Abstract
Background
Vitamin D deficiency and parathyroid hormone (PTH) excess are common among older adults and may adversely impact cardiovascular health. We evaluated associations of 25-hydroxyvitamin D (25-OHD) and PTH concentrations, separately, and in combination, with incident cardiovascular events and mortality during 14 years of follow-up in the Cardiovascular Health Study.
Methods and results
We studied 2,312 participants who were free of cardiovascular disease at baseline. We measured 25-OHD and intact PTH from previously frozen serum using mass spectrometry and a two-site immunoassay. Outcomes were adjudicated cases of myocardial infarction, heart failure, cardiovascular death, and all cause mortality. There were 384 participants (17%) who had serum 25-OHD concentrations <15 ng/ml and 570 (25%) who had serum PTH concentrations ≥ 65 pg/ml. After adjustment, each 10-ng/ml lower 25-OHD concentration was associated with a 9% greater (95% CI 2% to 17% greater) relative hazard of mortality and a 25% greater (95% CI 8% to 44% greater) relative hazard of myocardial infarction. Serum 25-OHD concentrations <15 ng/ml, were associated with a 29% greater (95% CI 5% to 55% greater) risk of mortality. Serum PTH concentrations ≥ 65 pg/ml were associated with a 30% greater risk of heart failure (95% CI 6% to 61% greater), but not other outcomes. There was no evidence of an interaction between serum 25-OHD and PTH concentrations and cardiovascular events.
Conclusions
Among older adults, 25-OHD deficiency is associated with myocardial infarction and mortality; PTH excess is associated with heart failure. Vitamin D and PTH might influence cardiovascular risk through divergent pathways.
Keywords: Vitamin D, parathyroid hormone, myocardial infarction, cardiovascular death, heart failure, mortality, mineral metabolism
INTRODUCTION
Disturbances in mineral metabolism are common among older adults and may adversely impact cardiovascular health.1, 2 Older age is associated with lower circulating concentrations of 25-hydroxyvitamin D (25-OHD), impaired vitamin D activation within the kidney, and a rise in serum parathyroid hormone (PTH) concentrations.3,4 Disturbances in vitamin D and PTH metabolic axes may increase cardiovascular risk through diverse pathways.1, 5, 6 In experimental models, vitamin D deficiency activates the renin-angiotensin system, stimulates inflammatory cytokines, and promotes cardiomyocyte growth.7–9 PTH excess raises intracellular calcium in target tissues and is associated with hypertension, cardiac valve calcification, and left ventricular hypertrophy.10, 11
Previous studies of mineral metabolism and cardiovascular risk have generally focused on middle-aged populations, and have evaluated 25-OHD and PTH concentrations separately. In the present study, we evaluate 25-OHD and PTH concentrations together in a general population of 2,312 ambulatory older adults who were free of clinical cardiovascular disease at baseline. We assess associations of mineral metabolism biomarkers with adjudicated cases of incident myocardial infarction, incident heart failure, cardiovascular death, and all cause mortality during 14-years of follow-up. We hypothesized that lower 25-OHD and higher PTH levels would be associated with cardiovascular events, and that associations would be strongest in the presence of both disturbances, because PTH represents an endogenous biologic marker of inadequate vitamin D stores.12, 13
METHODS
Study population
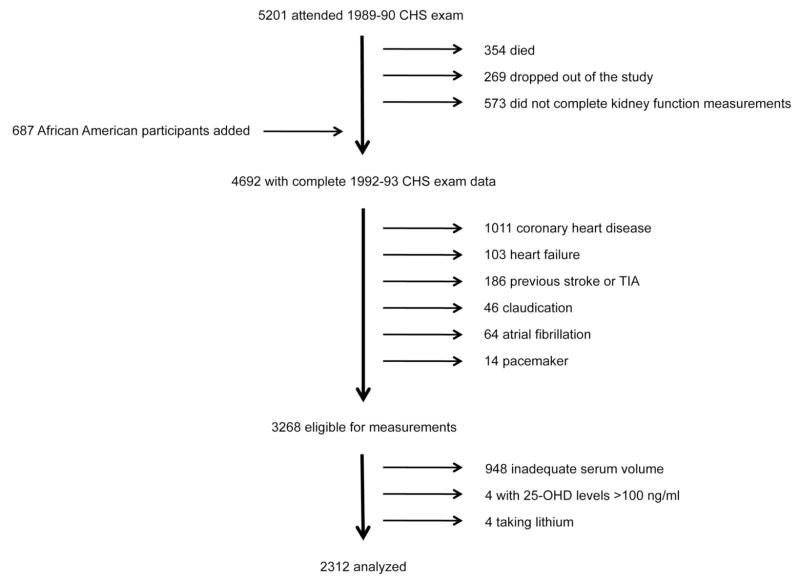
The Cardiovascular Health Study (CHS) is a prospective cohort study of clinical and subclinical cardiovascular disease among older individuals.14 In 1989–1990, the CHS enrolled 5201 ambulatory men and women ages 65 and older from Medicare eligibility lists in Forsyth County, North Carolina; Sacramento County, California; Washington County, Maryland; and Pittsburgh, Pennsylvania. CHS enrolled an additional 687 African American participants in 1992–1993. Exclusion criteria included the use of a wheelchair in the home, institutionalization, the need for a proxy respondent to provide informed consent, plans to move from the area within 3 years, or current treatment for cancer. Each center’s institutional review board approved the study and all participants provided informed consent.
