Abstract
Background
Adolescence is a common time for the onset of anorexia nervosa (AN), a condition associated with long-term medical and hormonal consequences.
Objective
The objective was to compare the nutrient intakes of community-dwelling girls with AN with those of healthy adolescents and to describe the associations between specific nutrient intakes and nutritionally dependent hormones.
Design
Nutrient intakes in 39 community-dwelling girls with AN and 39 healthy adolescents aged 12.1–18.7 y were determined by using 4-d food records. Fasting adiponectin, leptin, ghrelin, insulin, and insulin-like growth factor I (IGF-I) concentrations were measured. Indirect calorimetry was used to assess respiratory quotient and resting energy expenditure.
Results
In contrast with the control group, the AN group consumed fewer calories from fats (P < 0.0001) and more from carbohydrates (P = 0.0009) and proteins (P < 0.0001). Intake of individual fat components was lower and of dietary fiber higher in the AN group. No significant between-group differences were observed in dietary intakes of calcium, zinc, and iron; however, total intake was greater in the AN group because of greater supplement use (P = 0.006, 0.02, and 0.01, respectively). The AN group had greater intakes of vitamins A, D, and K and of most of the B vitamins, and significantly more girls with AN met the Dietary Reference Intake for calcium (P = 0.01) and vitamin D (P = 0.02) from supplement use. Fat intake predicted ghrelin, insulin, and IGF-I concentrations; carbohydrate intake predicted adiponectin. Resting energy expenditure was lower (P < 0.0001) and leisure activity levels higher in the AN group.
Conclusions
Despite outpatient follow-up, community-dwelling girls with AN continue to have lower fat and higher fiber intakes than do healthy adolescents, which results in lower calorie intakes. Nutritionally related hormones are associated with specific nutrient intakes.
Keywords: Anorexia nervosa, nutrient intake, vitamin D, energy expenditure, adolescent girls
INTRODUCTION
Eating disorders are the third most common chronic illness in girls under the age of 18 y (1). Anorexia nervosa (AN), in particular, occurs in 0.7–1.3% of female adolescents (2), and 50% of 5th to 12th grade adolescents report dieting in an effort to improve their appearance (3). In adolescents with AN, many medical consequences, such as cardiac arrhythmias, electrolyte imbalances, vitamin and mineral deficiencies, osteoporosis, and hormonal changes, have been identified (4–9). Studies have examined dietary patterns in adult women with AN (10). However, few studies have examined nutrient intakes, particularly micro-nutrient intakes, in adolescent girls with AN. Health care professionals are often unaware of specific eating patterns associated with this condition. AN patients may then have not only insufficient intakes of calories but also inadequate intakes of certain macronutrients or micronutrients. Even fewer data are available regarding nutrient intakes in community-dwelling girls with AN being followed by caregivers as outpatients. Pathologic patterns of nutrient intake may persist despite outpatient intervention and are important to determine to develop further strategies for nutritional counseling and to optimize weight rehabilitation. In addition, although specific hormone abnormalities have been reported, the relation between nutrient intake and nutritionally related hormones has not been explored. We previously reported abnormalities in leptin, ghrelin, adiponectin, insulin, and insulin-like growth factor I (IGF-I) secretion in 20 girls with AN and 21 healthy adolescents (11–13), but not in relation to nutrient intake.
This study aims to describe patterns of macronutrient and micronutrient intakes in community-dwelling girls with AN. A detailed analysis of food records, including micronutrient intakes in a large population of girls with AN and control subjects, and the relation with hormones affected by nutritional status are presented. Given the high incidence of low bone density in AN (4, 5, 7, 8), intakes of vitamins and minerals that relate to bone health were investigated.
SUBJECTS AND METHODS
Seventy-eight adolescent girls were enrolled in the study between July 1997 and October 2002 [n = 39 girls with AN meeting DSM-IV (Diagnostic and Statistical Manual) criteria and 39 healthy adolescents). Data regarding clinical characteristics, and partial data regarding macronutrient, calcium, and vitamin D intakes of 17 girls with AN and of 18 control subjects were reported earlier by our group (7). The mean chronologic age of the girls with AN was 15.9 ± 0.3 y and of the healthy adolescents was 15.0 ± 0.3 y. The groups did not differ significantly in bone age (AN group: 15.4 ± 0.2 y; control group: 15.5 ± 0.3 y). The mean (±SEM) duration of illness was 11.3 ± 1.9 mo in the AN group, and the 39 girls with AN were community-dwelling girls consecutively referred to our study. The AN subjects were being followed by providers on an outpatient basis and were recruited from Eating Disorder Units at Massachusetts General Hospital (MGH) and MGH-affiliated and community centers. Healthy adolescents were recruited through mailings to primary care pediatric practices in and around Boston and via newspaper advertisements and had no history of eating disorders. The study protocol was approved by our Institutional Review Board, and written informed assent and consent was obtained from all subjects and their legal guardians.
Subjects were assessed during an outpatient visit at our General Clinical Research Center. Height was measured with a single stadiometer (average of 3 measurements), and weight was measured with an electronic scale while the subjects were wearing a hospital gown. Body mass index (BMI) was calculated as the ratio of weight (in kg) to height squared (in m). Bone age was determined by using the methods of Greulich and Pyle (14) to confirm that the groups were of comparable maturity. After the subjects fasted overnight, blood samples were obtained for the measurement of adiponectin, leptin, ghrelin, insulin, and IGF-I concentrations. Leptin and IGF-I data were available for all subjects, and ghrelin, insulin, and adiponectin values were available for 20 girls with AN and 21 control subjects.
Resting energy expenditure (REE) was determined by indirect calorimetry (Vmax 29N; SensorMedics, Loma Linda, CA) (15, 16). Subjects sat quietly in a thermal neutral room for ≥15 min before the study began. Oxygen consumption and carbon dioxide production were measured continuously. Estimated REE was also determined by using the Harris-Benedict equation (17), the modified Harris-Benedict equation for AN (18), and World Health Organization (WHO) (19) recommendations. Finally, we used an equation incorporating weight, height, age, and physical activity to calculate estimated energy expenditure (EER) (20, 21). A modifiable activity questionnaire was administered to determine activity level (22).
Nutritional intake
All participants were instructed by a registered dietitian on the completion of a 4-d food (inclusive of beverages) record of 3 weekdays and 1 weekend day, and written and verbal guidelines were provided for the estimation of food portions. As part of the instructional session, the participants were asked to describe portions used and food preparation details for a minimum of 2 prior meals to clarify understanding. All participants were encouraged to depict typical food consumption. A research dietitian reviewed the completed food records for clarification of portions and food preparation. Nutrient intakes were calculated from the 4-d food records by using the Minnesota Nutrition Data System software (version 4.03; nutrient database 31).
Hormonal evaluations
Radioimmunoassay was used to measure leptin (Linco Diagnostics Inc, St Charles, MO; sensitivity: 0.5 μg/L; CV: 4.6–5.7%), ghrelin (Phoenix Pharmaceuticals, Belmont, CA; sensitivity: 2 pg/mL; CV: 9.9–10.5%), insulin (Diagnostics Products, Los Angeles, CA; CV: 4.7–7.7%), and adiponectin (Linco Diagnostics Inc; lowest detectable concentration: 1 μg/L; CV: 6.4–8.4%). An immunoradiometric assay was used to determine IGF-I concentrations (Nichols Institute Diagnostics Inc, San Juan Capistrano, CA), with a detection limit of 30 μg/L and a CV of 3.1–4.6%.
