Tissue factor profile of human monocytic cells, afforded by alternative splicing, can be greatly influenced by the activity of SR proteins.
Keywords: SRp40, SC35, alternative splicing
Abstract
Following recruitment to solid tissues, peripheral blood monocytes express a number of proinflammatory molecules including TF, a trigger of coagulation that also promotes cell–cell interactions and tissue remodeling. Monocytes express two forms of TF: flTF, a highly coagulant transmembrane form, and asTF, a highly proangiogenic, soluble TF form. Biosynthesis of the two TF forms occurs via alternative processing of exon 5 during pre-mRNA splicing. Its inclusion results in flTF mRNA and its exclusion, asTF mRNA. We developed a splicing reporter system recently and determined that two spliceosomal constituents, SR proteins ASF/SF2 and SRp55, play a pivotal role in exon 5 inclusion. In this report, we show for the first time that two other SR proteins expressed in human monocytes, SRp40 and SC35, antagonize ASF/SF2 and SRp55 by competing for binding to certain sites in exon 5, thereby promoting TF exon 5 exclusion, an event unique to asTF biosynthesis. We also show that the intron preceding TF exon 5 possesses characteristics rarely found in U2 introns. Our findings indicate that modulation of TF pre-mRNA splicing can be accomplished via modification of SR proteins’ activity, facilitating development of novel therapeutic strategies to modulate the “TF profile” of monocytes/macrophages.
Introduction
Human monocytes are the major source of TF, the trigger of coagulation [1]. TF protein is expressed in two forms: flTF, a transmembrane molecule, and asTF, which lacks a transmembrane domain as a result of exclusion of exon 5 [2, 3]. Human monocytes are capable of expressing flTF as well as asTF [2] and were shown to increase production of both TF forms in response to LPS: flTF and asTF mRNA levels in freshly isolated peripheral blood monocytes rose at similar rates to reach approximately fourfold over the baseline after 3 h of LPS stimulation [4], indicating that monocytes maintain the variable processing of TF exon 5 under basal and stimulated conditions. Although flTF appears to exhibit a greater procoagulant potential compared with secreted asTF when assessed in static assays [5], asTF was shown recently to carry a greater proangiogenic potential compared with flTF [6]. It was proposed that the maintenance of the flTF/asTF ratio in blood is critical to normal hemostasis [7], and elucidation of the regulatory mechanisms controlling the expression of the two TF forms is thus of interest to translational research focusing on the pathophysiological crossroads of coagulation and inflammation. Regulated pre-mRNA splicing plays a prominent role in monocyte biology [8], yet little is known about the mechanisms controlling this process in this cell type. The functional properties of SR proteins, spliceosomal regulators, recently received increasing attention. Two SR proteins expressed in human monocytes, termed ASF/SF2 and SRp55, play a pivotal role in the TF exon 5 definition, an event essential for flTF biosynthesis. Using a reporter system comprising the variable splicing region of the human TF gene and a web resource ESEfinder (a search tool for SR protein-binding motifs, ESE) [9], recently, we identified functional ESE for ASF/SF2 and SRp55 in TF exon 5 and confirmed their physical association with the corresponding SR proteins [10]. Although this sheds some light on TF exon 5 processing, ASF/SF2 and SRp55 are not likely to fully account for the monocytes’ ability to variably splice TF pre-mRNA. Other SR proteins may also be involved, and it remains to be determined whether there exists a functional interplay between SR proteins in TF exon 5 definition. In 1997, a pioneering study by Jumaa and Nielsen [11] demonstrated convincingly that in a murine B lymphoma cell line K46, ASF/SF2 and SRp20 play opposing roles in the variable processing of SRp20 exon 4.