We evaluated CHS participants at the time of their 1992–1993 examination. To focus on incident cardiovascular events, we excluded participants who had prevalent cardiovascular disease at the time of the 1992–1993 CHS exam, defined by any one of the following conditions: coronary heart disease, heart failure, stroke, transient ischemic attack, claudication, atrial fibrillation, pacemaker, or implantable cardioverter defibrillator (Figure 1). CHS investigators determined prevalent cardiovascular conditions by review of medical records, electrocardiographic findings, participant responses to questionnaires, and interim events that occurred between the baseline and 1992–1993 CHS examinations.15 We further excluded 948 participants who had inadequate serum volume (<500 ul) to perform the mineral metabolism measurements, 4 participants who had implausible 25-OHD concentrations (>100 ng/ml), and 4 participants who were taking lithium, which may alter calcium metabolism, resulting in a final study sample of 2,312 participants. Compared to participants excluded due to inadequate sample volume, included subjects were older (73.9 versus 71.9 years) and more likely to be Caucasian (85% versus 75%).
Figure 1. Flow diagram of the study population.
Exclusions are shown on the right hand side of the figure; additions to the study population are shown on the left hand side.
Measurement of mineral metabolism variables
The Laboratory for Clinical Biochemistry Research at the University of Vermont stored serum samples at −70°C using established methods, which have demonstrated long-term stability for serum markers of coagulation, fibrinolysis, and inflammation.16 The University of Washington Clinical Nutrition Research Unit (CNRU) performed mineral metabolism measurements from serum collected during the 1992–1993 CHS exam. Total 25-OHD (25-OHD2 + 25-OHD3) was measured using high performance liquid chromatography-tandem mass spectrometry on a Waters Quattro Micro mass spectrometer. The interassay coefficient of variation (CV) was <3.4%. Intact serum PTH was quantified using a two-site immunoassay on a Beckman Unicell DxI clinical analyzer. The reference range is 17–66 pg/mL as determined from the central 95% of values from 43 normal laboratory personnel with normal 25-OHD concentrations in March 2005. The interassay CV for PTH was <4.5% at 37 pg/mL. Serum non-ionized total calcium levels were measured using indirect potentiometry, and serum phosphorus levels were measured using a timed-rate colorimetric reaction method with ammonium molybdate on a Beckman DxC Synchron analyzer.
Ascertainment of cardiovascular outcomes
Study outcomes were all-cause mortality, cardiovascular mortality, incident heart failure, and incident myocardial infarction. CHS investigators identified potential cardiovascular events semi-annually via telephone surveillance, and annually by repeat interviews and examinations. The CHS Events Committee adjudicated cardiovascular events using available hospital discharge summaries, diagnostic test reports, surgery and radiology findings, consultation reports, autopsies, and death certificates.17 The committee defined heart failure by a physician diagnosis of heart failure plus either documentation of symptoms and signs of heart failure, pulmonary edema on chest X-ray, or specific medical treatment for heart failure. Echocardiography results, when available, were also considered during adjudication. The committee defined acute myocardial infarction using an algorithm that included elements of chest pain, cardiac enzymes, and electrocardiographic changes. The committee defined cardiovascular mortality as death due to acute myocardial infarction, atherosclerotic coronary heart disease, ischemic or hemorrhagic stroke, or other cardiovascular cause (ruptured aortic aneurysm, peripheral vascular disease, valvular heart disease, or pulmonary embolism). Cardiovascular event data were complete through June 30, 2007. Because participant data are linked with Medicare records and the National Death Index, follow-up is considered to be 100% complete for each of the study outcomes evaluated here.
Measurement of other study variables
Trained CHS study personnel conducted standardized interviews, which queried demographics, health status, smoking status, and alcohol use.14 Study participants were asked about the frequency and duration of 15 common leisure time activities during the previous two weeks, and CHS investigators calculated a total weighted leisure time physical activity score based on these responses. CHS study personnel assessed prescription and over the counter medication use, including vitamin D supplements, by instructing participants to bring in all of their medications and directly transcribing the medication bottle labels. We queried medication lists to identify vitamin D and calcium supplement use. CHS study personnel measured blood pressure in triplicate 5 minutes apart with the participant seated, and performed phlebotomy under fasting conditions.
CHS investigators defined diabetes by a fasting glucose level >7.0 mmol/L or the use of insulin or an oral hypoglycemic medication. We calculated body mass index as height/(weight)2 where height and weight were measured in meters and kilograms, respectively. We analyzed education level as none-grade 9, high school, and professional/vocational. The Laboratory for Clinical Biochemistry Research analyzed blood specimens for albumin, total and high-density lipoprotein cholesterol, creatinine, and C-reactive protein (CRP). Low-density lipoprotein cholesterol was calculated using the Friedewald equation. CHS investigators measured cystatin C levels from previously collected serum samples stored at −70°C using a particle-enhanced immunonephelometric assay with a nephelometer (BNII, Siemens Healthcare Diagnostics Inc).18 We calculated estimated GFR using the equation:
This equation, derived in a recent pooling study of 3418 adults who underwent simultaneous cystatin C measurements and gold-standard radionucleotide measurements of GFR, explains approximately 82% of the variation in directly measured GFR in the setting of chronic kidney disease.19
Statistical analysis
We analyzed 25-OHD as a continuous variable and according to previously published categories,20–22 because functional analyses revealed linear associations of serum 25-OHD concentrations with study outcomes. We analyzed serum PTH concentrations as <65 versus ≥65 pg/ml, because functional analyses revealed threshold associations of serum PTH concentration ≥65 pg/ml with each of the study outcomes, and because 65 pg/ml represents the upper limit of normal for this assay based on the central 95% of values from healthy subjects who had normal 25-OHD concentrations. We defined primary hyperparathyroidism by a serum PTH concentration ≥65 pg/ml plus a serum calcium concentration >10.2 mg/dl, as previously published.23 We used Spearman’s correlation to describe joint associations between serum 25-OHD and PTH concentrations.