Body composition and bone density
Body composition was determined with both whole-body dual-energy X-ray absorptiometry (DXA) (Hologic QDR-4500; Hologic Inc, Waltham, MA), which has been validated for body-composition measurements (23, 24). Lumbar spine bone mineral density (BMD) was measured by DXA with the Hologic QDR-4500. The applet of Bachrach, Hastie, and Narasimhan (Internet: http://www-stat-class.stanford.edu/pediatric-bones/) was used to calculate z scores of lumbar spine BMD.
Statistical analysis
Data are described as means ± SEMs. Student’s t test was used to determine whether differences between the groups were significant, and the chi-square test or Fisher’s exact test was used to determine significant differences between proportions. When the data were not normally distributed, Wilcoxon’s rank-sum test was used to compare the groups. A P value <0.05 was considered significant. Correlational analyses were used to determine relations between variables, which was followed by stepwise regression analysis to determine independent predictors. Data were analyzed by using the JMP program (version 4; SAS Institute Inc, Cary, NC).
RESULTS
Clinical characteristics
Compared with healthy adolescent girls, girls with AN had a significantly lower BMI (16.5 ± 0.2 compared with 21.8 ± 0.5; P < 0.0001), fat mass (8.3 ± 0.5 compared with 17.9 ± 0.8 kg; P < 0.0001), and lean body mass (35.3 ± 0.6 compared with 38.1 ± 0.9 kg; P = 0.01). Lumbar spine BMD z scores were also lower in the AN group (−0.755 ± 0.164 compared with −0.088 ± 0.157; P = 0.004).
Nutrient intakes
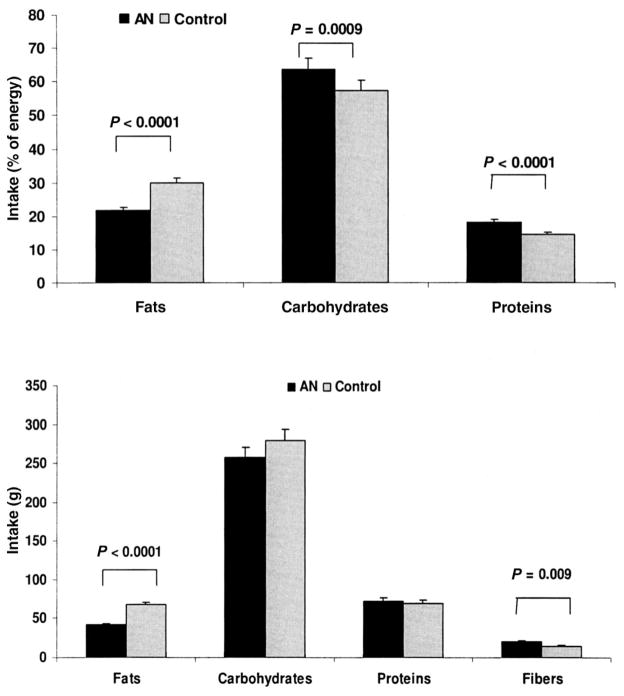
The girls with AN consumed significantly fewer calories than did the healthy adolescents (1649 ± 110 compared with 1970 + 91 kcal; P = 0.03) based on average daily calorie intakes from the 4-d food record. The percentage of energy derived from fat and absolute intakes of fats were lower, and the percentage of energy derived from carbohydrates and proteins was higher in the AN group than in the control group (Figure 1). Intakes of all forms of fat (ie, saturated, monounsaturated, and polyunsaturated) were lower in the AN group than in the control group, and measured 42.0%, 39.7%, and 27.0% of the values in the control group, respectively (Table 1). Intakes of n – 3 and n – 6 fatty acids were also lower in the AN group.
FIGURE 1.
Mean (±SEM) macronutrient intakes in 39 girls with anorexia nervosa (AN) and in 39 healthy control subjects. The girls with AN had a lower absolute intake of fats and a higher intake of fibers than did the control subjects. Compared with the control group, the percentage of energy derived from fats was lower and the percentage of energy derived from carbohydrates and proteins was greater in the AN group. Student’s t test was used to compare the data between groups.
TABLE 1.
Intakes of fats, carbohydrates, proteins, and fiber from the diet in 39 adolescent girls with anorexia nervosa (AN) and in 39 healthy adolescents1
| Control group (n = 39) | AN group (n = 39) | P2 | |
|---|---|---|---|
| SFA (g) | 24.7 ± 1.83 | 14.3 ± 1.7 | 0.0001 |
| Monounsaturated fatty acids (g) | 24.7 ± 1.7 | 14.9 ± 1.5 | <0.0001 |
| PUFA (g) | 12.8 ± 0.9 | 9.3 ± 1.0 | 0.01 |
| trans Fatty acids (g) | 4.5 ± 0.5 | 2.6 ± 0.3 | 0.002 |
| Cholesterol (mg) | 216.2 ± 18.7 | 128.5 ± 15.4 | 0.0005 |
| PUFA:SFA (g) | 0.60 ± 0.04 | 0.87 ± 0.08 | 0.003 |
| n – 3 Fatty acids (g) | 1.3 ± 0.1 | 0.9 ± 0.1 | 0.02 |
| n – 6 Fatty acids (g) | 11.4 ± 0.9 | 8.3 ± 0.9 | 0.02 |
| Fructose (g) | 30.4 ± 2.9 | 25.3 ± 2.7 | NS |
| Galactose (g) | 0.45 ± 0.17 | 1.02 ± 0.48 | NS |
| Glucose (g) | 33.7 ± 2.9 | 26.1 ± 2.9 | 0.07 |
| Lactose (g) | 19.2 ± 2.3 | 25.8 ± 2.5 | 0.05 |
| Maltose (g) | 3.3 ± 0.5 | 4.0 ± 0.8 | NS |
| Sucrose (g) | 52.2 ± 4.2 | 48.9 ± 4.8 | NS |
| Starch (g) | 116.4 ± 6.2 | 96.8 ± 7.6 | 0.05 |
| Animal proteins (g) | 44.9 ± 3.2 | 45.9 ± 4.6 | NS |
| Vegetable proteins (g) | 24.7 ± 1.5 | 26.7 ± 1.7 | NS |
| Soluble dietary fiber (g) | 5.3 ± 0.4 | 6.6 ± 0.5 | 0.03 |
| Insoluble dietary fiber (g) | 9.4 ± 0.6 | 14.0 ± 1.1 | 0.0003 |
| Pectin (g) | 1.6 ± 0.2 | 2.5 ± 0.3 | 0.003 |
| Oxalates | 220.7 ± 40.2 | 404.7 ± 53.1 | 0.009 |
| Phytates | 514.8 ± 58.6 | 732.1 ± 76.7 | 0.03 |
Data were derived from 4-d food records. PUFA, polyunsaturated fatty acids; SFA, saturated fatty acids; DRI, Dietary Reference Intake.
Student’s t test.
x̄ ± SEM (all such values).