MATERIALS AND METHODS
Cell culture
Human monocytic cell line THP-1 was purchased from American Cell Type Collection (Manassas, VA, USA) and maintained at 37°C in a CO2 incubator (5%) in RPMI-1640 medium supplemented with 10% FBS, 10 mM HEPES, 1 mM sodium pyruvate (all HyClone, Logan, UT, USA), and 50 uM β-ME (Sigma-Aldrich, St. Louis, MO, USA). To achieve minimal toxicity and consistency of the transient transfection procedure, no antibiotics or differentiating/stimulating agents were added to the cell medium. To ensure consistency of the cell culture phenotype, the purchased cells were expanded for 2 weeks and frozen in multiple aliquots of 2 × 106 cells/ml, which were expanded subsequently, one at a time, for 2–3 weeks and then used for all experiments.
Transfections, site-directed mutagenesis, RT-PCR, and Western blotting
All were performed as described previously [10]; amplicons were quantified using Image J software v. 1.42 (National Institutes of Health, Bethesda, MD, USA).
RNA mobility shift assay
Uniformly labeled RNA probes comprising bases 4–77 and bases 57–120 of the human TF exon 5 (probes 1 and 2, respectively) were synthesized as described previously [10]. Nuclear extracts of THP-1 cells were prepared on the day of the experiment, as per Andrews and Faller [12], and protein concentrations were determined using the Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA). RNA band-shift gels were prepared and run as described previously [10]. After each run, band-shift gels were dried and hereafter, exposed to a phosphoscreen; quantification of protein-RNA complexes was performed using the Typhoon TRIO variable mode imager and ImageQuant TL software v. 2005 (GE Healthcare, Waukesha, WI, USA).
RESULTS
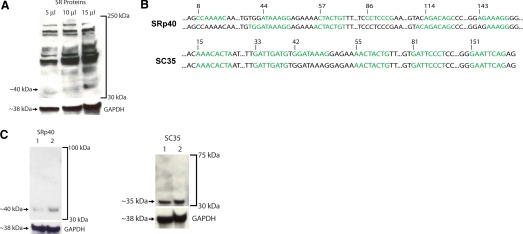
Western blotting of THP-1 cells, a human monocytic line long preferred to study TF biology [13] using the mAb 16H3, revealed that these cells express a large number of SR proteins (Fig. 1A), including proteins with the apparent molecular weights corresponding to those of ASF/SF2, SRp55, and SRp75, the three SR proteins shown recently to play a major role in the regulated TF pre-mRNA splicing in the vasculature [10, 14]. The pattern of higher molecular weight protein species detected in THP-1 cells was similar to that observed in the course of the study comprising the initial characterization of 16H3 immunoreactivity [15]. We also detected a protein whose apparent molecular weight was close to that of SRp40, the SR protein detected recently along with the SR protein SC35, in human peripheral white blood cells [16] (Fig. 1A). Using ESEfinder [10], we analyzed TF exon 5 for potential binding sites for SRp40 and SC35. Six potential sites for SRp40 were identified, and one of these sites, at base 44, overlapped with the known SRp55 ESE at base 39, critical for TF exon 5 inclusion [10]. Also overlapping with this SRp55 ESE was one of the six putative sites for SC35, at base 33, and the putative SC35 site at base 81 overlapped with the known ESE for ASF/SF2 at base 87, raising the possibility that SRp40 and SC35 may interfere with the binding of SRp55 and/or ASF/SF2 to TF exon 5 (Fig. 1B). Another search tool for SR binding motifs, termed Splicing Rainbow, also detected several putative SRp40 and SC35 sites in TF exon 5 (Fig. 1B). The antibody 16H3 recognizes SRp40 but not SC35. To ascertain whether SRp40 and SC35 are expressed in THP-1 cells, we used SRp40- and SC35-specific antibodies for Western blotting and primers specific for SRp40 and SC35 for RT-PCR. SRp40 and SC35 mRNA (not shown) and protein (Fig. 1C) were detectable in THP-1 cells.
Figure 1.