We defined time-at-risk as the elapsed time from the 1992–1993 examination until the first occurrence of each outcome of interest. We censored analyses of non-fatal outcomes for mortality, and censored analyses of cardiovascular mortality for non-cardiovascular death. We constructed nested Cox proportional hazards models to estimate the relative hazard of each study outcome after adjustment for relevant confounding variables.24 A basic model included 25-OHD and PTH levels and adjusted for age, race, sex, season of the year, and clinic site. A second model added cardiovascular risk factors (diabetes, anti-hypertensive medications, smoking, education, physical activity, body mass index, systolic blood pressure, C-reactive protein, total and HDL-cholesterol), and serum concentrations of calcium and phosphorus. A third model added GFR, estimated by serum cystatin C concentrations, to separately describe the confounding influence of kidney function. We analyzed kilocalories of physical activity, body mass index, systolic blood pressure, and levels of CRP, lipids, calcium, phosphorus, and estimated GFR as continuous variables in the multivariate models. We assessed the functional associations of 25-OHD and PTH concentrations with study outcomes by constructing spline models after basic adjustment for covariates specified in model 1. The proportional hazards assumption was satisfied for all models (p-values >0.2) indicating statistically similar relative risks of each study outcome over time.
We looked for evidence of an interaction between 25-OHD deficiency and PTH excess by calculating the relative excess risk of interaction (RERI). An RERI value of 0 indicates no additive biological interaction. We performed stratified analyses to explore whether associations of 25-OHD with study outcomes might differ according to baseline characteristics. We conducted analyses using S-Plus (version 8.0, Tibco, Seattle, WA) and SPSS statistical software (version 15.0.1.1, SPSS, Inc., Chicago, IL).
RESULTS
Description of vitamin D and PTH concentrations
Serum 25-OHD concentrations were normally distributed with a mean value of 25.2 ng/ml (standard deviation 10.2; interquartile range [IQR] 17.8, 31.5 ng/ml). The prevalences of vitamin D deficiency (<15 ng/ml) and insufficiency (15–30 ng/ml) were 16.6% and 53.9%, respectively. Serum 25-OHD concentrations were highest among participants from the Sacramento site (mean 26.4 ±10.5 ng/ml) and lowest among participants from the Pittsburgh site (mean 24.4 ±11.5 ng/ml). The distribution of serum PTH concentrations was rightward-skewed with a median value of 51 pg/ml (standard deviation 29.7; IQR 39, 65 pg/ml). Serum 25-OHD concentrations were inversely correlated with serum PTH concentrations (correlation coefficient = -0.317).
Associations of vitamin D and PTH with baseline characteristics
Lower serum 25-OHD concentrations were related to African American race, female sex, measurement during winter months, prevalent diabetes, current smoking, greater body mass index, lesser physical activity, higher systolic blood pressure, and higher serum CRP concentrations (Table 1). The prevalence of vitamin D deficiency was more than 3-fold greater among African Americans compared to Caucasians (42.9% versus 12.2%). Serum 25-OHD concentrations were not associated with estimated GFR. Higher serum PTH concentrations were associated with African American race, female sex, higher systolic blood pressure, and lower estimated GFR. Serum calcium and phosphorus concentrations did not vary across categories of 25-OHD, though serum phosphorus concentrations were slightly lower among participants who had serum PTH concentrations ≥65 pg/ml.
Table 1.
Baseline characteristics by parathyroid hormone and 25-hydroxyvitamin D concentrations.
| 25-hydroxyvitamin D (ng/ml) | PTH (pg/ml) | ||||
|---|---|---|---|---|---|
| >30 | 15–30 | <15 | <65 | ≥65 | |
| Number of participants | 681 (29) | 1247 (54) | 384 (17) | 1742 (75) | 570 (25) |
| Demographic data | |||||
| Age | 73 ±4 | 74 ±5 | 74 ±6 | 74 ±5 | 75 ±6 |
| African American | 30 (4) | 160 (13) | 142 (37) | 226 (13) | 107 (19) |
| Male | 287 (42) | 335 (27) | 79 (21) | 555 (32) | 146 (26) |
| Season | |||||
| Winter | 112 (16) | 303 (24) | 169 (44) | 413 (24) | 171 (30) |
| Spring | 109 (16) | 302 (24) | 118 (31) | 386 (22) | 143 (25) |
| Summer | 286 (42) | 339 (27) | 45 (12) | 538 (31) | 132 (23) |
| Autumn | 174 (26) | 303 (24) | 52 (14) | 405 (23) | 124 (22) |
| Site | |||||
| Forsyth County, North Carolina | 195 (29) | 387 (31) | 103 (27) | 513 (30) | 172 (30) |
| Sacramento County, California | 199 (29) | 264 (21) | 94 (24) | 390 (22) | 167 (29) |
| Washington County, Maryland | 152 (22) | 334 (27) | 76 (20) | 449 (26) | 113 (20) |
| Pittsburgh, Pennsylvania | 135 (20) | 262 (21) | 111 (29) | 390 (22) | 118 (21) |
| Diabetes | 55 (8) | 134 (11) | 76 (20) | 196 (11) | 69 (12) |
| Medication use | |||||
| Any anti-hypertension agent | 252 (37) | 486 (39) | 187 (49) | 663 (38) | 262 (46) |
| Thiazide diuretic | 56 (8) | 131 (11) | 53 (14) | 183 (11) | 57 (10) |
