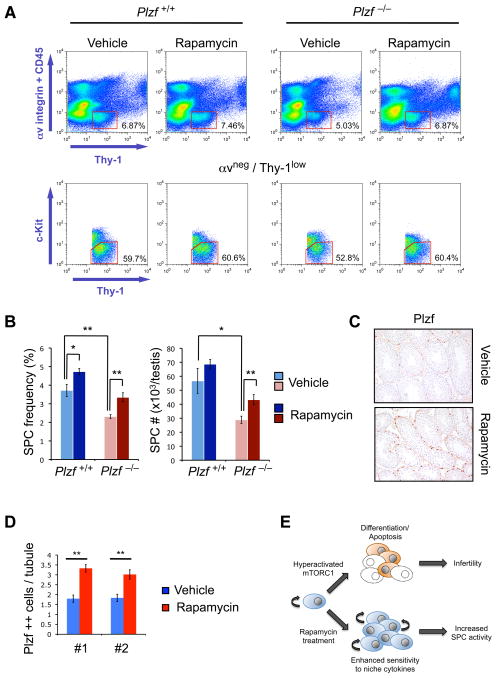
Figure 6. mTORC1 inhibition attenuates SPC maintenance defect of Plzf −/− mice.
(A) Top panels: Representative αv-integrin/Thy-1 flow profiles of testis cells from Plzf +/+ and Plzf −/− mice treated with Vehicle or Rapamycin from 10 days postnatal age for a period of 1 week and analyzed the day after completing treatment. Bottom panels: Flow cytometry analysis of c-Kit expression within the corresponding αv-integrin negative, Thy-1 low fractions.
(B) Quantification of frequency and absolute numbers of αv integrin negative, Thy-1 low, c-Kit negative testis cells from mice of the indicated genotypes treated with Vehicle or Rapamycin as in (A) (n≥5 per genotype and treatment group, **P<0.02, *P<0.05).
(C) WT juvenile mice (2–3 weeks postnatal) were treated daily for 2 weeks with Rapamycin or Vehicle. Testes were harvested and subject to immunohistochemistry for Plzf. Representative images are 20×.
(D) Quantification of cells showing strong positivity for Plzf in seminiferous tubules of testis from (C), representing SPCs. Results from duplicate experiments (#1, #2) are shown together with SEM (**P<0.01).
(E) Illustration of the effects of aberrant mTORC1 activity or inhibition of physiological mTORC1 activation with Rapamycin on SPC numbers and function.
See also Figure S6.
Intriguingly, Rapamycin also increased the frequency of SPCs in wildtype mice (Figure 6B), suggesting that mTORC1 inhibition stimulates SPC function in a wildtype setting. Indeed, in wildtype SPCs, Rapamycin enhanced Akt activation in response to GDNF (Figures 5A and 5B) and increased GDNF receptor expression (Figures 5D and 5E). Importantly, prolonged Rapamycin treatment of juvenile wildtype mice increased the number of cells with high levels of Plzf expression in the testis (Figures 6C, 6D and S6B) and enhanced GDNF receptor expression (Figure S6C), suggesting that physiological mTORC1 activity controls size and function of the SPC pool in vivo.
DISCUSSION
Disruption of genes encoding for negative regulators of the PI3K/Akt/mTORC1 pathway (e.g. Pten, Tsc1 and Pml) leads to loss of hematopoietic stem cell (HSC) quiescence and subsequent exhaustion. Rapamycin prevents HSC depletion, demonstrating that aberrant mTORC1 activation is responsible for stem cell loss (Chen et al., 2008; Gan et al., 2008; Ito et al., 2008; Yilmaz et al., 2006). The negative effects of mTORC1 on HSC maintenance have been attributed to its downstream targets in cell growth pathways (Chen et al., 2008) and aberrant mTORC1 activity elicits activation of tumor suppressive mechanisms that can contribute to stem cell failure (Figure S6D) (Alimonti et al., 2010; Lee et al., 2007a). By studying Plzf function in SPCs, we now implicate negative feedback responses from the mTORC1 pathway to receptors required to transduce niche-derived self-renewal signals in the loss of stem cell potential (Figure S6E).