SRp40 and SC35 are expressed in human monocytic cells. (A) Western blotting of THP-1 lysates, anti-SR protein mAb 16H3 (Zymed, San Francisco, CA, USA). The blot was reprobed with anti-GAPDH polyclonal antibody (Trevigen, Gaithersburg, MD, USA). (B) In silico analysis of TF exon 5 using web resources ESEfinder (http://rulai.cshl.edu/tools/ESE2/) and Splicing Rainbow (http://www.ebi.ac.uk/asd-srv/wb.cgi?method=8). Potential binding sites for SRp40 (upper panel) and SC35 (lower panel) are in green; in both panels, the upper row shows the sites identified by ESEfinder and the lower row, by Splicing Rainbow. Numbers indicate base positions in TF exon 5 (length=160 bp). (C) Detection of SRp40 and SC35 proteins in THP-1 cells. The amount of lysate in Lane 1 is half of that in Lane 2; the polyclonal anti-SRp40 antibody (USB Corp., Cleveland, OH, USA) and the polyclonal anti-SC35 antibody (Sigma-Aldrich) were used. Blots were stripped and reprobed with polyclonal anti-GAPDH antibodies as described [10].
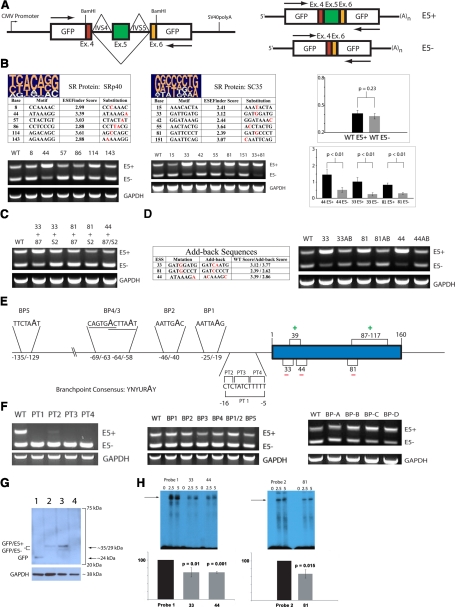
The splicing reporter system pGL-hTF [10] comprises the open-reading frame of the GFP driven by the CMV promoter, modified via insertion of human TF exon 5 flanked by its endogenous introns 4 and 5 (IVS4 and IVS5, respectively); the schematic of pGL-hTF is shown in Figure 2A. Splicing of the pre-mRNA, produced by pGL-hTF, yields two mRNA species: the longer one with exon 5 and the shorter one without exon 5, representing flTF and asTF, respectively (Fig. 2A). pGL-hTF was used successfully to delineate the ESE experimentally for ASF/SF2 and SRp55, in our study examining the role of these SR proteins in TF exon 5 definition [10]. To investigate the functionality of the putative sites for SRp40 and SC35, we designed base substitutions, selectively weakening each site, and introduced them into pGL-hTF by site-directed mutagenesis, which yielded six mutants each for SRp40 and SC35. When SRp40 mutants were expressed transiently in THP-1 cells, only one SRp40 mutant, with a weakened site 44, exhibited a splicing pattern clearly distinct from that of the wild-type pGL-hTF. Enhanced inclusion of exon 5 was observed (Fig. 2B). Two SC35 mutants, with weakened sites 33 and 81, also exhibited a change in the wild-type splicing pattern analogous to that of the SRp40 mutant 44; further, a pGL-hTF mutant featuring mutagenized SC35 sites 33 and 81 exhibited a more severe pattern of enhanced exon 5 inclusion with a drastic reduction of the mRNA species lacking exon 5 (Fig. 2B). All three sites, whose weakening enhances exon 5 inclusion, overlap with SRp55 ESE 39 and/or ASF/SF2 ESE 87, weakening of which elicits the opposite effect [10]. This indicates that a functional interplay between ASF/SF2 and SRp55 on one hand and SRp40 and SC35 on the other hand likely accounts for the concomitant production of asTF and flTF mRNA variants, afforded by competitive binding of these SR proteins to their position-overlapping sites in exon 5, whereby the sites for SRp40 and SC35 act as ESS. To confirm our findings, we generated combinatorial pGL-hTF mutants featuring the weakened sites for SRp40 and SC35 and the weakened ASF/SF2 ESE at positions 87–117, critical for exon 5 inclusion [10]. These mutants exhibited the splicing pattern close to that of the wild-type pGL-hTF, attesting to the ESS