The AN group derived a significantly greater percentage of their energy intake from proteins and carbohydrates than did the control group, but absolute intakes of these macronutrients did not differ significantly between groups (Figure 1). No significant between-group differences were noted in intakes of vegetable or animal protein or in the proportion of proteins derived from animal and vegetable sources (59.6 ± 2.6% and 39.9 ± 2.6% in the AN group compared with 62.4 ± 2.2% and 37.2 ± 2.3% in the control group, respectively). The girls with AN did not differ from the control subjects in absolute intakes of fructose, galactose, and sucrose. However, intakes of glucose and starch trended lower and of lactose higher in the AN group (Table 1). No significant differences were noted in the proportion of carbohydrates consumed as fructose, galactose, maltose, sucrose, or starch. Compared with the control group, the girls with AN consumed a lower proportion of carbohydrates as glucose (9.6 ± 0.8% compared with 11.8 ± 0.9%; P = 0.06) and a higher proportion as lactose (10.2 ± 0.9% compared with 6.8 ± 0.7%; P = 0.003). No between-group differences in intakes of individual amino acids were observed (data not shown).
Intake of dietary fiber was significantly higher in the girls with AN. Soluble and insoluble fiber intakes were higher by 24.0% and 49.6%, respectively, in the AN group than in the healthy adolescents. The AN group consumed more phytates and oxalates than did the control subjects. We observed a higher intake of aspartame in the AN group than in the control group (91.4 ± 29.8 compared with 22.1 ± 8.1 mg; P = 0.03), although the intake of saccharin did not differ significantly between groups (7.7 ± 5.0 compared with 1.3 ± 0.9).
Vitamin, mineral, and trace element intakes
Dietary intakes of vitamins A, C, and K and the B vitamins (including riboflavin, pantothenic acid, vitamin B-6, and folate) were higher in the AN group than in the control group, whereas the dietary intake of other vitamins did not differ significantly between the groups (Table 2). Supplement use contributed to a higher intake of vitamins A and D and of most of the B vitamins in the AN group than in the control group. Total intakes of vitamins A, D, and K and of most of the B vitamins (except niacin) were thus higher in the AN group than in the control group. A higher proportion of girls with AN than of the control subjects met the dietary reference intake (DRI) for vitamins A and D. The proportion of girls with AN who met the DRI for pantothenic acid and folate was also higher than was the proportion of healthy adolescents who met the DRI.
TABLE 2.
Vitamin intakes from the diet and supplements in 39 adolescent girls with anorexia nervosa (AN) and in 39 healthy adolescents and the proportion of these subjects meeting the Dietary Reference Intake (DRI) for these nutrients1
| Diet alone
|
Supplements alone
|
Total (diet + supplements)
|
||||
|---|---|---|---|---|---|---|
| Control group (n = 39) | AN group (n = 39) | Control group (n = 9) | AN group (n = 18) | Control group (n = 39) | AN group (n = 39) | |
| Vitamin A | ||||||
| (IU) | 6359 ± 8362 | 11521 ± 13473 | 775 ± 269 | 2303 ± 5784 | 7133 ± 917 | 13823 ± 14055 |
| (% meeting DRI) | 67.5 | 84.6 | — | — | 70.0 | 89.74 |
| B vitamins | ||||||
| Thiamine | ||||||
| (mg) | 1.8 ± 0.1 | 2.0 ± 0.2 | 0.3 ± 0.1 | 0.9 ± 0.33 | 2.1 ± 0.2 | 2.9 ± 0.34 |
| (% meeting DRI) | 97.5 | 84.6 | — | — | 97.5 | 92.3 |
| Riboflavin | ||||||
| (mg) | 2.2 ± 0.1 | 2.7 ± 0.24 | 0.3 ± 0.1 | 1.1 ± 0.34 | 2.5 ± 0.2 | 3.7 ± 0.43 |
| (% meeting DRI) | 95.0 | 87.2 | — | — | 95.0 | 92.3 |
| Niacin | ||||||
| (mg) | 21.0 ± 1.2 | 24.7 ± 2.4 | 7.9 ± 5.0 | 9.3 ± 2.3 | 28.9 ± 5.2 | 34.0 ± 3.3 |
| (% meeting DRI) | 85.0 | 76.9 | — | — | 85.0 | 82.1 |
| Pantothenic acid | ||||||
| (mg) | 4.6 ± 0.3 | 6.4 ± 0.84 | 1.5 ± 0.6 | 4.5 ± 1.24 | 6.1 ± 0.7 | 10.9 ± 1.43 |
| (% meeting DRI) | 37.5 | 53.9 | — | — | 47.5 | 71.84 |
| Vitamin B-6 | ||||||
| (mg) | 1.7 ± 0.1 | 2.2 ± 0.24 | 0.3 ± 0.1 | 1.2 ± 0.33 | 2.0 ± 0.2 | 3.4 ± 0.43 |
| (% meeting DRI) | 82.5 | 82.1 | — | — | 82.5 | 87.2 |
| Folate | ||||||
| (μg) | 342 ± 20 | 447 ± 363 | 65 ± 22 | 177 ± 444 | 406 ± 29 | 624 ± 546 |
| (% meeting DRI) | 37.5 | 56.4 | — | — | 45.0 | 74.43 |
| Vitamin B-12 | ||||||
| (μg) | 4.3 ± 0.6 | 4.7 ± 0.6 | 1.0 ± 0.3 | 3.2 ± 0.84 | 5.3 ± 0.7 | 7.9 ± 0.94 |
| (% meeting DRI) | 82.5 | 74.4 | — | — | 85.0 | 87.2 |
| Vitamin C | ||||||
| (mg) | 120 ± 11 | 159 ± 164 | 53 ± 22 | 29 ± 7 | 173 ± 25 | 188 ± 17 |
| (% meeting DRI) | 82.5 | 76.9 | — | — | 82.5 | 82.1 |
| Vitamin D | ||||||
| (μg) | 5.0 ± 0.6 | 6.1 ± 0.7 | 1.7 ± 0.6 | 5.2 ± 1.23 | 6.7 ± 0.9 | 11.3 ± 1.33 |
| (% meeting DRI) | 45.0 | 51.3 | — | — | 50.0 | 76.94 |
| Vitamin E | ||||||
| (μg) | 7.8 ± 0.7 | 10.4 ± 1.6 | 14.6 ± 8.8 | 8.5 ± 2.3 | 22.3 ± 8.8 | 18.9 ± 2.7 |
| (% meeting DRI) | 17.5 | 20.5 | — | — | 30.0 | 48.7 |
| α-Tocopherol (mg) | — | — | — | — | 22.2 ± 8.7 | 19.0 ± 2.7 |
| β-Tocopherol (mg) | — | — | — | — | 0.25 ± 0.02 | 0.33 ± 0.04 |
| γ-Tocopherol (mg) | — | — | — | — | 14.3 ± 1.3 | 9.2 ± 1.03 |
| δ-Tocopherol (mg) | — | — | — | — | 2.6 ± 0.3 | 1.6 ± 0.23 |
| Vitamin K | ||||||
| (μg) | 66.9 ± 7.1 | 165.9 ± 47.64 | 0.0 ± 0.0 | 1.6 ± 1.6 | 66.9 ± 7.1 | 167.5 ± 47.54 |
| (% meeting DRI) | 34.8 | 48.0 | — | — | 34.8 | 52.0 |
Data were derived from 4-d food records.
x̄ ± SEM (all such values).