HSC maintenance is also linked to niche signals acting through growth factor receptors (Arai et al., 2004; Yoshihara et al., 2007) thus it will be of interest to translate our findings into the hematopoietic system. In addition, the decreased sensitivity of Plzf −/− SPCs to GDNF mimics a reduced production of niche-signals; the decline in stem cell numbers in aging mouse testis is due partly to declining niche function and GDNF production (Ryu et al., 2006; Zhang et al., 2006), thus loss of Plzf expression can represent a premature aging phenotype. Our studies therefore provide further insight into the association between stem cell maintenance, mTORC1 and aging (Harrison et al., 2009; Rossi et al., 2008).
A correlation between aging and cancer is also apparent, likely due to the accumulation of multiple genetic hits over time for tumor development (Rossi et al., 2008). As stem cells are resident long-term in tissues and possess self-renewal capability (a feature of cancer stem cells), they are suggested to be the source of many cancers. However, loss of tumor suppressors (e.g. Pten, Pml) trigger stem cell depletion (Ito et al., 2009) and subsequent cancer development is associated with additional genetic hits (Guo et al., 2008). It is proposed that oncogenic hits in stem cells elicit activation of fail-safe mechanisms that have to be evaded to allow cancer development (Figure S6D). These fail-safe mechanisms prevent propagation of clones of cells derived from stem cells with oncogenic mutations and the consequent risk of acquiring additional mutations that lead to cancer. The mTORC1 hyper-activation that occurs in response to loss of tumor suppressors such as Pten and Pml is likely a trigger of this fail-safe mechanism as Rapamycin prevents stem cell exhaustion. Based on our new model, mTORC1 hyperactivation in response to oncogenic stimulation may also desensitize the cancer stem cell to niche-signals that are required for self-renewal; thus additional mutations supporting niche-independent self-renewal could allow cancer to develop (Figure S6E).
PLZF was first identified from involvement in acute promyelocytic leukemia (APL) (Chen et al., 1993). Thus, our model of Plzf-dependent SPC maintenance can have implications for leukemia pathogenesis. The ability of Plzf to inhibit mTORC1 through Redd1 induction may be relevant in this context given that perturbed PLZF function and elevated mTORC1 activity are associated with leukemia development (He et al., 2000; Martelli et al., 2007; McConnell and Licht, 2007). In addition, REDD1 is involved in leukemic and myeloid cell differentiation (Gery et al., 2007; Nishioka et al., 2009), suggesting that this gene can be a relevant PLZF target in hematopoietic cells. PML, also identified from its involvement in chromosomal translocations with the RARA gene in APL, is required for HSC maintenance and inhibits mTORC1 (Bernardi et al., 2006; Ito et al., 2008); thus illustrating a striking commonality of function between the RARA partners of APL.
We have identified a role for Plzf-mediated Redd1 expression in inhibiting mTORC1 activation in SPCs. As mTORC1 activity has a significant impact on the response of SPCs to GDNF, the tight regulation of this pathway is critical. Redd1 is also induced in response to cellular stress and in particular, by hypoxia (Brugarolas et al., 2004; Shoshani et al., 2002). Interestingly, the HSC niche has been suggested to exist in a hypoxic state (Kubota et al., 2008; Parmar et al., 2007) that, if the SPC niche had similar characteristics, could be responsible for Redd1 induction. However, SPCs reside close to the vasculature in vivo (Yoshida et al., 2007) and Redd1 expression is maintained when SPCs are cultured at atmospheric oxygen levels. Thus Redd1 expression in this case depends on a Plzf tonic transcriptional activity and its induction is seemingly independent of hypoxia.
As mTORC1 inhibition enhances SPC function even in a wildtype setting, our data also have important therapeutic implications. It is tempting to speculate that Rapamycin could be used to enhance SPC activity in humans; potentially allowing improved SPC culture from testicular biopsies and generation of therapeutically relevant MASCs (Figure 6E). Furthermore, our findings provide a novel and straightforward explanation for the requirement of Plzf in germline maintenance.