nature of the SRp40 site 44 and the SC35 sites 33 and 81 (Fig. 2C). To confirm these findings further, we generated add-back pGL-hTF mutants featuring nonendogenous-binding motifs in place of the weakened ESS, whose ESEfinder scores are similar to those of the corresponding wild-type ESS. Reintroduction of a site for SRp40 or SC35 in its endogenous location is expected to revert the splicing pattern to that of the wild-type pGL-hTF. All three add-back mutants exhibited the wild-type splicing pattern (Fig. 2D), allowing us to conclude that the SRp40 site 44 and the SC35 sites 33 and 81 are functional, acting as ESS. The SR protein-dependent definition of TF exon 5 is a characteristic of internal exons preceded by weak 3′ splice sites [17]. Exon 5 fits this description, as intron 4 features a short PT (Fig. 2E). In the course of pre-mRNA splicing, SR proteins bound to exons interact with the proteins bound to the PT located at the end of a preceding intron, e.g., U2AF65/35 [17]; thus, investigation of the structural elements in TF intron 4 is instrumental in elucidating the mechanics of TF exon 5 definition. To assess the major functional properties of intron 4, we first generated pGL-hTF mutants featuring pyrimidine-to-purine substitutions throughout the entire PT (PT1) or in its three sequential areas (PT2, -3, and -4). These mutants produced virtually no exon 5 containing mRNA when expressed in THP-1 cells (Fig. 2F), indicating that the PT of intron 4 plays a pivotal role in SR protein-dependent exon 5 definition. We also sought to identify the BP of intron 4. Guanine residues in pGL-hTF replaced the adenine residues within each potential BP consensus motif [18] (total of five motifs, two of them overlapping). To our surprise, no discernible changes in the splicing pattern were observed (Fig. 2F). We then proceeded to generate four mutants designated BP-A, BP-B, BP-C, and BP-D, in which all adenines in the increments of 20 bases within the last 100 bases of intron 4, excluding the PT, were replaced by guanines. None of these mutants exhibited an appreciable change in the splicing pattern (Fig. 2F), indicating that intron 4 features a noncanonical BP that does not use an adenine to form a lariat, a rare type of BP in mammalian U2 introns [18]. To confirm that the changes in the pGL-hTF pre-mRNA splicing pattern result in the analogous changes on the protein level, we transfected a panel of representative constructs in THP-1 cells and performed Western blotting using an anti-GFP mAb. As expected, weakening of the SC35 sites 33 and 81 augmented substantially the levels of the modified GFP protein species containing the TF extracellular segment encoded by exon 5, whereas weakening of the PT of exon 5 elicited the opposite effect (Fig. 2G). Still, the results described thus far do not comprise direct evidence that the identified ESS serve as bona fide binding sites for SRp40 and SC35. To verify that SR proteins physically associate with the sites 33, 44, and 81, we performed RNA mobility shift assays using the wild-type probes featuring these sites and the probes in which these three motifs were weakened by single base substitutions (Fig. 2B). As expected, the mutant probes exhibited a significantly weakened association with SR proteins compared with their wild-type counterparts (Fig. 2H).
Figure 2.