Significantly different from the control group (Student’s t test): 3P ≤ 0.01, 4P ≤ 0.05, 5P ≤ 0.0001, 6P ≤ 0.001.
Dietary intakes of calcium, phosphorus, iron, zinc, copper, selenium, and sodium did not differ significantly between the girls with AN and the control subjects (Table 3). Calcium, iron, and zinc intakes from supplements were higher in the AN group than in the control group, as were total calcium, magnesium, iron, zinc, and potassium intake from the diet and supplements. The proportion of girls with AN meeting the DRI for total calcium intake, but not for dietary calcium, was significantly higher than that of the healthy adolescents (P = 0.01).
TABLE 3.
Minerals and trace elements from the diet and supplements in 39 adolescent girls with anorexia nervosa (AN) and in 39 healthy adolescents and the proportion of these subjects meeting the Dietary Reference Intake (DRI) for these nutrients1
| Diet alone
|
Supplements alone
|
Total (diet + supplements)
|
||||
|---|---|---|---|---|---|---|
| Control group (n = 39) | AN group (n = 39) | Control group (n = 9) | AN group (n = 18) | Control group (n = 39) | AN group (n = 39) | |
| Calcium | ||||||
| (mg) | 981 ± 882 | 1169 ± 88 | 79 ± 33 | 277 ± 743 | 1060 ± 92 | 1446 ± 994 |
| (% meeting DRI) | 25.0 | 38.5 | 30.0 | 59.04 | ||
| Phosphorus | ||||||
| (mg) | 1216 ± 77 | 1333 ± 82 | 9 ± 4 | 25 ± 10 | 1225 ± 78 | 1358 ± 83 |
| (% meeting DRI) | 42.5 | 48.7 | 42.5 | 51.3 | ||
| Magnesium | ||||||
| (mg) | 254 ± 14 | 313 ± 203 | 6 ± 3 | 25 ± 11 | 260 ± 15 | 337 ± 214 |
| (% meeting DRI) | 30.0 | 43.6 | 35.0 | 51.3 | ||
| Iron | ||||||
| (mg) | 15.0 ± 0.9 | 17.4 ± 1.4 | 2.2 ± 1.0 | 6.8 ± 2.14 | 17.2 ± 1.5 | 24.2 ± 2.44 |
| (% meeting DRI) | 52.5 | 59.0 | 57.5 | 74.4 | ||
| Zinc | ||||||
| (mg) | 9.3 ± 0.5 | 10.9 ± 1.1 | 1.6 ± 0.7 | 5.0 ± 1.6 | 10.9 ± 0.9 | 15.9 ± 1.93 |
| (% meeting DRI) | 42.5 | 53.9 | 55.0 | 69.2 | ||
| Copper | ||||||
| (mg) | 1.1 ± 0.1 | 1.2 ± 0.1 | 0.2 ± 0.1 | 0.5 ± 0.2 | 1.3 ± 0.1 | 1.7 ± 0.2 |
| (% meeting DRI) | 72.5 | 76.9 | 72.5 | 76.9 | ||
| Selenium | ||||||
| (μg) | 101.9 ± 6.0 | 92.7 ± 6.9 | 1.2 ± 0.7 | 6.4 ± 5.2 | 103.1 ± 6.0 | 99.1 ± 9.0 |
| (% meeting DRI) | 100.0 | 79.54 | 100.0 | 84.64 | ||
| Sodium (mg) | 2997 ± 137 | 2879 ± 220 | 0.1 ± 0.1 | 0.7 ± 0.5 | 2997 ± 137 | 2880 ± 220 |
| Potassium (mg) | 2421 ± 142 | 2866 ± 181 | 3.4 ± 2.2 | 7.0 ± 3.5 | 2424 ± 142 | 2873 ± 1813 |
Data were derived from 4-d food records.
x̄ ± SEM (all such values).
Significantly different from the control group (Student’s t test): 3P ≥ 0.05, 4P ≥ 0.01.
Eighteen girls with AN took some form of supplement compared with 9 healthy adolescents. Twelve girls with AN took multivitamins with calcium, 4 took multivitamins alone, and 2 took calcium with or without vitamin D. Five healthy adolescents took multivitamins with calcium, and 3 each took either multi-vitamins alone or calcium with or without vitamin D.
Indirect calorimetry and activity questionnaire
Adolescents with AN had a significantly lower REE than did healthy adolescents (1104 ± 48 compared with 1488 ± 49 kcal; P < 0.0001), and the difference between the groups persisted despite correction for lean mass and fat mass (data not shown). Predicted REE from the Harris-Benedict equation (REE-HB) and from the WHO criteria (REE-WHO) were both significantly lower in the AN group than in the control group [1314 ± 9 compared with 1430 ± 16 (P < 0.0001) and 1293 ± 10 compared with 1443 ± 20 kcal (P < 0.0001)]. REE-HB correlated strongly with REE-WHO (r = 0.99, P < 0.0001). Significant correlations were also noted between REE from the indirect calorimetry (REE-IC) and REE-HB (r = 0.46, P < 0.0001) and between REE-IC and REE-WHO (r = 0.45, P < 0.0001). When the Harris-Benedict equation (unmodified) was used for the control subjects and the modified Harris-Benedict equation was used for the AN group, the correlation between predicted REE-HB and REE-IC improved (r = 0.57, P < 0.0001). In the AN group, REE-IC differed significantly from REE-HB (mean difference: −209 ± 47 kcal), the modified Harris-Benedict equation (difference of 122 ± 47 kcal), and REE-WHO (−189 ± 47 kcal). However, the difference was least with the modified Harris-Benedict equation. In the control subjects, REE-IC did not differ from REE-HB or REE-WHO.
For the group as a whole, REE-IC correlated directly with BMI (r = 0.47, P < 0.0001) and fat mass(r = 0.44, P = 0.0001) and weakly with lean body mass (r = 0.22, P = 0.06). On stepwise regression including BMI, fat mass, and lean mass, BMI was the only significant predictor of REE-IC, which contributed to 21.8% of the variability. A positive correlation was noted between REE-IC and calorie intake in all subjects (r = 0.47, P < 0.0001), the AN group (r = 0.44, P = 0.005), and the control subjects (r = 0.39, P = 0.02).
Calorie intake from food was appropriately higher than that measured by REE-IC in both the girls with AN (1649 ± 110 compared with 1104 ± 48 kcal; P < 0.0001) and the control group (1982 ± 93 compared with 1488 ± 49 kcal; P < 0.0001). The difference between caloric intake from food and REE-IC was not significantly different between the groups (AN group: 545 ± 99 kcal; control group: 476 ± 87 kcal).
EER did not differ significantly between the AN group and the control group (2273 ± 41 compared with 2315 ± 51 kcal) and did not correlate with REE-IC, REE-HB, or REE-WHO. EER also did not correlate with caloric intake for the groups taken together or individually.