EXPERIMENTAL PROCEDURES
Mouse maintenance and manipulation
Plzf −/− mice are previously described (Costoya et al., 2004). Mice were treated with Rapamycin (LC Laboratories) at a daily dose of 4mg/kg as described (Yilmaz et al., 2006). Transgenic mice expressing EGFP from the β-actin promoter were from The Jackson Laboratories. Testis transplant assays were performed as described (Seandel et al., 2007) with 50×103 unfractionated or 5×103 αvneg Thy-1low c-Kitneg testis cells injected per recipient testis. Teratoma formation assays in NOD/SCID mice (Jackson Laboratories) were performed as described (Seandel et al., 2007).
Antibody generation
Monoclonal anti-PLZF antibody (clone 9E12) was raised in Armenian hamster against a pool of Keyhole Limpet Hemocyanin (KLH)-conjugated peptides at the Memorial Sloan-Kettering Cancer Center (MSKCC) antibody facility and reacts to a peptide within the hinge domain of mouse Plzf (RSKEGPGTPTRRSVITSARE). Antibody was directly conjugated to Alexa 488 or Alexa 647 for use in flow cytometry (MSKCC antibody facility).
Flow cytometry
Single cell suspensions were prepared from testis as described (Ogawa et al., 1997) and resuspended in Phosphate Buffered Saline (PBS) containing 2% fetal bovine serum (FBS) for subsequent staining and analysis (Filipponi et al., 2007). For a detailed description of flow cytometry methods and antibodies used refer to the Supplemental Experimental Procedures.
Cell culture and treatment
SPC fractions from testis were plated directly onto mitotically inactivated MEF feeder cells. Medium for SPC culture was as previously described (Seandel et al., 2007) supplemented with 10ng/ml IGF-I (Peprotech). MASCs and mouse ESCs (v6.5) were cultured in embryonic stem cell medium on MEF feeders (Seandel et al., 2007). Knockdown of Redd1 expression in SPCs by shRNA was performed using pLKO.1 lentiviral vectors (Open Biosystems). A detailed description of methods is included in the Supplemental Experimental Procedures.
Immunofluorescence (IF), immunohistochemistry (IHC) and Western blot
Tissue and cultured cells were fixed in 4% paraformaldehyde prior to processing for IF and IHC as described (Costoya et al., 2004; Filipponi et al., 2007). For Western blot analysis, cells were lysed in RIPA buffer and processed as described (Costoya et al., 2008). Detailed methods are included in the Supplemental Experimental Procedures.
Chromatin immunoprecipitation (ChIP)
SPCs were trypsinized and resuspended in SPC medium then incubated in tissue culture dishes for 30 minutes to remove contaminating (adherent) MEFs. Preparation and immunoprecipitation of chromatin was performed using a SimpleChIP Enzymatic Chromatin IP kit (Cell Signaling Technology) according to manufacturers instructions and monoclonal anti-PLZF antibody (Calbiochem). DNA samples were analyzed by quantitative PCR using a QuantiTect SYBR Green PCR kit (Qiagen) and Light Cycler (Roche). Primer sequences for the Redd1 promoter are included in the Supplemental Experimental Procedures.
Luciferase assay
REDD1 promoter constructs are described (Lin et al., 2005) and were cloned from U2OS genomic DNA into the pGL3-enhancer vector (Promega). Luciferase assays were performed with 293HEK cells as described (Barna et al., 2002). Please refer to Supplemental Experimental Procedures for details.
Quantitative reverse transcription polymerase chain reaction (qRT-PCR)
RNA was harvested using Trizol reagent (Invitrogen) then used for first strand cDNA synthesis and subsequent quantitative PCR analysis as described (Filipponi et al., 2007). For primer sequences and detailed methodology please see Supplemental Experimental Procedures.
Statistical analysis
Results from cell growth assays, testis transplants and flow cytometry analysis were assessed for statistical significance by a standard two-tailed t-test.