Spliceosomal definition of TF exon 5: SR protein-dependent mechanisms predominate. (A) Schematic of the reporter system pGL-hTF. Arrows indicate positions of primers used in conventional RT-PCR. Exon 5-containing amplicon (E5+), 432 bp; exon 5-lacking amplicon (E5-), 272 bp. (B) SRp40 promotes TF exon 5 exclusion via the ESS at position 44 and SC35 via ESS at positions 33 and 81. At the top of each table are pictograms of the degenerate motifs for SRp40 and SC35 [9]; mutagenized positions in each putative site are shown in red. Representative RT-PCR (n≥3) of the resultant expression patterns is below each table. In the graphs, the change in the degree of exon 5 definition as a result of ESS weakening results in the change of the splicing pattern. At least three independent transfection assays were normalized to GAPDH, quantified using ImageJ software v.1.42, and averaged; error bars are sd. WT, Wild-type. (C) Representative RT-PCR (n≥4), the expression pattern of the combinatorial mutants featuring weakened ASF/SF2 ESE and weakened SRp40/SC35 ESS. (D) Representative RT-PCR (n≥4), expression pattern of the “add-back” mutants whose sequences and scores are shown in the table, featuring nonendogenous SRp40 and SC35 ESS. (E) The diagram depicting the opposing roles of SR proteins in TF exon 5 definition and the potential structural elements in TF intron 4. (F, left panel) Representative RT-PCR (n≥4), the expression pattern of the pGL-hTF mutants featuring purine substitutions in the PT of intron 4. (Middle panel) Representative RT-PCR (n≥4), the expression pattern of the pGL-hTF mutants featuring adenine-to-guanine substitutions in the potential BP motifs of intron 4. (Right panel) Representative RT-PCR (n≥4), pGL-hTF mutants featuring multiple adenine-to-guanine substitutions clustered within the last 100 bases of intron 4. (G) Representative Western blot (n≥3), THP-1 was transiently transfected for 60 h with pGL (Lane 1), pGL-hTF wild-type (Lane 2), pGL-hTF harboring weakened SC35 sites 33 and 81 (Lane 3), and pGL-hTF harboring an expensively weakened PT of exon 5 (PT1, Lane 4), lysed and probed with anti-GFP mAb (Roche Life Sciences, Indianapolis, IN, USA). The blot was stripped and reprobed with polyclonal anti-GAPDH antibodies as described [10]. (H, upper panels) Representative radiograms (n=3) of RNA mobility shift assays using wild-type and mutagenized probes featuring the ESS 33, 44, and 81. Numbers on top indicate the amounts of nuclear protein (in μg). (Lower panels) The results of three independent assays were quantified as described in Materials in Methods and averaged. (In both graphs, the intensity of the major RNA-protein complexes produced by wild-type probes was treated as 100%; error bars are sd.)
DISCUSSION
We here demonstrate for the first time that competitive binding of several SR proteins to TF exon 5 appears to play a major role in determining the flTF/asTF mRNA ratio in human monocytic cells. SR proteins are known to work antagonistically. Most recently, SC35 and ASF/SF2 were found to exert opposing functions in the splicing of GH pre-mRNA [19]. The full-length form of GH is produced when all five exons are included; when GH exon 3 is skipped, the resultant protein acts by preventing the secretion of the full-length GH. ASF/SF2 promotes exon 3 inclusion, and SC35 promotes exon 3 exclusion. ASF/SF2 and SC35 bind to certain sites in GH exon 3—ESE2 and a site immediately after ESE2, respectively. Competition between SC35 and ASF/SF2 for binding to their respective sites, which are in close proximity, results in the variable processing of exon 3 [19]. Specific to our study is the competition between SRp40/SC35 and SRp55 and between SC35 and ASF/SF2. ASF/SF2 and SRp55 promote biosynthesis of flTF mRNA, and SRp40 and SC35 appear to promote biosynthesis of asTF mRNA. The binding sites affording this functional interplay are overlapping, analogously to what was described for SC35 and ASF/SF2 [19]. Most recently, Eisenreich et al. [14] reported that the SR protein SRp75 plays an important role in the variable TF pre-mRNA splicing in human endothelial cells. Although it is not yet known whether SRp75 physically associates with TF exon 5 or alternatively, exerts its activity via binding to other TF pre-mRNA regions, it is highly likely that SRp75, a protein expressed abundantly in THP-1 cells (Fig. 1A), may also be a substantial contributor to the protein network that affords the spliceosomal definition of this variable cassette exon in the human TF pre-mRNA, along with other spliceosomal constituents such as the SR protein 9G8, U170K, and heterogeneous nuclear ribonucleoprotein family of proteins [17].