Respiratory quotient (RQ) did not differ significantly between the groups (0.96 ± 0.02 in both). However, an inverse correlation was noted between RQ and REE in the group as a whole (r = −0.23, P = 0.04) and also in the control subjects considered separately (r = −0.35, P = 0.03) but not in the AN group taken alone. We found no significant difference between the AN group (15.7 ± 1.6) and the control group (12.9 ± 1.5) in the total activity score as determined from the exercise questionnaire. However, leisure activity was higher for girls with AN than for control subjects [13.6 ± 1.5 compared with 9.5 ± 1.2 metabolic equivalent (MET)-h/wk; P = 0.04]. In the group as a whole, leisure activity correlated inversely with fat mass (r = −0.27, P = 0.02) and percentage body fat (r = −0.27, P = 0.02) and weakly with BMI (r = −0.21, P = 0.06). These correlations were lost in the 2 groups when considered separately. In the AN group, a weak positive correlation was noted between total activity and REE (r = 0.029, P = 0.07) and between leisure activity and REE (r = 0.28, P = 0.08). No relation was noted between total activity and caloric intake in either group.
Relation of food intake with body composition and bone density
A positive correlation was observed between fat mass and intake of total dietary fat (r = 0.27, P = 0.02), saturated fat (r = 0.26, P = 0.02), and monounsaturated fat (r = 0.29, P = 0.01). Fat mass correlated positively with the percentage of energy derived from fats (r = 0.28, P = 0.01) and inversely with the percentage of energy derived from proteins (r = −0.26, P = 0.02) and carbohydrates (r = −0.24, P = 0.04). No relation was observed between polyunsaturated fat intake and body composition. Fat mass also correlated positively with glucose (r = 0.32, P = 0.005) and fructose (r = 0.25, P = 0.03) intakes and inversely with dietary fiber intake.
Protein intake has been shown to be correlated with bone strength (25). A positive correlation was observed between protein intake and lumbar BMD z scores in the girls with AN (r = 0.46, P = 0.003). No relation was observed in the healthy adolescents. Calcium and vitamin D intakes did not predict bone density measures in the AN or control group. No significant differences were seen in lumbar spine BMD or BMD z scores when the girls with AN who met the DRI for calcium or vitamin D were compared with those who did not meet the DRI for these nutrients.
Relation between food intake and hormone concentrations
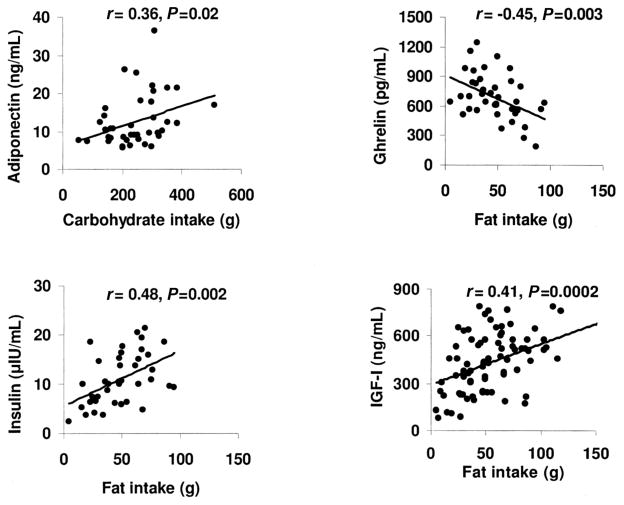
The girls with AN did not differ significantly from the control subjects in concentrations of adiponectin (12.6 ± 5.8 and 12.5 ± 7.7 ng/mL, respectively). Adiponectin concentrations were predicted by total calorie intake (r = 0.30, P = 0.05) and the total carbohydrate content of the diet (r = 0.36, P = 0.02) but not by total fat or protein intake. A positive correlation was noted between concentrations of adiponectin and intake of fructose (r = 0.34, P = 0.03), glucose (r = 0.36, P = 0.02), and lactose and sucrose (r = 0.30, P = 0.05 for both). Multiple regression analysis with BMI and components of food intake entered into the model showed that the most significant predictors of adiponectin were intakes of glucose and lactose, which contributed to 12.9% and 8.6% of the variability of adiponectin, respectively (total: 21.5% of the variability).
Fasting ghrelin concentrations were higher in the girls with AN than in the control subjects (786 ± 206 compared with 588 ± 209 pg/mL; P = 0.004) and were predicted by total fat intake (r = −0.45, P = 0.003), percentage of energy from fat (r = −0.37, P = 0.02), percentage of energy from protein (r = 0.47, P = 0.002), and intakes of saturated fats (r = −0.40, P = 0.009), monounsaturated fats (r = −0.40, P = 0.009), and poly-unsaturated fats (r = −0.42, P = 0.006). On multiple regression analysis with BMI and components of food intake entered into the model, the most significant predictor of fasting ghrelin was total fat intake, which contributed to 20% of the variability.
Concentrations of fasting insulin were lower (P < 0.0001) in the girls with AN (6.7 ± 2.6 μIU/mL) than in the control subjects (14.5 ± 4.1 μIU/mL) and were predicted by total fat intake (r = 0.48, P = 0.002) but not by protein or carbohydrate intake. A stronger correlation was observed with the percentage of energy from fat (r = 0.52, P = 0.0008). Individual components of fat intake also predicted insulin concentrations; the strongest correlations were observed between insulin and trans fatty acids in diet (r = 0.48, P = 0.003), dietary monounsaturated fats (r = 0.47, P = 0.003), and saturated fats (r = 0.45, P = 0.005); a relatively less strong correlation was observed with polyunsaturated fat (r = 0.37, P = 0.02). Of the carbohydrate components in diet, only starch intake predicted insulin concentrations (r = 0.46, P = 0.004). Weak correlations were observed between insulin concentrations and dietary total (r = −0.32, P = 0.05) and insoluble (−0.34, P = 0.03) fiber. Regression modeling including BMI, fat mass, and dietary components showed that the most significant predictors of insulin concentrations were percentage of energy from fat in the diet, percentage of body fat, and starch intake, which contributed to 32%, 12.5%, and 13.7% of the variability, respectively (total: 58% of the variability).
IGF-I concentrations were lower in the AN group than in the control subjects (311 ± 142 compared with 551 ± 142 ng/mL; P < 0.0001) and correlated with total energy intake (r = 0.33, P = 0.003) and total fat intake (r = 0.41, P = 0.0002) but not with carbohydrate or protein intake. Multiple regression analysis with BMI and various food components entered into the model showed that the most significant predictors of IGF-I concentrations were BMI, percentage of energy from fat, and intakes of starch and insoluble fiber, which contributed to 30.1%, 8.7%, 3.9%, and 2.9% of the variability, respectively (total variability: 46.6%).
Leptin concentrations were lower in the girls with AN than in the control subjects (3.5 ± 2.4 compared with 14.3 ± 6.3 ng/mL; P < 0.0001) and were predicted by total calorie intake (r = 0.25, P = 0.03), total fat intake (r = 0.30, P = 0.007), and individual components of fat intake, such as saturated fat intake (r = 0.29, P = 0.01) and monounsaturated fat intake (r = 0.33, P = 0.003) but not polyunsaturated fat, total carbohydrate, or protein intakes. Of the components of carbohydrate intake, leptin concentrations were predicted by intakes of fructose (r = 0.25, P = 0.02) and glucose (r = 0.33, P = 0.003). Leptin correlated inversely with intake of total fiber (r = −0.33, P = 0.003) and with intake of both soluble fiber (r = −0.24, P = 0.03) and insoluble fiber (r = −0.36, P = 0.001). On multiple regression analysis with BMI and intake of different food components entered into the model, the most significant predictors of leptin were BMI and percentage of energy from saturated fat, which contributed to 65.7% and 1.1% of the variability, respectively (total: 66.8% of the variability). When fat mass was entered into the model instead of BMI, fat mass contributed to 70.5% of the variability in leptin concentrations.