Supplementary Material
Acknowledgments
We thank current and past members of the Pandolfi lab and in particular Takahiro Maeda and Rosa Bernardi for helpful discussion and advice. We would also like to thank Antonella Papa for generating luciferase reporters, Sharmila Fagoonee for experimental help, Leif Ellisen for Redd1 promoter constructs, and the flow cytometry facilities of BIDMC and MSKCC for expert support. M.S. is a Stanley and Fiona Druckenmiller Fellow of the New York Stem Cell Foundation. This work was supported by NIH grants to P.P.P.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alimonti A, Nardella C, Chen Z, Clohessy JG, Carracedo A, Trotman LC, Cheng K, Varmeh S, Kozma SC, Thomas G, et al. A novel type of cellular senescence that can be enhanced in mouse models and human tumor xenografts to suppress prostate tumorigenesis. J Clin Invest. 2010;120:681–693. doi: 10.1172/JCI40535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, Ito K, Koh GY, Suda T. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Barna M, Merghoub T, Costoya JA, Ruggero D, Branford M, Bergia A, Samori B, Pandolfi PP. Plzf mediates transcriptional repression of HoxD gene expression through chromatin remodeling. Dev Cell. 2002;3:499–510. doi: 10.1016/s1534-5807(02)00289-7. [DOI] [PubMed] [Google Scholar]
- Bernardi R, Guernah I, Jin D, Grisendi S, Alimonti A, Teruya-Feldstein J, Cordon-Cardo C, Simon MC, Rafii S, Pandolfi PP. PML inhibits HIF-1alpha translation and neoangiogenesis through repression of mTOR. Nature. 2006;442:779–785. doi: 10.1038/nature05029. [DOI] [PubMed] [Google Scholar]
- Brown EJ, Albers MW, Shin TB, Ichikawa K, Keith CT, Lane WS, Schreiber SL. A mammalian protein targeted by G1-arresting rapamycin-receptor complex. Nature. 1994;369:756–758. doi: 10.1038/369756a0. [DOI] [PubMed] [Google Scholar]
- Brugarolas J, Lei K, Hurley RL, Manning BD, Reiling JH, Hafen E, Witters LA, Ellisen LW, Kaelin WG., Jr Regulation of mTOR function in response to hypoxia by REDD1 and the TSC1/TSC2 tumor suppressor complex. Genes Dev. 2004;18:2893–2904. doi: 10.1101/gad.1256804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buaas FW, Kirsh AL, Sharma M, McLean DJ, Morris JL, Griswold MD, de Rooij DG, Braun RE. Plzf is required in adult male germ cells for stem cell self-renewal. Nat Genet. 2004;36:647–652. doi: 10.1038/ng1366. [DOI] [PubMed] [Google Scholar]
- Castilho RM, Squarize CH, Chodosh LA, Williams BO, Gutkind JS. mTOR mediates Wnt-induced epidermal stem cell exhaustion and aging. Cell Stem Cell. 2009;5:279–289. doi: 10.1016/j.stem.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Zheng P. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205:2397–2408. doi: 10.1084/jem.20081297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Brand NJ, Chen A, Chen SJ, Tong JH, Wang ZY, Waxman S, Zelent A. Fusion between a novel Kruppel-like zinc finger gene and the retinoic acid receptor-alpha locus due to a variant t(11;17) translocation associated with acute promyelocytic leukaemia. EMBO J. 1993;12:1161–1167. doi: 10.1002/j.1460-2075.1993.tb05757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corradetti MN, Inoki K, Guan KL. The stress-inducted proteins RTP801 and RTP801L are negative regulators of the mammalian target of rapamycin pathway. J Biol Chem. 2005;280:9769–9772. doi: 10.1074/jbc.C400557200. [DOI] [PubMed] [Google Scholar]
- Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, Orwig KE, Wolgemuth DJ, Pandolfi PP. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet. 2004;36:653–659. doi: 10.1038/ng1367. [DOI] [PubMed] [Google Scholar]
- Costoya JA, Hobbs RM, Pandolfi PP. Cyclin-dependent kinase antagonizes promyelocytic leukemia zinc-finger through phosphorylation. Oncogene. 2008 doi: 10.1038/onc.2008.7. [DOI] [PubMed] [Google Scholar]