Our splicing reporter system was able to reveal a heretofore unknown complexity of the SR protein interplay in the variable processing of exon 5 under nonstimulated conditions. Until now, SR proteins were thought to promote exon 5 inclusion exclusively [10, 14]. We note that the reporter system pGL-hTF comprises only a portion of the human TF gene and as such, may not optimally recapitulate the entire scope of the spliceosomal dynamics in the course of the native TF pre-mRNA splicing, especially under the conditions leading to transcriptional up-regulation of the TF gene, e.g., LPS stimulation [4]. That notwithstanding, our findings suggest that the signaling pathways regulating SR protein activity are likely to play a major role in the shaping of the monocyte TF profile, as was shown recently to be the case in human endothelial cells. Eisenreich et al. [14] demonstrated that in HUVEC, regulated splicing of the TF pre-mRNA synthesized de novo in response to TNF-α is largely controlled by the Clk and the PI-3/Akt intracellular signaling pathways, two major mechanisms of SR protein-mediated, alternative splicing regulation [17, 20]. Interestingly, Jiang et al. [21] reported recently that Akt is able to phosphorylate Clk, which in turn, leads to a change in the phosphorylation status and activity of the distinct SR protein subsets controlled by these two kinases. Our future studies will address whether down-regulation and/or overexpression of select SR proteins controlled by these kinases, including SRp40 and SC35, will elicit significant changes in the levels of total and surface-localized TF activity in peripheral blood monocytes, which express low levels of TF, and THP-1 cells, whose constitutive TF levels are substantially higher [10].
The concomitant expression of the two TF forms is an established feature of monocyte physiology [2, 4], and SR proteins appear to act as the “molecular enablers” of this phenomenon under steady-state conditions; thus, aberrations in the relative amounts or the activity levels of SR proteins are likely to have a major impact on regulated TF biosynthesis. In light of the pronounced, systemic pre-mRNA splicing aberrations in malignancies [22], it is worth noting a study examining TF levels in patients with APL. The standard of treatment for APL, all-trans retinoic acid, reduced flTF and asTF mRNA levels over a period of 2 weeks, and asTF was the predominant TF species in one of the two representative de novo cases at the treatment’s onset [23]. As flTF and asTF possess distinct functional properties, with flTF more procoagulant and asTF more proiangiogenic [6], novel therapeutic strategies affording a targeted change in the flTF/asTF ratio hold promise in the management of disease states linked to monocyte TF, most notably, plaque neovascularization, intravascular thrombosis, and proliferative retinopathy in diabetes mellitus. Cardiovascular disease remains a major cause of morbidity and mortality among diabetic patients, and the continuous activation of circulating monocytes and resident plaque macrophages/foam cells is a known contributor to cardiovascular diabetic disease progression, afforded in large part by heightened TF expression by these cells [24]. Of note, Akt2 is the central kinase in insulin action [21], and it is not known currently whether its splicing regulatory functions are altered in circulating monocytes of poorly controlled diabetic patients. Future studies should address whether a specific form of TF might be responsible for such debilitating diabetic vasculopathies as propensity to thrombosis, as well as abnormal angiogenesis in the retina and developing atherosclerotic plaques.
AUTHORSHIP
S. C., G. D., and J. G. T. designed and performed experiments, analyzed data, and wrote the paper; V. Y. B. conceived the research, performed experiments, analyzed data, and wrote the paper.
ACKNOWLEDGMENTS
This study was supported by the National Institutes of Health (grant DK065752 to V. Y. B.) and the American Society of Hematology (2009 Trainee Research Award to S. C.). The authors thank Professor David H. Bechhofer (Mount Sinai School of Medicine) for helpful discussions.