Thus, fat intake predicted concentrations of all nutrition-related hormones (ghrelin, leptin, insulin, and IGF-I), except adiponectin, which was predicted by carbohydrate intake (Figure 2).
FIGURE 2.
Relation between food intake and concentrations of hormones affected by nutrition in adolescent girls with anorexia nervosa (AN) and healthy adolescents. Correlational analysis was used to determine the associations between food intake and nutritionally modulated hormones. Associations of adiponectin with carbohydrate intake and of ghrelin and insulin with fat intake are reported for 20 girls with AN and 21 control subjects; associations of insulin-like growth factor I (IGF-I) with fat intake are reported for 39 girls with AN and 39 control subjects. Adiponectin concentrations correlated positively with carbohydrate intake. A positive association was observed between fat intake and concentrations of insulin and IGF-I, and an inverse association was observed between fat intake and ghrelin concentrations.
DISCUSSION
We observed lower fat and higher fiber intakes in community-dwelling girls with AN than in healthy adolescents, despite comparable protein and carbohydrate intakes. All fats were consumed in lesser quantities by the girls with AN, with the greatest reductions in the proportion of saturated fats consumed. A greater proportion of the girls with AN than of the control subjects met the DRI for vitamins A and D and calcium because of higher supplement consumption. Fat intake predicted serum leptin, ghrelin, IGF-I, and insulin, and carbohydrate intake predicted adiponectin concentrations.
Although dietary patterns in adults with AN have been reported (10, 26–28), few studies have examined dietary patterns in adolescents with AN, especially community-dwelling girls with AN. In adults with AN, lower fat intakes have been reported (10, 26–28), with a higher proportion of energy being derived from proteins (26) or carbohydrates (10, 28) or without any difference in the proportion of energy derived from either (27). One study reported a lower proportion of energy from fat intake in 8 adolescents with AN, with no difference in energy derived from proteins or carbohydrates (29). In our study, we report lower intakes of fats and a lower percentage of energy from fats in the girls with AN than in the control subjects. In contrast with the other study in adolescents, we observed that the AN group derived a significantly higher proportion of their caloric intake from carbohydrates and proteins. Our data suggest that outpatient interventional strategies are not sufficient to normalize calorie intakes in community-dwelling adolescents with AN; intakes of dietary fat remain particularly low. Few data describing dietary intakes of specific forms of fats, proteins, and carbohydrates in AN are available. We report a lower intake of all types of fat in AN. Protein intake for weight was higher in the AN group, but intakes of specific types of proteins did not differ between groups. Although sucrose intakes did not differ between groups, a lower proportion of carbohydrates was obtained from glucose and a higher proportion from lactose in the AN group. Fiber intake in the AN group was high.
Decreased dietary intakes of calcium and vitamin D have been proposed to contribute to low bone density in AN. Beaumont et al (26) reported that most adults with AN do not meet the DRI for calcium intake. In a previous study, we reported that calcium and vitamin D intakes did not differ significantly between 17 subjects with AN and 18 control subjects (7). In this larger study, the total intakes of calcium, magnesium, and vitamin D was higher in the AN group, and a higher proportion of the girls with AN than of the healthy adolescents fulfilled the DRI for vitamin D, calcium, and magnesium. Because supplements contributed greatly to the higher intakes of these nutrients in the AN group, our data suggest an increasing awareness of the risk of low bone density in AN on the part of caregivers and an effort to decrease this risk with the use of supplements. Studies in healthy adolescents have shown the importance of optimal calcium and vitamin D intakes for bone mineral accrual (30, 31). The higher proportion of carbohydrate intake from lactose in the AN group also suggests an effort to optimize calcium intake through consumption of dairy products. Of note, calcium intake may be lower in certain subgroups of patients with AN, such as vegans. This was not examined in our study. Despite the higher intakes of minerals and vitamin D in the AN group, lumbar spine BMD z scores remained low and did not differ between the AN groups who met or did not meet the DRI for calcium and vitamin D. We previously showed that calcium and vitamin D supplementation alone is insufficient to improve bone density in adults with AN (32). Although optimum calcium and vitamin D intakes from diet and through supplements are to be encouraged, alone they are not sufficient to preserve bone density. Low bone density in AN is likely a consequence of decreased muscle mass and of known endocrine alterations including hypogonadism, hypercortisolemia, and low IGF-I concentrations (8, 13) and not of a decreased intake of calcium or vitamin D. Of concern, however, is the fact that only 35% and 26% of our healthy girls met the DRI for calcium and vitamin D, respectively.
Many studies have reported a lower REE in AN than in controls (7, 33–38), although few studies have been performed in adolescents (7, 38). Previous reports suggested that REE correlates with fat-free mass (39, 40), although more recent studies have reported changes in REE with weight loss independent of fat-free mass (37). We observed markedly decreased measured REE in the AN group, which persisted despite correction for lean body mass or fat mass, which indicated increased efficiency of energy expenditure at rest, independent of tissue loss. The only significant body-composition predictor of REE was BMI on regression modeling; when REE was corrected for BMI, the difference between the groups was no longer significant. The WHO and Harris-Benedict equations overpredicted REE in the AN group, which is similar to the findings of other reports (18, 41) in subjects with AN but not in control subjects. The modified Harris-Benedict equation, suggested for use in AN (18), under-predicted REE in AN. Our data showed that the WHO or Harris-Benedict equations are accurate predictors of measured REE in healthy adolescents but do not accurately estimate REE in AN. Although REE was lower in the AN group, EER did not differ between the groups, likely because of higher activity levels in the AN group. The use of EER rather than REE may be necessary when calculating the caloric needs of those with AN to prevent underestimation of calorie requirements. RQ did not differ significantly between the groups in our study, although some studies (36, 41) have shown higher RQs in those with AN after refeeding, presumably as a consequence of changes in body metabolism and substrate utilization (representing lipogenesis, an energetically expensive process). We did observe that lower measured REE predicted higher RQs. As expected, increased reported leisure time physical activity was associated with decreases in fat mass.
Our data showed that adiponectin is predicted by carbohydrate intake, whereas fasting ghrelin, insulin, and IGF-I concentrations are predicted by fat intake. Only modest effects of nutrient intake on adiponectin concentrations have been shown (42), and scant data exist regarding the relation between nutrient intake and ghrelin. We observed positive associations between adiponectin and mono- and disaccharide intakes, and carbohydrate intake was an independent predictor of adiponectin on regression modeling. Interestingly, adiponectin did not differ significantly between the AN and control groups, and one may speculate that this was because carbohydrate intakes did not differ significantly between the groups. We observed inverse associations between ghrelin and fat intake. Fat intake also predicted insulin and IGF-I independent of BMI or fat mass. Other investigators have reported inverse associations between leptin and calorie or fat intake (42, 43). The most significant predictor of leptin in our study was fat mass, consistent with other reports, and the associations between leptin concentrations and specific nutrient intakes did not hold up after fat mass was controlled for. More studies are necessary to better understand the relations between food intake and hormones related to nutritional status.