| Loop diuretic | 15 (2) | 45 (4) | 16 (4) | 39 (2) | 37 (7) |
| Vitamin D supplement | 10 (1.5) | 0 (0) | 0 (0) | 8 (0.5) | 2 (0.4) |
| Calcium supplement | 7 (1.0) | 9 (0.7) | 2 (0.5) | 16 (0.9) | 2 (0.4) |
| Current smoking | 54 (8) | 116 (10) | 58 (16) | 180 (10) | 48 (9) |
| Education level | |||||
| None -- grade 9 | 73 (11) | 207 (17) | 87 (23) | 262 (15) | 105 (18) |
| High school | 256 (38) | 469 (38) | 145 (38) | 654 (38) | 216 (38) |
| Professional/vocational | 349 (51) | 569 (46) | 151 (39) | 823 (47) | 246 (43) |
| Physical activity (Kcal/week) | 2479 ±2421 | 1772 ±1911 | 1243 ±1676 | 1964 ±2111 | 1672 ±1978 |
| Physical examination data | |||||
| Body mass index | 25.5 ±3.9 | 27.1 ±4.8 | 27.9 ±5.5 | 26.4 ±4.5 | 27.8 ±5.4 |
| Systolic blood pressure (mm/Hg) | 134 ±20 | 136 ±21 | 140 ±21 | 135 ±20 | 142 ±23 |
| Serum measurements | |||||
| Parathyroid hormone (pg/mL) | 49 ±27 | 57 ±26 | 71 ±39 | 44 ±12 | 94 ±37 |
| 25-hydroxyvitamin D (ng/mL) | 37 ±8 | 23 ±4 | 11 ±3 | 27 ±10 | 21 ±9 |
| Calcium (mg/dL) | 9.5 ±0.3 | 9.5 ±0.4 | 9.5 ±0.4 | 9.5 ±0.4 | 9.5 ±0.4 |
| Phosphorus (mg/dL) | 3.6 ±0.5 | 3.6 ±0.5 | 3.6 ±0.5 | 3.6 ±0.5 | 3.5 ±0.5 |
| Estimated GFRcystatin C (ml/min/1.73m2) | 75 ±18 | 76 ±18 | 76 ±19 | 77 ±17 | 72 ±20 |
| C-reactive protein (mg/L) | 4.3 ±8.5 | .4.7±7.9 | 6.1 ±9.2 | 4.7 ±8.6 | 5.0 ±7.5 |
| Total cholesterol (mg/dL) | 210 ±37 | 211 ±35 | 212 ±39 | 210 ±36 | 214 ±39 |
| High density lipoprotein (mg/dL) | 57 ±16 | 55 ±14 | 56 ±14 | 56 ±15 | 54 ±14 |
All values in the table expressed as number of participants (percent) or mean ± standard deviation.
GFR = glomerular filtration rate.
Associations of 25-OHD with study outcomes
During a median follow-up of 14.0 years (IQR 8.5, 14.6 years), there were 1226 deaths, of which 389 (32%) were classified as cardiovascular, 504 incident cases of heart failure, and 299 incident myocardial infarctions. In a minimally adjusted model that included serum PTH concentration, age, race, sex, season, and clinic site, each 10 ng/ml lower serum 25-OHD concentration was associated with all-cause mortality and incident myocardial infarction, but not with cardiovascular death or incident heart failure (Table 2). Further adjustment for traditional cardiovascular risk factors plus serum calcium and phosphorus modestly attenuated associations of 25-OHD with mortality and myocardial infarction; adjustment for estimated kidney function did not appreciably alter these associations. After full adjustment, each 10-ng/ml lower 25-OHD concentration was associated with a 9% greater relative risk of all-cause mortality (95% CI 2% to 17% greater; p= 0.012) and a 25% greater relative risk of incident myocardial infarction (95% CI 8% to 44% greater; p= 0.002). These associations were not appreciably altered by additional adjustment for calcium and vitamin D supplementation. Neither serum calcium nor serum phosphorus concentrations were associated with any of the cardiovascular outcomes in this study population.
Table 2.
Associations of serum 25-hydroxyvitamin D concentration with study outcomes.
| Number | Events | Adjusted Hazard Ratio (95% confidence interval) | |||
|---|---|---|---|---|---|
| Model 11 | Model 22 | Model 33 | |||
| All cause mortality | 2312 | 1226 | |||
| 25-OHD >30ng/ml | 681 | 329 | 1.0 | 1.0 | 1.0 |
| 25-OHD 15–30 ng/ml | 1247 | 668 | 1.14 (0.99, 1.31) | 1.10 (0.95, 1.27) | 1.15 (1.00, 1.33) |
| 25-OHD <15 ng/ml | 384 | 229 | 1.36 (1.12, 1.66) | 1.21 (0.99, 1.48) | 1.29 (1.05, 1.57) |
| Continuous per 10 ng/ml lower 25-OHD | 1.10 (1.03, 1.17) | 1.05 (0.99, 1.13) | 1.09 (1.02, 1.17) | ||
| P-value (continuous) | 0.005 | 0.122 | 0.012 | ||
| Cardiovascular mortality | 2312 | 389 | |||
| 25-OHD >30ng/ml | 681 | 107 | 1.0 | 1.0 | 1.0 |
| 25-OHD 15–30 ng/ml | 1247 | 207 | 1.00 (0.78, 1.29) | 0.95 (0.74, 1.23) | 1.01 (0.78, 1.30) |
| 25-OHD <15 ng/ml | 384 | 75 | 1.24 (0.88, 1.76) | 1.08 (0.76, 1.54) | 1.17 (0.83, 1.67) |
| Continuous per 10 ng/ml lower 25-OHD | 1.07 (0.95, 1.20) | 1.02 (0.90, 1.14) | 1.06 (0.94, 1.19) | ||
| P-value (continuous) | 0.247 | 0.795 | 0.356 | ||
| Incident heart failure | 2312 | 504 | |||
| 25-OHD >30ng/ml | 681 | 107 | 1.0 | 1.0 | 1.0 |
| 25-OHD 15–30 ng/ml | 1247 | 207 | 1.00 (0.78, 1.29) | 0.95 (0.74, 1.23) | 1.01 (0.78, 1.30) |
| 25-OHD <15 ng/ml | 384 | 75 | 1.24 (0.88, 1.76) | 1.08 (0.76, 1.54) | 1.17 (0.83, 1.67) |
| Continuous per 10 ng/ml lower 25-OHD | 0.99 (0.90, 1.09) | 0.92 (0.83, 1.02) | 0.95 (0.86, 1.05) | ||
| P-value (continuous) | 0.861 | 0.100 | 0.303 | ||
| Incident myocardial infarction | 2312 | 299 | |||
| 25-OHD >30ng/ml | 681 | 88 | 1.0 | 1.0 | 1.0 |
| 25-OHD 15–30 ng/ml | 1247 | 161 | 1.17 (0.89, 1.54) | 1.17 (0.88, 1.55) | 1.20 (0.90, 1.59) |
| 25-OHD <15 ng/ml | 384 | 50 | 1.41 (0.94, 2.11) | 1.37 (0.90, 2.07) | 1.40 (0.93, 2.12) |
| Continuous per 10 ng/ml lower 25-OHD | 1.25 (1.09, 1.43) | 1.23 (1.07, 1.42) | 1.25 (1.08, 1.44) | ||
| P-value (continuous) | 0.001 | 0.004 | 0.002 | ||
25-OHD = 25-hydroxyvitamin D; PTH = parathyroid hormone.