Footnotes
Abbreviations: APL=acute promyelocytic leukemia, asTF=alternatively spliced tissue factor, BP=branch point, ESE=exonic splicing enhancer, ESS=exonic splicing silencer, flTF=full-length tissue factor, GH=growth hormone, PT=polypyrimidine tract, SR=serine/arginine-rich, TF=tissue factor
References
- Østerud B., Bjørklid E. Sources of tissue factor. Semin Thromb Hemost. 2006;32:11–23. doi: 10.1055/s-2006-933336. [DOI] [PubMed] [Google Scholar]
- Bogdanov V. Y., Balasubramanian V., Hathcock J., Vele O., Lieb M., Nemerson Y. Alternatively spliced human tissue factor: a circulating, soluble, thrombogenic protein. Nat Med. 2003;9:458–462. doi: 10.1038/nm841. [DOI] [PubMed] [Google Scholar]
- Bogdanov V. Y., Kirk R. I., Miller C., Hathcock J. J., Vele S., Gazdoiu M., Nemerson Y., Taubman M. B. Identification and characterization of murine alternatively spliced tissue factor. J Thromb Haemost. 2006;4:158–167. doi: 10.1111/j.1538-7836.2005.01680.x. [DOI] [PubMed] [Google Scholar]
- Bajaj M. S., Ghosh M., Bajaj S. P. Fibronectin-adherent monocytes express tissue factor and tissue factor pathway inhibitor whereas endotoxin-stimulated monocytes primarily express tissue factor: physiologic and pathologic implications. J Thromb Haemost. 2007;5:1493–1499. doi: 10.1111/j.1538-7836.2007.02604.x. [DOI] [PubMed] [Google Scholar]
- Szotowski B., Antoniak S., Poller W., Schultheiss H. P., Rauch U. Procoagulant soluble tissue factor is released from endothelial cells in response to inflammatory cytokines. Circ Res. 2005;96:1233–1239. doi: 10.1161/01.RES.0000171805.24799.fa. [DOI] [PubMed] [Google Scholar]
- Hobbs J. E., Zakarija A., Cundiff D. L., Doll J. A., Hymen E., Cornwell M., Crawford S. E., Liu N., Signaevsky M., Soff G. A. Alternatively spliced human tissue factor promotes tumor growth and angiogenesis in a pancreatic cancer tumor model. Thromb Res. 2007;120(Suppl. 2):S13–S21. doi: 10.1016/S0049-3848(07)70126-3. [DOI] [PubMed] [Google Scholar]
- Brüggemann L. W., Drijfhout J. W., Reitsma P. H., Spek C. A. Alternatively spliced tissue factor in mice: induction by Streptococcus pneumoniae. J Thromb Haemost. 2006;4:918–920. doi: 10.1111/j.1538-7836.2006.01870.x. [DOI] [PubMed] [Google Scholar]
- Jaresová I., Rozková D., Spísek R., Janda A., Brázová J., Sedivá A. Kinetics of Toll-like receptor-4 splice variants expression in lipopolysaccharide-stimulated antigen presenting cells of healthy donors and patients with cystic fibrosis. Microbes Infect. 2007;9:1359–1367. doi: 10.1016/j.micinf.2007.06.009. [DOI] [PubMed] [Google Scholar]
- Cartegni L., Wang J., Zhu Z., Zhang M. Q., Krainer A. R. ESEfinder: a web resource to identify exonic splicing enhancers. Nucleic Acids Res. 2003;31:3568–3571. doi: 10.1093/nar/gkg616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardos J. G., Eisenreich A., Deikus G., Bechhofer D. H., Chandradas S., Zafar U., Rauch U., Bogdanov V. Y. SR proteins ASF/SF2 and SRp55 participate in tissue factor biosynthesis in human monocytic cells. J Thromb Haemost. 2008;6:877–884. doi: 10.1111/j.1538-7836.2008.02946.x. [DOI] [PubMed] [Google Scholar]