The limitations of this study were those inherent to the use of food records. Individuals may consciously or unconsciously overestimate or underestimate their nutrient intakes. Half of the subjects in this study also completed a 24-h food recall, which correlated strongly with data from the 4-d food records, particularly in girls with AN (data not shown). This suggests that both methods are reasonable estimations of intake yielding similar results, but are still self-reported. Food records also document intake over a few days, which may not truly represent an individual’s long-term intake. However, long-term measurement of observed food intake in a free-living community setting is difficult to perform. Food-frequency questionnaires would be an alternative, but these are also self-reported. In addition, our subjects were community-dwelling girls being followed by caregivers on an outpatient basis. Variations in intake patterns may relate to variations in intensity of intervention by caregivers. However, it is of concern that, despite follow-up, intake of higher-calorie food components such as fat intake remained low in the AN group, whereas intake of dietary fiber remained high. There is the risk of overreporting calorie intakes in AN. However, this would result in a further dilution of the results, and significant differences in fat and fiber intakes between the AN and control groups, despite self-report, suggest that true differences may be even greater. Follow-up of food records over time would be useful to determine whether intake patterns change with continued intervention.
We showed specific dietary patterns in AN that differ from those of healthy adolescents, including a markedly low fat intake, especially intake of saturated fat, and an increased intake of dietary fiber. Community-dwelling girls with AN continue to show decreased calorie intakes compared with healthy adolescents, despite outpatient follow-up by caregivers, and particularly show a reduction in the intake of high-calorie food components.
Supported in part by NIH grants M01-RR-01066, 1RO1 DK 062249, and K23 RR018851; NIH Nutrition Training grant DK07703; and an Eli Lilly fellowship grant.
MM helped design and conduct the study, analyze the data, and write the manuscript. PT helped conduct the study, write the manuscript, and review the data. EJA and JLH performed and reviewed the nutrient analysis and conducted the indirect calorimetry. KG helped conduct the study and analyze the data. LAS, KKM, DBH, and AK helped design and conduct the study. All authors reviewed the manuscript. The authors had no conflicts of interest to report.
References
- 1.Whitaker A. An epidemiological study of anorectic and bulimic symptoms in adolescent girls: implications of pediatricians. Pediatr Ann. 1992;21:752–9. doi: 10.3928/0090-4481-19921101-10. [DOI] [PubMed] [Google Scholar]
- 2.Rastam M, Gillberg C, Garton M. Anorexia nervosa in a Swedish urban region. A population-based study. Br J Psychiatr. 1989;155:642–6. doi: 10.1192/s0007125000018134. [DOI] [PubMed] [Google Scholar]
- 3.Neumark-Sztainer D, Hannan P. Weight-related behaviors among adolescent girls and boys: results from a national survey. Arch Pediatr Adolesc Med. 2000;154:569–77. doi: 10.1001/archpedi.154.6.569. [DOI] [PubMed] [Google Scholar]
- 4.Bachrach L, Guido D, Katzman D, Litt I, Marcus R. Decreased bone density in adolescent girls with anorexia nervosa. Pediatrics. 1990;86:440–7. [PubMed] [Google Scholar]
- 5.Kooh S, Noriega E, Leslie K, Muller C, Harrison J. Bone mass and soft tissue composition in adolescents with anorexia nervosa. Bone. 1996;19:181–8. doi: 10.1016/8756-3282(96)00162-7. [DOI] [PubMed] [Google Scholar]
- 6.Castro J, Lazaro L, Pons F, Halperin I, Toro J. Predictors of bone mineral density reduction in adolescents with anorexia nervosa. J Am Acad Child Adolesc Psychiatr. 2000;39:1365–70. doi: 10.1097/00004583-200011000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Soyka L, Grinspoon S, Levitsky L, Herzog D, Klibanski A. The effects of anorexia nervosa on bone metabolism in female adolescents. J Clin Endocrinol Metab. 1999;84:4489–96. doi: 10.1210/jcem.84.12.6207. [DOI] [PubMed] [Google Scholar]
- 8.Soyka L, Misra M, Frenchman A, et al. Abnormal bone mineral accrual in adolescent girls with anorexia nervosa. J Clin Endocrinol Metab. 2002;87:4177–85. doi: 10.1210/jc.2001-011889. [DOI] [PubMed] [Google Scholar]
- 9.Palla B, Litt I. Medical complications of eating disorders in adolescents. Pediatrics. 1988;81:613–22. [PubMed] [Google Scholar]
- 10.Hadigan C, Anderson E, Miller K, et al. Assessment of macronutrient and micronutrient intake in women with anorexia nervosa. Int J Eating Disord. 2000;28:284–92. doi: 10.1002/1098-108x(200011)28:3<284::aid-eat5>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 11.Misra M, Miller KK, Almazan C, et al. Hormonal and body composition predictors of soluble leptin receptor, leptin, and free leptin index in adolescent girls with anorexia nervosa and controls and relation to insulin sensitivity. J Clin Endocrinol Metab. 2004;89:3486–95. doi: 10.1210/jc.2003-032251. [DOI] [PubMed] [Google Scholar]
- 12.Misra M, Miller KK, Herzog DB, et al. Growth hormone and ghrelin responses to an oral glucose load in adolescent girls with anorexia nervosa and controls. J Clin Endocrinol Metab. 2004;89:1605–12. doi: 10.1210/jc.2003-031861. [DOI] [PubMed] [Google Scholar]
- 13.Misra M, Miller KK, Bjornson J, et al. Alterations in growth hormone secretory dynamics in adolescent girls with anorexia nervosa and effects on bone metabolism. J Clin Endocrinol Metab. 2003;88:5615–23. doi: 10.1210/jc.2003-030532. [DOI] [PubMed] [Google Scholar]
- 14.Greulich W, Pyle S. Radiographic atlas of skeletal development of the hand and wrist. 2. Stanford, CA: Stanford University Press; 1959. [Google Scholar]
- 15.Segal K. Comparison of indirect calorimetric measurements of resting energy expenditure with a ventilated hood, face mask, and mouthpiece. Am J Clin Nutr. 1987;45:1420–3. doi: 10.1093/ajcn/45.6.1420. [DOI] [PubMed] [Google Scholar]
- 16.Ritz R, Cunningham J. Indirect calorimetry. In: Kacmarek RM, Hess D, Stroller JK, editors. Monitoring in respiratory care. St Louis, MO: Mosby-Yearbook; 1993. pp. 407–41. [Google Scholar]