Model 1 includes 25-OHD and PTH levels, and adjusted for age, race, sex, season of the year, and clinic site.
Model 2 adds diabetes, anti-hypertensive medications, smoking (never, current, former), education, kilocalories physical activity, body mass index, systolic blood pressure, levels of C-reactive protein, total, HDL-cholesterol, calcium, and phosphorus.
Model 3 adds estimated GFRcystatin C.
To further address the possibility of confounding and to explore potential interactions, we estimated associations of lower 25-OHD concentrations with mortality and myocardial infarction across categories of physical activity level, body mass index, age, race, sex, and estimated kidney function (Figure 2). Associations were generally similar, with widely overlapping confidence intervals.
Figure 2. Association of 25-OHD concentration with mortality and myocardial infarction by subgroups.
Continuous associations of 10 ng/ml lower 25-OHD concentration with all-cause mortality and incident myocardial infarction by subgroups, adjusted for the covariates in model 3.
Associations of PTH with study outcomes
After basic adjustment, serum PTH concentrations ≥65 pg/ml were associated with cardiovascular death and incident heart failure, but not with all-cause mortality or myocardial infarction (Table 3, model 1). Associations of PTH excess with cardiovascular outcomes were confounded by kidney function; adjustment for estimated GFR removed the statistical association with cardiovascular death and attenuated the association with heart failure (Table 3, model 3). After full adjustment, PTH concentrations ≥65 pg/ml remained associated with an estimated 30% greater risk of heart failure (95% confidence interval 6% to 61% greater). This association was not altered by excluding 21 subjects who met the definition of primary hyperparathyroidism (adjusted hazard ratio 1.29; 95% confidence interval 1.04, 1.59) or by additional adjustment for thiazide diuretic use (adjusted hazard ratio 1.29; 95% confidence interval 1.04, 1.61). When analyzed as a continuous variable, serum PTH was not associated with any of the study outcomes.
Table 3.
Associations of serum PTH concentration with study outcomes.
| Number | Events | Adjusted Hazard Ratio (95% confidence interval) | |||
|---|---|---|---|---|---|
| Model 11 | Model 22 | Model 33 | |||
| All cause mortality | |||||
| PTH <65 pg/ml | 1742 | 899 | 1.0 | 1.0 | 1.0 |
| PTH ≥ 65 pg/ml | 570 | 327 | 1.11 (0.97, 1.28) | 1.14 (0.99, 1.31) | 1.07 (0.93, 1.23) |
| P-value | 0.125 | 0.035 | 0.341 | ||
| Cardiovascular mortality | |||||
| PTH <65 pg/ml | 1742 | 273 | 1.0 | 1.0 | 1.0 |
| PTH ≥65 pg/ml | 570 | 116 | 1.30 (1.03, 1.65) | 1.30 (1.01, 1.66) | 1.16 (0.90, 1.50) |
| P-value | 0.030 | 0.040 | 0.213 | ||
| Incident heart failure | |||||
| PTH <65 pg/ml | 1742 | 351 | 1.0 | 1.0 | 1.0 |
| PTH ≥65 pg/ml | 570 | 153 | 1.47 (1.19, 1.80) | 1.40 (1.13, 1.73) | 1.30 (1.05, 1.61) |
| P-value | <0.001 | 0.003 | 0.018 | ||
| Incident myocardial infarction | |||||
| PTH <65 pg/ml | 1742 | 223 | 1.0 | 1.0 | 1.0 |
| PTH ≥65 pg/ml | 570 | 76 | 1.05 (0.79, 1.40) | 1.01 (0.76, 1.35) | 0.98 (0.74, 1.31) |
| P-value | 0.719 | 0.994 | 0.793 | ||
25-OHD = 25-hydroxyvitamin D; PTH = parathyroid hormone.
Model 1 includes 25-OHD and PTH levels, and adjusted for age, race, sex, season of the year, and clinic site.
Model 2 adds diabetes, anti-hypertensive medications, smoking (never, current, former), education, kilocalories physical activity, body mass index, systolic blood pressure, levels of C-reactive protein, total, HDL-cholesterol, calcium, and phosphorus.
Model 3 adds estimated GFRcystatin C.
Combined associations of 25-OHD and PTH with study outcomes
The combination of vitamin D deficiency and PTH excess tended to be associated with greater risks of cardiovascular outcomes; however, these differences were not statistically significant (Table 4). We further looked for evidence of additive interactions between serum 25-OHD and PTH concentrations and cardiovascular events. The relative excess risk of interaction was not statistically significant for any of the four cardiovascular outcomes (all p-values >0.3).
Table 4.