- David G, Alland L, Hong SH, Wong CW, DePinho RA, Dejean A. Histone deacetylase associated with mSin3A mediates repression by the acute promyelocytic leukemia-associated PLZF protein. Oncogene. 1998;16:2549–2556. doi: 10.1038/sj.onc.1202043. [DOI] [PubMed] [Google Scholar]
- de Rooij DG, Russell LD. All you wanted to know about spermatogonia but were afraid to ask. J Androl. 2000;21:776–798. [PubMed] [Google Scholar]
- Doulatov S, Notta F, Rice KL, Howell L, Zelent A, Licht JD, Dick JE. PLZF is a regulator of homeostatic and cytokine-induced myeloid development. Genes Dev. 2009;23:2076–2087. doi: 10.1101/gad.1788109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellisen LW, Ramsayer KD, Johannessen CM, Yang A, Beppu H, Minda K, Oliner JD, McKeon F, Haber DA. REDD1, a developmentally regulated transcriptional target of p63 and p53, links p63 to regulation of reactive oxygen species. Mol Cell. 2002;10:995–1005. doi: 10.1016/s1097-2765(02)00706-2. [DOI] [PubMed] [Google Scholar]
- Encinas M, Tansey MG, Tsui-Pierchala BA, Comella JX, Milbrandt J, Johnson EM., Jr c-Src is required for glial cell line-derived neurotrophic factor (GDNF) family ligand-mediated neuronal survival via a phosphatidylinositol-3 kinase (PI-3K)-dependent pathway. J Neurosci. 2001;21:1464–1472. doi: 10.1523/JNEUROSCI.21-05-01464.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan QW, Cheng C, Knight ZA, Haas-Kogan D, Stokoe D, James CD, McCormick F, Shokat KM, Weiss WA. EGFR signals to mTOR through PKC and independently of Akt in glioma. Sci Signal. 2009;2:ra4. doi: 10.1126/scisignal.2000014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipponi D, Hobbs RM, Ottolenghi S, Rossi P, Jannini EA, Pandolfi PP, Dolci S. Repression of kit expression by Plzf in germ cells. Mol Cell Biol. 2007;27:6770–6781. doi: 10.1128/MCB.00479-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16:1472–1487. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Kohlhase J, Bohm D, Schweiger B, Hoffmann D, Heitmann M, Horsthemke B, Wieczorek D. Biallelic loss of function of the promyelocytic leukaemia zinc finger (PLZF) gene causes severe skeletal defects and genital hypoplasia. J Med Genet. 2008;45:731–737. doi: 10.1136/jmg.2008.059451. [DOI] [PubMed] [Google Scholar]
- Gan B, DePinho RA. mTORC1 signaling governs hematopoietic stem cell quiescence. Cell Cycle. 2009;8:1003–1006. doi: 10.4161/cc.8.7.8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan B, Sahin E, Jiang S, Sanchez-Aguilera A, Scott KL, Chin L, Williams DA, Kwiatkowski DJ, DePinho RA. mTORC1-dependent and -independent regulation of stem cell renewal, differentiation, and mobilization. Proc Natl Acad Sci U S A. 2008;105:19384–19389. doi: 10.1073/pnas.0810584105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gery S, Park DJ, Vuong PT, Virk RK, Muller CI, Hofmann WK, Koeffler HP. RTP801 is a novel retinoic acid-responsive gene associated with myeloid differentiation. Exp Hematol. 2007;35:572–578. doi: 10.1016/j.exphem.2007.01.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Lasky JL, Chang CJ, Mosessian S, Lewis X, Xiao Y, Yeh JE, Chen JY, Iruela-Arispe ML, Varella-Garcia M, et al. Multi-genetic events collaboratively contribute to Pten-null leukaemia stem-cell formation. Nature. 2008;453:529–533. doi: 10.1038/nature06933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamra FK, Chapman KM, Nguyen D, Garbers DL. Identification of neuregulin as a factor required for formation of aligned spermatogonia. J Biol Chem. 2007;282:721–730. doi: 10.1074/jbc.M608398200. [DOI] [PubMed] [Google Scholar]