- Jumaa H., Nielsen P. J. The splicing factor SRp20 modifies splicing of its own mRNA and ASF/SF2 antagonizes this regulation. EMBO J. 1997;16:5077–5085. doi: 10.1093/emboj/16.16.5077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews N. C., Faller D. V. A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acids Res. 1991;19:2499. doi: 10.1093/nar/19.9.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand K., Fowler B. J., Edgington T. S., Mackman N. Tissue factor mRNA in THP-1 monocytic cells is regulated at both transcriptional and posttranscriptional levels in response to lipopolysaccharide. Mol Cell Biol. 1991;11:4732–4738. doi: 10.1128/mcb.11.9.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenreich A., Bogdanov V. Y., Zakrzewicz A., Pries A., Antoniak S., Poller W., Schultheiss H. P., Rauch U. Cdc2-like kinases and DNA topoisomerase I regulate alternative splicing of tissue factor in human endothelial cells. Circ Res. 2009;104:589–599. doi: 10.1161/CIRCRESAHA.108.183905. [DOI] [PubMed] [Google Scholar]
- Neugebauer K. M., Stolk J. A., Roth M. B. A conserved epitope on a subset of SR proteins defines a larger family of pre-mRNA splicing factors. J Cell Biol. 1995;129:899–908. doi: 10.1083/jcb.129.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanuki T., Funato H., Uchida S., Matsubara T., Kobayashi A., Wakabayashi Y., Otsuki K., Nishida A., Watanabe Y. Increased expression of splicing factor SRp20 mRNA in bipolar disorder patients. J Affect Disord. 2008;110:62–69. doi: 10.1016/j.jad.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Long J. C., Caceres J. F. The SR protein family of splicing factors: master regulators of gene expression. Biochem J. 2009;417:15–27. doi: 10.1042/BJ20081501. [DOI] [PubMed] [Google Scholar]
- Gesteland R. F., Cech T. R., Atkins J. F., editors. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY, USA: The RNA World. (Second Edition) 1999 [Google Scholar]
- Solis A. S., Peng R., Crawford J. B., Phillips J. A., III, Patton J. G. Growth hormone deficiency and splicing fidelity: two serine/arginine-rich proteins, ASF/SF2 and SC35, act antagonistically. J Biol Chem. 2008;283:23619–23626. doi: 10.1074/jbc.M710175200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenreich A., Malz R., Pepke W., Ayral Y., Poller W., Schultheiss H. P., Rauch U. Role of the phosphatidylinositol 3-kinase/protein kinase B pathway in regulating alternative splicing of tissue factor mRNA in human endothelial cells. Circ J. 2009;73:1746–1752. doi: 10.1253/circj.cj-99-0225. [DOI] [PubMed] [Google Scholar]
- Jiang K., Patel N. A., Watson J. E., Apostolatos H., Kleiman E., Hanson O., Hagiwara M., Cooper D. R. Akt2 regulation of Cdc2-like kinases (Clk/Sty), serine/arginine-rich (SR) protein phosphorylation, and insulin-induced alternative splicing of PKCβII messenger ribonucleic acid. Endocrinology. 2009;150:2087–2097. doi: 10.1210/en.2008-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables J. P. Unbalanced alternative splicing and its significance in cancer. Bioessays. 2006;28:378–386. doi: 10.1002/bies.20390. [DOI] [PubMed] [Google Scholar]
- Zhu J., Guo W. M., Yao Y. Y., Zhao W. L., Pan L., Cai X., Ju B., Sun G. L., Wang H. L., Chen S. J., Chen G. Q., Caen J., Chen Z., Wang Z. Y. Tissue factors on acute promyelocytic leukemia and endothelial cells are differently regulated by retinoic acid, arsenic trioxide and chemotherapeutic agents. Leukemia. 1999;13:1062–1070. doi: 10.1038/sj.leu.2401448. [DOI] [PubMed] [Google Scholar]
- Khechai F., Ollivier V., Bridey F., Amar M., Hakim J., de Prost D. Effect of advanced glycation end product-modified albumin on tissue factor expression by monocytes Role of oxidant stress and protein tyrosine kinase activation. Arterioscler Thromb Vasc Biol. 1997;17:2885–2890. doi: 10.1161/01.atv.17.11.2885. [DOI] [PubMed] [Google Scholar]