- 17.Harris J, Benedict F. A biometric study of basal metabolism in man. Washington, DC: Carnegie Institute of Washington; 1919. (Publication no. 279.) [Google Scholar]
- 18.Schebendach J, Golden N, Jacobson M, et al. Indirect calorimetry in the nutritional management of eating disorders. Int J Eat Disord. 1995;17:59–66. doi: 10.1002/1098-108x(199501)17:1<59::aid-eat2260170108>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 19.Energy and protein requirements. World Health Organ Tech Rep Ser. 1985. Report of a Joint FAO/WHO/UNU Expert Consultation; p. 724. [PubMed] [Google Scholar]
- 20.Barr S. Introduction to dietary reference intakes. Appl Physiol Nutr Metab. 2006;31:61–5. doi: 10.1139/h05-019. [DOI] [PubMed] [Google Scholar]
- 21.Zello G. Dietary Reference Intakes for the macronutrients and energy: considerations for physical activity. Appl Physiol Nutr Metab. 2006;31:74–9. doi: 10.1139/h05-022. [DOI] [PubMed] [Google Scholar]
- 22.Kriska A, Caspersen CE. A collection of physical activity questionnaires for health-related research. J Am Coll Sports Med. 1997;29(suppl):73S–82S. [PubMed] [Google Scholar]
- 23.Kelly T, Berger N, Richardson T. DXA body composition: theory and practice. Appl Radiat Isot. 1998;49:511–3. doi: 10.1016/s0969-8043(97)00226-1. [DOI] [PubMed] [Google Scholar]
- 24.Visser M, Kiel D, Langlois J, et al. Muscle mass and fat mass in relation to bone mineral density in very old men and women: the Framingham Heart Study. Appl Radiat Isot. 1998;49:745–47. doi: 10.1016/s0969-8043(97)00101-2. [DOI] [PubMed] [Google Scholar]
- 25.Alexy U, Remer T, Manz F, Neu CM, Schoenau E. Long-term protein intake and dietary potential renal acid load are associated with bone modeling and remodeling at the proximal radius in healthy children. Am J Clin Nutr. 2005;82:1107–14. doi: 10.1093/ajcn/82.5.1107. [DOI] [PubMed] [Google Scholar]
- 26.Beaumont P, Chambers T, Rouse L, Abraham S. The diet composition and nutritional knowledge of patients with anorexia nervosa. J Hum Nutr. 1981;35:265–73. doi: 10.3109/09637488109143052. [DOI] [PubMed] [Google Scholar]
- 27.Gwirtsman H, Kaye W, Curtis S, Lyter L. Energy intake and dietary macronutrient content in women with anorexia nervosa and volunteers. J Am Diet Assoc. 1989;89:54–7. [PubMed] [Google Scholar]
- 28.Fernstrom M, Weltzin T, Neuberger S, Srinivasagam N, Kaye W. Twenty-four-hour food intake in patients with anorexia nervosa and in healthy control subjects. Biol Psychiatr. 1994;36:696–702. doi: 10.1016/0006-3223(94)91179-7. [DOI] [PubMed] [Google Scholar]
- 29.Affenito S, Dohm F, Crawford P, Daniels S, Striegel-Moore R. Macro-nutrient intake in anorexia nervosa: the National Heart, Lung, and Blood Institute Growth and Health Study. J Pediatr. 2002;141:701–5. doi: 10.1067/mpd.2002.129840. [DOI] [PubMed] [Google Scholar]
- 30.Johnston CJ, Miller J, Slemenda C, et al. Calcium supplementation and increases in bone mineral density in children. N Engl J Med. 1992;327:82–7. doi: 10.1056/NEJM199207093270204. [DOI] [PubMed] [Google Scholar]
- 31.Teegarden D, Lyle R, McCabe G, et al. Dietary calcium, protein, and phosphorus are related to bone mineral density and content in young women. Am J Clin Nutr. 1998;68:749–54. doi: 10.1093/ajcn/68.3.749. [DOI] [PubMed] [Google Scholar]
- 32.Klibanski A, Biller B, Schoenfeld D, Herzog D, Saxe V. The effects of estrogen administration on trabecular bone loss in young women with anorexia nervosa. J Clin Endocrinol Metab. 1995;80:898–904. doi: 10.1210/jcem.80.3.7883849. [DOI] [PubMed] [Google Scholar]
- 33.Casper R, Schoeller D, Kushner R, Hnilicka J, Gold S. Total daily energy expenditure and activity level in anorexia nervosa. Am J Clin Nutr. 1991;53:1143–50. doi: 10.1093/ajcn/53.5.1143. [DOI] [PubMed] [Google Scholar]
- 34.Vaisman N, Rossi M, Corey M, Clarke R, Goldberg E, Pencharz P. Effect of refeeding on the energy metabolism of adolescent girls who have anorexia nervosa. Eur J Clin Nutr. 1991;45:527–37. [PubMed] [Google Scholar]
- 35.Scalfi L, Di Biase G, Coltorti A, Contaldo F. Bioimpedance analysis and resting energy expenditure in undernourished and refed anorectic patients. Eur J Clin Nutr. 1993;47:61–7. [PubMed] [Google Scholar]
- 36.Russell J, Baur L, Beumont P, et al. Altered energy metabolism in anorexia nervosa. Psychoneuroendocrinology. 2001;26:51–63. doi: 10.1016/s0306-4530(00)00036-6. [DOI] [PubMed] [Google Scholar]
- 37.Polito A, Fabbri A, Ferro-Luzzi A, et al. Basal metabolic rate in anorexia nervosa: relation to body composition and leptin concentrations. Am J Clin Nutr. 2000;71:1495–502. doi: 10.1093/ajcn/71.6.1495. [DOI] [PubMed] [Google Scholar]
- 38.Satoh Y, Shimizu T, Lee T, Nishizawa K, Iijima M, Yamashiro Y. Resting energy expenditure and plasma leptin levels in adolescent girls with anorexia nervosa. Int J Eat Disord. 2003;34:156–61. doi: 10.1002/eat.10158. [DOI] [PubMed] [Google Scholar]
- 39.de Zwaan M, Aslam Z, Mitchell J. Research on energy expenditure in individuals with eating disorders: a review. Int J Eat Disord. 2002;32:127–34. doi: 10.1002/eat.10074. [DOI] [PubMed] [Google Scholar]
- 40.Melchior J, Rigaud D, Rozen R, Malon D, Apfelbaum M. Energy expenditure economy induced by decrease in lean body mass in anorexia nervosa. Eur J Clin Nutr. 1989;43:793–9. [PubMed] [Google Scholar]
- 41.Krahn D, Rock C, Dechert R, Nairn K, Hasse S. Changes in resting energy expenditure and body composition in anorexia nervosa patients during refeeding. J Am Diet Assoc. 1993;93:434–8. doi: 10.1016/0002-8223(93)92291-5. [DOI] [PubMed] [Google Scholar]
- 42.Havel PJ. Update on adipocyte hormones: regulation of energy balance and carbohydrate/lipid metabolism. Diabetes. 2004;53(suppl):S143–51. doi: 10.2337/diabetes.53.2007.s143. [DOI] [PubMed] [Google Scholar]
- 43.Yannakoulia M, Yiannakouris N, Bluher S, Matalas A-L, Klimis-Zacas D, Mantzoros CS. Body fat mass and macronutrient intake in relation to circulating soluble leptin receptor, free leptin index, adiponectin, and resistin concentrations in healthy humans. J Clin Endocrinol Metab. 2003;88:1730–6. doi: 10.1210/jc.2002-021604. [DOI] [PubMed] [Google Scholar]