Combined associations of 25-hydroxyvitamin D and parathyroid hormone concentrations with study outcomes.
| Adjusted hazard ratio (95% CI) | P-value for interaction | |||
|---|---|---|---|---|
| PTH <65 pg/ml | PTH ≥65 pg/ml | |||
| All-cause mortality | 25-OHD >30 ng/ml | 1.0 | 1.15 (0.82, 1.60) | |
| 25-OHD 15–30 ng/ml | 1.18 (1.01, 1.37) | 1.21 (0.98, 1.49) | ||
| 25-OHD <15 ng/ml | 1.27 (1.00, 1.61) | 1.43 (1.11, 1.85) | 0.775 | |
| Cardiovascular mortality | 25-OHD >30 ng/ml | 1.0 | 0.97 (0.54, 1.76) | |
| 25-OHD 15–30 ng/ml | 0.96 (0.73, 1.27) | 1.20 (0.85, 1.71) | ||
| 25-OHD <15 ng/ml | 1.15 (0.76, 1.74) | 1.32 (0.83, 2.08) | 0.745 | |
| Heart failure | 25-OHD >30 ng/ml | 1.0 | 1.35 (0.85, 2.15) | |
| 25-OHD 15–30 ng/ml | 1.03 (0.81, 1.32) | 1.39 (1.02, 1.88) | ||
| 25-OHD <15 ng/ml | 0.85 (0.57, 1.26) | 0.95 (0.61, 1.46) | 0.774 | |
| Myocardial infarction | 25-OHD >30 ng/ml | 1.0 | 1.09 (0.58, 2.02) | |
| 25-OHD 15–30 ng/ml | 1.25 (0.92, 1.70) | 1.05 (0.68, 1.62) | ||
| 25-OHD <15 ng/ml | 1.30 (0.79, 2.13) | 1.55 (0.91, 2.62) | 0.562 | |
25-OHD = 25-hydroxyvitamin D; PTH = parathyroid hormone.
Hazard ratios adjusted for age, race, sex, season of the year, clinic site, diabetes, anti-hypertensive medications, smoking (never, current, former), education, kilocalories physical activity, body mass index, systolic blood pressure, serum levels of C-reactive protein, total and HDL-cholesterol, calcium, and phosphorus, and estimated GFRcystatin C.
DISCUSSION
In this community-based cohort of ambulatory older adults without clinical cardiovascular disease, lower serum 25-OHD concentrations were associated with all-cause mortality and incident myocardial infarction, whereas higher serum PTH concentrations were associated with incident heart failure. We did not detect an interaction of 25-OHD and PTH on cardiovascular outcomes in this study population. This study has several important strengths, which include a generally healthy study population that was free of clinical cardiovascular disease at baseline, more than twice the length of follow-up compared to previous studies, a large number of adjudicated cardiovascular events and cardiovascular causes of death, and the use of standardized methods to assess mineral metabolism markers, cardiovascular risk factors, kidney function, and co-morbid conditions.
In middle-aged populations, lower 25-OHD concentrations are associated with incident myocardial infarction and all cause mortality.20, 25, 26 Associations of lower 25-OHD concentrations with cardiovascular death, defined using administrative codes, have recently been reported in older populations from the United States and Europe.27, 28 However, diagnosis codes and/or death certificate data are known to misclassify cardiovascular events and causes of death in older people.29, 30
We found higher serum PTH levels to be associated with an estimated 18% greater risk of cardiovascular death; however, this result was not statistically significant (95% confidence interval 0.92, 1.50). In contrast, Hagström et al. reported a statistically significant association of higher PTH concentrations with cardiovascular death among older Swedish men.31 The relatively healthy make-up of our study populations and more specific definition of cardiovascular death in CHS may have reduced study power to detect associations with this outcome.
Lower serum 25-OHD concentrations represent decreased vitamin D stores, whereas serum PTH excess reflects, in part, inadequate biologic vitamin D activity.5, 32, 33 Based on these relationships, we hypothesized that associations of lower 25-OHD concentrations with cardiovascular outcomes would be strongest in the presence of concomitant PTH excess. However, our findings do not support such an interaction. Moreover, 25-OHD and PTH concentrations tended to be associated with different cardiovascular outcomes in this study. These findings are more compatible with the hypothesis that 25-OHD and PTH might influence cardiovascular risk through divergent pathways.
The biologic actions of 25-OHD and PTH may explain their associations with myocardial infarction and heart failure. In previous studies, lower 25-OHD concentrations are associated with metabolic risk factors for atherosclerosis, including diabetes, hypertension, and inflammation.34–36 Cell culture studies and in vivo animal models demonstrate immunomodulatory actions of vitamin D,8, 37 and vitamin D increases insulin receptor expression and insulin responsiveness for glucose transport in cultured human promonocytic cells.38 Hyperparathyroidism a state of chronic PTH excess, has been linked with arterial stiffness and hypertension. The prevalence of hypertension among individuals with primary hyperparathyroidism ranges from 30 to 70%, and blood pressure decreases following surgical parathyroidectomy.39–41 PTH exerts a trophic effect on cardiomyocytes, with an increase in total cellular mass, and higher serum PTH concentrations are associated with left ventricular hypertrophy in the general population.11, 42
These observational data cannot prove that 25-OHD deficiency or PTH excess play causal roles in the development of cardiovascular disease. These serologic markers may be indicators of health status, in particular 25-OHD deficiency, which may reflect co-morbidity, less time spent outdoors, and inadequate nutrient intake. We attempted to address potential confounding in this study by excluding individuals with pre-existing cardiovascular diseases and by adjusting for lifestyle factors, co-morbidity, and cardiovascular risk factors that were measured using uniform methods. Adjusted models included some factors that could reside on the hypothesized causal pathway between mineral metabolism disturbances and cardiovascular events, such as hypertension, diabetes, and inflammation. Adjustment for estimated GFR using cystatin C levels was particularly important for appreciating the confounding influence of kidney function on the associations of PTH with cardiovascular mortality. A rise in serum PTH is one of the first detectable mineral metabolism disturbances of chronic kidney disease,43 which is highly prevalent in older adults and may be difficult to detect using traditional serologic markers.
A second limitation of this study is the use of a single measurement of 25-OHD and PTH later in life. Biologic variation in these markers within an individual over time is expected. The prospective design of this study favors non-differential misclassification of 25-OHD and PTH concentrations, which would be expected to dilute the observed associations. Future studies of mineral metabolism markers and cardiovascular disease outcomes would benefit from multiple measurements within an individual over time. CHS focused exclusively on older adults; associations of mineral metabolism markers with cardiovascular events may differ in younger and more ethnically diverse populations.