- Harrington LS, Findlay GM, Gray A, Tolkacheva T, Wigfield S, Rebholz H, Barnett J, Leslie NR, Cheng S, Shepherd PR, et al. The TSC1-2 tumor suppressor controls insulin-PI3K signaling via regulation of IRS proteins. J Cell Biol. 2004;166:213–223. doi: 10.1083/jcb.200403069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington LS, Findlay GM, Lamb RF. Restraining PI3K: mTOR signalling goes back to the membrane. Trends Biochem Sci. 2005;30:35–42. doi: 10.1016/j.tibs.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature. 2009;460:392–395. doi: 10.1038/nature08221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He LZ, Bhaumik M, Tribioli C, Rego EM, Ivins S, Zelent A, Pandolfi PP. Two critical hits for promyelocytic leukemia. Mol Cell. 2000;6:1131–1141. doi: 10.1016/s1097-2765(00)00111-8. [DOI] [PubMed] [Google Scholar]
- Hennige AM, Lammers R, Arlt D, Hoppner W, Strack V, Niederfellner G, Seif FJ, Haring HU, Kellerer M. Ret oncogene signal transduction via a IRS-2/PI 3-kinase/PKB and a SHC/Grb-2 dependent pathway: possible implication for transforming activity in NIH3T3 cells. Mol Cell Endocrinol. 2000;167:69–76. doi: 10.1016/s0303-7207(00)00283-5. [DOI] [PubMed] [Google Scholar]
- Huang J, Manning BD. The TSC1-TSC2 complex: a molecular switchboard controlling cell growth. Biochem J. 2008;412:179–190. doi: 10.1042/BJ20080281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Bernardi R, Morotti A, Matsuoka S, Saglio G, Ikeda Y, Rosenblatt J, Avigan DE, Teruya-Feldstein J, Pandolfi PP. PML targeting eradicates quiescent leukaemia-initiating cells. Nature. 2008;453:1072–1078. doi: 10.1038/nature07016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Bernardi R, Pandolfi PP. A novel signaling network as a critical rheostat for the biology and maintenance of the normal stem cell and the cancer-initiating cell. Curr Opin Genet Dev. 2009;19:51–59. doi: 10.1016/j.gde.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Inoue K, Lee J, Yoshimoto M, Ogonuki N, Miki H, Baba S, Kato T, Kazuki Y, Toyokuni S, et al. Generation of pluripotent stem cells from neonatal mouse testis. Cell. 2004a;119:1001–1012. doi: 10.1016/j.cell.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Ogonuki N, Inoue K, Miki H, Ogura A, Toyokuni S, Shinohara T. Long-term proliferation in culture and germline transmission of mouse male germline stem cells. Biol Reprod. 2003;69:612–616. doi: 10.1095/biolreprod.103.017012. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Toyokuni S, Shinohara T. CD9 is a surface marker on mouse and rat male germline stem cells. Biol Reprod. 2004b;70:70–75. doi: 10.1095/biolreprod.103.020867. [DOI] [PubMed] [Google Scholar]
- Kubota H, Avarbock MR, Brinster RL. Spermatogonial stem cells share some, but not all, phenotypic and functional characteristics with other stem cells. Proc Natl Acad Sci U S A. 2003;100:6487–6492. doi: 10.1073/pnas.0631767100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H, Avarbock MR, Brinster RL. Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 2004;101:16489–16494. doi: 10.1073/pnas.0407063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Takubo K, Suda T. Bone marrow long label-retaining cells reside in the sinusoidal hypoxic niche. Biochem Biophys Res Commun. 2008;366:335–339. doi: 10.1016/j.bbrc.2007.11.086. [DOI] [PubMed] [Google Scholar]
- Labbaye C, Quaranta MT, Pagliuca A, Militi S, Licht JD, Testa U, Peschle C. PLZF induces megakaryocytic development, activates Tpo receptor expression and interacts with GATA1 protein. Oncogene. 2002;21:6669–6679. doi: 10.1038/sj.onc.1205884. [DOI] [PubMed] [Google Scholar]
- Lee CH, Inoki K, Karbowniczek M, Petroulakis E, Sonenberg N, Henske EP, Guan KL. Constitutive mTOR activation in TSC mutants sensitizes cells to energy starvation and genomic damage via p53. EMBO J. 2007a;26:4812–4823. doi: 10.1038/sj.emboj.7601900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kanatsu-Shinohara M, Inoue K, Ogonuki N, Miki H, Toyokuni S, Kimura T, Nakano T, Ogura A, Shinohara T. Akt mediates self-renewal division of mouse spermatogonial stem cells. Development. 2007b;134:1853–1859. doi: 10.1242/dev.003004. [DOI] [PubMed] [Google Scholar]
- Lin L, Stringfield TM, Shi X, Chen Y. Arsenite induces a cell stress-response gene, RTP801, through reactive oxygen species and transcription factors Elk-1 and CCAAT/enhancer-binding protein. Biochem J. 2005;392:93–102. doi: 10.1042/BJ20050553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- Martelli AM, Tazzari PL, Evangelisti C, Chiarini F, Blalock WL, Billi AM, Manzoli L, McCubrey JA, Cocco L. Targeting the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin module for acute myelogenous leukemia therapy: from bench to bedside. Curr Med Chem. 2007;14:2009–2023. doi: 10.2174/092986707781368423. [DOI] [PubMed] [Google Scholar]
- McConnell MJ, Licht JD. The PLZF gene of t (11;17)-associated APL. Curr Top Microbiol Immunol. 2007;313:31–48. doi: 10.1007/978-3-540-34594-7_3. [DOI] [PubMed] [Google Scholar]
- Meng X, Lindahl M, Hyvonen ME, Parvinen M, de Rooij DG, Hess MW, Raatikainen-Ahokas A, Sainio K, Rauvala H, Lakso M, et al. Regulation of cell fate decision of undifferentiated spermatogonia by GDNF. Science (New York, NY. 2000;287:1489–1493. doi: 10.1126/science.287.5457.1489. [DOI] [PubMed] [Google Scholar]
- Nagano M, Avarbock MR, Brinster RL. Pattern and kinetics of mouse donor spermatogonial stem cell colonization in recipient testes. Biol Reprod. 1999;60:1429–1436. doi: 10.1095/biolreprod60.6.1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka C, Ikezoe T, Yang J, Gery S, Koeffler HP, Yokoyama A. Inhibition of mammalian target of rapamycin signaling potentiates the effects of all-trans retinoic acid to induce growth arrest and differentiation of human acute myelogenous leukemia cells. Int J Cancer. 2009;125:1710–1720. doi: 10.1002/ijc.24472. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Avarbock MR, Brinster RL. Glial cell line-derived neurotrophic factor regulation of genes essential for self-renewal of mouse spermatogonial stem cells is dependent on Src family kinase signaling. The Journal of biological chemistry. 2007;282:25842–25851. doi: 10.1074/jbc.M703474200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oatley JM, Avarbock MR, Telaranta AI, Fearon DT, Brinster RL. Identifying genes important for spermatogonial stem cell self-renewal and survival. Proc Natl Acad Sci U S A. 2006;103:9524–9529. doi: 10.1073/pnas.0603332103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Arechaga JM, Avarbock MR, Brinster RL. Transplantation of testis germinal cells into mouse seminiferous tubules. Int J Dev Biol. 1997;41:111–122. [PubMed] [Google Scholar]
- Ohbo K, Yoshida S, Ohmura M, Ohneda O, Ogawa T, Tsuchiya H, Kuwana T, Kehler J, Abe K, Scholer HR, et al. Identification and characterization of stem cells in prepubertal spermatogenesis in mice small star, filled. Dev Biol. 2003;258:209–225. doi: 10.1016/s0012-1606(03)00111-8. [DOI] [PubMed] [Google Scholar]
- Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S A. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- Ruvinsky I, Sharon N, Lerer T, Cohen H, Stolovich-Rain M, Nir T, Dor Y, Zisman P, Meyuhas O. Ribosomal protein S6 phosphorylation is a determinant of cell size and glucose homeostasis. Genes Dev. 2005;19:2199–2211. doi: 10.1101/gad.351605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu BY, Orwig KE, Oatley JM, Avarbock MR, Brinster RL. Effects of aging and niche microenvironment on spermatogonial stem cell self-renewal. Stem Cells. 2006;24:1505–1511. doi: 10.1634/stemcells.2005-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieke SM, Finkel T. Mitochondrial signaling, TOR, and life span. Biol Chem. 2006;387:1357–1361. doi: 10.1515/BC.2006.170. [DOI] [PubMed] [Google Scholar]