Consistent associations of 25-OHD concentrations with cardiovascular outcomes across multiple cohort studies and compelling biologic evidence for a beneficial effect of vitamin D on cardiovascular health support clinical trials of vitamin D therapy as the next step to test whether observed associations are truly causal. Associations of PTH excess with cardiovascular diseases are emerging and motivate further epidemiological work in diverse populations to better understand functional relationships and to determine specific populations in which associations may be strongest.
Acknowledgments
This work was supported by NHLBI award 1R01HL084443-01A2. CHS was supported by contracts N01-HC-35129, N01-HC-45133, N01-HC-75150, N01-HC-85079 through N01-HC-85086, N01 HC-15103, N01 HC-55222, and U01 HL080295 from the National Heart, Lung, and Blood Institute; by the National Institute of Neurological Disorders and Stroke; and by grant R01AG027002 from the National Institutes on Aging.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lips P. Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev. 2001;22:477–501. doi: 10.1210/edrv.22.4.0437. [DOI] [PubMed] [Google Scholar]
- 2.van der Wielen RP, Lowik MR, van den Berg H, de Groot LC, Haller J, Moreiras O, van Staveren WA. Serum vitamin D concentrations among elderly people in Europe. Lancet. 1995;346:207–210. doi: 10.1016/s0140-6736(95)91266-5. [DOI] [PubMed] [Google Scholar]
- 3.Brunette MG, Chan M, Ferriere C, Roberts KD. Site of 1,25(OH)2 vitamin D3 synthesis in the kidney. Nature. 1978;276:287–289. doi: 10.1038/276287a0. [DOI] [PubMed] [Google Scholar]
- 4.Kamycheva E, Sundsfjord J, Jorde R. Serum parathyroid hormone level is associated with body mass index. The 5th Tromso study. Eur J Endocrinol. 2004;151:167–172. doi: 10.1530/eje.0.1510167. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 6.Sherman SS, Hollis BW, Tobin JD. Vitamin D status and related parameters in a healthy population: the effects of age, sex, and season. J Clin Endocrinol Metab. 1990;71:405–413. doi: 10.1210/jcem-71-2-405. [DOI] [PubMed] [Google Scholar]
- 7.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Etten E, Mathieu C. Immunoregulation by 1,25-dihydroxyvitamin D3: basic concepts. J Steroid Biochem Mol Biol. 2005;97:93–101. doi: 10.1016/j.jsbmb.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288:E125–132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 10.Nainby-Luxmoore JC, Langford HG, Nelson NC, Watson RL, Barnes TY. A case-comparison study of hypertension and hyperparathyroidism. J Clin Endocrinol Metab. 1982;55:303–306. doi: 10.1210/jcem-55-2-303. [DOI] [PubMed] [Google Scholar]
- 11.Saleh FN, Schirmer H, Sundsfjord J, Jorde R. Parathyroid hormone and left ventricular hypertrophy. Eur Heart J. 2003;24:2054–2060. doi: 10.1016/j.ehj.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81:353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 13.Dawson-Hughes B, Heaney RP, Holick MF, Lips P, Meunier PJ, Vieth R. Estimates of optimal vitamin D status. Osteoporos Int. 2005;16:713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 14.Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. The Cardiovascular Health Study: design and rationale. Ann Epidemiol. 1991;1:263–276. doi: 10.1016/1047-2797(91)90005-w. [DOI] [PubMed] [Google Scholar]
- 15.Psaty BM, Kuller LH, Bild D, Burke GL, Kittner SJ, Mittelmark M, Price TR, Rautaharju PM, Robbins J. Methods of assessing prevalent cardiovascular disease in the Cardiovascular Health Study. Ann Epidemiol. 1995;5:270–277. doi: 10.1016/1047-2797(94)00092-8. [DOI] [PubMed] [Google Scholar]
- 16.Cushman M, Cornell ES, Howard PR, Bovill EG, Tracy RP. Laboratory methods and quality assurance in the Cardiovascular Health Study. Clin Chem. 1995;41:264–270. [PubMed] [Google Scholar]
- 17.Ives DG, Fitzpatrick AL, Bild DE, Psaty BM, Kuller LH, Crowley PM, Cruise RG, Theroux S. Surveillance and ascertainment of cardiovascular events. The Cardiovascular Health Study. Ann Epidemiol. 1995;5:278–285. doi: 10.1016/1047-2797(94)00093-9. [DOI] [PubMed] [Google Scholar]
- 18.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C. Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med. 2005;352:2049–2060. doi: 10.1056/NEJMoa043161. [DOI] [PubMed] [Google Scholar]
- 19.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, 3rd, Zhang YL, Greene T, Levey AS. Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis. 2008;51:395–406. doi: 10.1053/j.ajkd.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giovannucci E, Liu Y, Hollis BW, Rimm EB. 25-hydroxyvitamin D and risk of myocardial infarction in men: a prospective study. Arch Intern Med. 2008;168:1174–1180. doi: 10.1001/archinte.168.11.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ensrud KE, Taylor BC, Paudel ML, Cauley JA, Cawthon PM, Cummings SR, Fink HA, Barrett-Connor E, Zmuda JM, Shikany JM, Orwoll ES. Serum 25-hydroxyvitamin D levels and rate of hip bone loss in older men. J Clin Endocrinol Metab. 2009;94:2773–2780. doi: 10.1210/jc.2008-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holick MF, Chen TC. Vitamin D deficiency: a worldwide problem with health consequences. Am J Clin Nutr. 2008;87:1080S–1086S. doi: 10.1093/ajcn/87.4.1080S. [DOI] [PubMed] [Google Scholar]