- Schrans-Stassen BH, van de Kant HJ, de Rooij DG, van Pelt AM. Differential expression of c-kit in mouse undifferentiated and differentiating type A spermatogonia. Endocrinology. 1999;140:5894–5900. doi: 10.1210/endo.140.12.7172. [DOI] [PubMed] [Google Scholar]
- Seandel M, James D, Shmelkov SV, Falciatori I, Kim J, Chavala S, Scherr DS, Zhang F, Torres R, Gale NW, et al. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature. 2007;449:346–350. doi: 10.1038/nature06129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah OJ, Wang Z, Hunter T. Inappropriate activation of the TSC/Rheb/mTOR/S6K cassette induces IRS1/2 depletion, insulin resistance, and cell survival deficiencies. Curr Biol. 2004;14:1650–1656. doi: 10.1016/j.cub.2004.08.026. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- Shinohara T, Avarbock MR, Brinster RL. beta1- and alpha6-integrin are surface markers on mouse spermatogonial stem cells. Proc Natl Acad Sci U S A. 1999;96:5504–5509. doi: 10.1073/pnas.96.10.5504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T, Orwig KE, Avarbock MR, Brinster RL. Spermatogonial stem cell enrichment by multiparameter selection of mouse testis cells. Proc Natl Acad Sci U S A. 2000;97:8346–8351. doi: 10.1073/pnas.97.15.8346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoshani T, Faerman A, Mett I, Zelin E, Tenne T, Gorodin S, Moshel Y, Elbaz S, Budanov A, Chajut A, et al. Identification of a novel hypoxia-inducible factor 1-responsive gene, RTP801, involved in apoptosis. Mol Cell Biol. 2002;22:2283–2293. doi: 10.1128/MCB.22.7.2283-2293.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takubo K, Ohmura M, Azuma M, Nagamatsu G, Yamada W, Arai F, Hirao A, Suda T. Stem cell defects in ATM-deficient undifferentiated spermatogonia through DNA damage-induced cell-cycle arrest. Cell Stem Cell. 2008;2:170–182. doi: 10.1016/j.stem.2007.10.023. [DOI] [PubMed] [Google Scholar]
- Virtanen I, Kallajoki M, Narvanen O, Paranko J, Thornell LE, Miettinen M, Lehto VP. Peritubular myoid cells of human and rat testis are smooth muscle cells that contain desmin-type intermediate filaments. Anat Rec. 1986;215:10–20. doi: 10.1002/ar.1092150103. [DOI] [PubMed] [Google Scholar]
- Wu X, Oatley JM, Oatley MJ, Kaucher AV, Avarbock MR, Brinster RL. The POU Domain Transcription Factor POU3F1 Is an Important Intrinsic Regulator of GDNF-Induced Survival and Self-Renewal of Mouse Spermatogonial Stem Cells. Biol Reprod. 2010 doi: 10.1095/biolreprod.109.083097. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, Morrison SJ. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Sukeno M, Nabeshima Y. A vasculature-associated niche for undifferentiated spermatogonia in the mouse testis. Science. 2007;317:1722–1726. doi: 10.1126/science.1144885. [DOI] [PubMed] [Google Scholar]
- Yoshida S, Sukeno M, Nakagawa T, Ohbo K, Nagamatsu G, Suda T, Nabeshima Y. The first round of mouse spermatogenesis is a distinctive program that lacks the self-renewing spermatogonia stage. Development. 2006;133:1495–1505. doi: 10.1242/dev.02316. [DOI] [PubMed] [Google Scholar]
- Yoshihara H, Arai F, Hosokawa K, Hagiwara T, Takubo K, Nakamura Y, Gomei Y, Iwasaki H, Matsuoka S, Miyamoto K, et al. Thrombopoietin/MPL signaling regulates hematopoietic stem cell quiescence and interaction with the osteoblastic niche. Cell Stem Cell. 2007;1:685–697. doi: 10.1016/j.stem.2007.10.020. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bajraszewski N, Wu E, Wang H, Moseman AP, Dabora SL, Griffin JD, Kwiatkowski DJ. PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J Clin Invest. 2007;117:730–738. doi: 10.1172/JCI28984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Ebata KT, Robaire B, Nagano MC. Aging of male germ line stem cells in mice. Biol Reprod. 2006;74:119–124. doi: 10.1095/biolreprod.105.045591. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.