- 23.Eastell R, Arnold A, Brandi ML, Brown EM, D’Amour P, Hanley DA, Rao DS, Rubin MR, Goltzman D, Silverberg SJ, Marx SJ, Peacock M, Mosekilde L, Bouillon R, Lewiecki EM. Diagnosis of asymptomatic primary hyperparathyroidism: proceedings of the third international workshop. J Clin Endocrinol Metab. 2009;94:340–350. doi: 10.1210/jc.2008-1758. [DOI] [PubMed] [Google Scholar]
- 24.Fried LP, Kronmal RA, Newman AB, Bild DE, Mittelmark MB, Polak JF, Robbins JA, Gardin JM. Risk factors for 5-year mortality in older adults: the Cardiovascular Health Study. Jama. 1998;279:585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 25.Melamed ML, Michos ED, Post W, Astor B. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med. 2008;168:1629–1637. doi: 10.1001/archinte.168.15.1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS. Vitamin D deficiency and risk of cardiovascular disease. Circulation. 2008;117:503–511. doi: 10.1161/CIRCULATIONAHA.107.706127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ginde AA, Scragg R, Schwartz RS, Camargo CA., Jr Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older U.S. adults. J Am Geriatr Soc. 2009;57:1595–1603. doi: 10.1111/j.1532-5415.2009.02359.x. [DOI] [PubMed] [Google Scholar]
- 28.Pilz S, Dobnig H, Nijpels G, Heine RJ, Stehouwer CD, Snijder MB, van Dam RM, Dekker JM. Vitamin D and mortality in older men and women. Clin Endocrinol (Oxf) 2009;71:666–672. doi: 10.1111/j.1365-2265.2009.03548.x. [DOI] [PubMed] [Google Scholar]
- 29.Schellenbaum GD, Heckbert SR, Smith NL, Rea TD, Lumley T, Kitzman DW, Roger VL, Taylor HA, Psaty BM. Congestive heart failure incidence and prognosis: case identification using central adjudication versus hospital discharge diagnoses. Ann Epidemiol. 2006;16:115–122. doi: 10.1016/j.annepidem.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 30.Ives DG, Samuel P, Psaty BM, Kuller LH. Agreement between nosologist and cardiovascular health study review of deaths: implications of coding differences. J Am Geriatr Soc. 2009;57:133–139. doi: 10.1111/j.1532-5415.2008.02056.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagstrom E, Hellman P, Larsson TE, Ingelsson E, Berglund L, Sundstrom J, Melhus H, Held C, Lind L, Michaelsson K, Arnlov J. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation. 2009;119:2765–2771. doi: 10.1161/CIRCULATIONAHA.108.808733. [DOI] [PubMed] [Google Scholar]
- 32.Bikle D. Nonclassic actions of vitamin D. J Clin Endocrinol Metab. 2009;94:26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Szabo A, Merke J, Beier E, Mall G, Ritz E. 1,25(OH)2 vitamin D3 inhibits parathyroid cell proliferation in experimental uremia. Kidney Int. 1989;35:1049–1056. doi: 10.1038/ki.1989.89. [DOI] [PubMed] [Google Scholar]
- 34.Forman JP, Curhan GC, Taylor EN. Plasma 25-hydroxyvitamin D levels and risk of incident hypertension among young women. Hypertension. 2008;52:828–832. doi: 10.1161/HYPERTENSIONAHA.108.117630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.de Boer IH, Ioannou GN, Kestenbaum B, Brunzell JD, Weiss NS. 25-Hydroxyvitamin D levels and albuminuria in the Third National Health and Nutrition Examination Survey (NHANES III) Am J Kidney Dis. 2007;50:69–77. doi: 10.1053/j.ajkd.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 36.Lind L, Hanni A, Lithell H, Hvarfner A, Sorensen OH, Ljunghall S. Vitamin D is related to blood pressure and other cardiovascular risk factors in middle-aged men. Am J Hypertens. 1995;8:894–901. doi: 10.1016/0895-7061(95)00154-H. [DOI] [PubMed] [Google Scholar]
- 37.Reichel H, Koeffler HP, Tobler A, Norman AW. 1 alpha,25-Dihydroxyvitamin D3 inhibits gamma-interferon synthesis by normal human peripheral blood lymphocytes. Proc Natl Acad Sci U S A. 1987;84:3385–3389. doi: 10.1073/pnas.84.10.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Maestro B, Molero S, Bajo S, Davila N, Calle C. Transcriptional activation of the human insulin receptor gene by 1,25-dihydroxyvitamin D(3) Cell Biochem Funct. 2002;20:227–232. doi: 10.1002/cbf.951. [DOI] [PubMed] [Google Scholar]
- 39.Chan AK, Duh QY, Katz MH, Siperstein AE, Clark OH. Clinical manifestations of primary hyperparathyroidism before and after parathyroidectomy. A case-control study. Ann Surg. 1995;222:402–412. doi: 10.1097/00000658-199509000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piovesan A, Molineri N, Casasso F, Emmolo I, Ugliengo G, Cesario F, Borretta G. Left ventricular hypertrophy in primary hyperparathyroidism. Effects of successful parathyroidectomy. Clin Endocrinol (Oxf) 1999;50:321–328. doi: 10.1046/j.1365-2265.1999.00651.x. [DOI] [PubMed] [Google Scholar]
- 41.Jorde R, Sundsfjord J, Haug E, Bonaa KH. Relation between low calcium intake, parathyroid hormone, and blood pressure. Hypertension. 2000;35:1154–1159. doi: 10.1161/01.hyp.35.5.1154. [DOI] [PubMed] [Google Scholar]
- 42.Schluter KD, Piper HM. Trophic effects of catecholamines and parathyroid hormone on adult ventricular cardiomyocytes. Am J Physiol. 1992;263:H1739–1746. doi: 10.1152/ajpheart.1992.263.6.H1739. [DOI] [PubMed] [Google Scholar]
- 43.Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, Andress DL. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71:31–38. doi: 10.1038/sj.ki.5002009. [DOI] [PubMed] [Google Scholar]