Abstract
Anaerobic reductive dehalogenation of commercial PCBs such as Aroclor 1260 has a critical role of transforming highly chlorinated congeners to less chlorinated congeners that are then susceptible to aerobic degradation. The efficacy of bioaugmentation with the dehalorespiring bacterium “Dehalobium chlorocoercia” DF1 was tested in 2-liter laboratory mesocosms containing sediment contaminated with weathered Aroclor 1260 (1.3 ppm) from Baltimore Harbor, MD. Total penta- and higher chlorinated PCBs decreased by approximately 56% (by mass) in bioaugmented mesocosms after 120 days compared with no activity observed in unamended controls. Bioaugmentation with DF-1 enhanced the dechlorination of doubly flanked chlorines and stimulated the dechlorination of single flanked chlorines as a result of an apparent synergistic effect on the indigenous population. Addition of granulated activated carbon had a slight stimulatory effect indicating that anaerobic reductive dechlorination of PCBs at low concentrations was not inhibited by a high background of inorganic carbon that could affect bioavailability. The total number of dehalorespiring bacteria was reduced by approximately half after 60 days. However, a steady state level was maintained that was greater than the indigenous population of putative dehalorespiring bacteria in untreated sediments and DF1 was maintained within the indigenous population after 120 days. The results of this study demonstrate that bioaugmentation with dehalorespiring bacteria has a stimulatory effect on the dechlorination of weathered PCBs and supports the feasibility of using in situ bioaugmentation as an environmentally less invasive and lower cost alternate to dredging for treatment of PCB impacted sediments.
INTRODUCTION
Polychlorinated biphenyls (PCBs) are persistent organic pollutants that are globally dispersed as a result of cycling between air, water, and soil 1–3. PCBs are hydrophobic and bioaccumulate throughout the food chain by absorption in the fatty tissue of animals and humans where they have been reported to act as endocrine disrupters 4 and possible carcinogens 5. The most widely applied technologies for treating PCB-impacted sites are removal by dredging and subsequent transfer to hazardous waste landfills or in situ containment in subaqueous sites by capping 6. In addition to being cost prohibitive for treating large areas of contamination in rivers, lakes and coastal sediments, these technologies are disruptive to environmentally sensitive areas such as marshes and wetlands. Development of an in situ treatment system that utilizes microbial activity, bioaugmentation, could potentially reduce high costs and negative environmental impacts associated with dredging and capping.
There have been reports on the potential of aerobic bioaugmentation with bacteria, fungi and plants, but these processes have limited capacity to attack highly chlorinated congeners often found in PCB impacted sites 7. Microbial reductive dechlorination of higher chlorinated PCB congeners has the potential to complement these processes, but there have been very few studies to date describing the use of anaerobic bioaugmentation to stimulate in situ treatment of PCB impacted sediments. Wu and Weigel 8 and Bedard et al. 9 observed only slight or no stimulation of weathered Aroclor 1260 in Housatanic River sediments bioaugmented with PCB enriched soil and sediment slurries. Natarajan at al 10 reported dechlorination of weathered Aroclor 1242 and 1248 in microcosms inoculated with microbial granules from a UASB digestor. However, the possibility could not be ruled out that the observed activity resulted from stimulation of indigenous PCB dechlorinating bacteria by hydrogen generated from fermentation of wood powder added an electron donor rather than the result of bioaugmentation with dehalorespiring bacteria. More recently Dehalococcoides spp. and related species within the Chloroflexi have been identified that are capable of reductively dechlorinating PCBs by utilizing PCBs as terminal electron acceptors, a process termed dehalorespiration, and several strains with specific dechlorinating capabilities have been reported 11–14. The commercial PCB mixture Aroclor 1260 was reported to be significantly dechlorinated by a consortium consisting of one or more phylotypes enriched from sediment microcosms 15, sediment-free microcosms 11 and by an individual species, “Dehalococcoides” sp. CBDB1 16. A recent report showed that “Dehalobium chlorocoercia” DF-1, bacterium o-17, phylotypes SF1 and DEH10, each with different PCB congener specificities for dechlorination, would alter the overall pathway for dechlorination of spiked Aroclor 1260 differently depending on which microorganisms were added to sediment microcosms 17. Bedard et al. 11 observed in a sediment-free enrichment culture that a critical mass of cells was required before reductive dechlorination of spiked Aroclor 1260 was detected and proposed that low indigenous numbers of dehalorespiring bacteria might explain why substantial attenuation of PCBs is rarely observed in the environment.
Although these combined reports suggest that bioaugmentation could be a potential strategy for treatment of PCB-impacted sediments, several questions need to be addressed to determine if in situ treatment of weathered PCBs by bioaugmentation is possible. Bedard 9 demonstrated that bioaugmentation with sediment slurries enriched for PCB dechlorinating bacteria combined with PCB 116 as a primer stimulated dechlorination of relatively high levels (50 ppm) of weathered Aroclor 1260 from Housatonic River sediment. However, the effectiveness of bioaugmentation for dechlorinating low levels of PCBs most commonly associated with weathered PCB contaminated sites where both kinetic and availability issues might affect the effectiveness of in situ PCB transformation is currently unknown 18–19. A recent report by May et al. 20 showed that bioaugmentation with DF-1 stimulated the reductive dechlorination of weathered Aroclor 1260 (4.6 ppm) in contaminated soil microcosms, and Krumins, 21 found that the addition of “D. ethenogenes” and pentachloronitrobenzene stimulated the dechlorination of weathered Aroclors 1248, 1254 and 1260 (2.1 ppm) in sediment microcosms. Combined, these results suggest that using bioaugmentation for low levels of weathered PCBs is feasible. Another unknown is whether dehalorespiring microorganisms used for in situ bioaugmentation can successfully compete with indigenous populations and be sufficiently sustainable to have a significant impact on reduction of higher chlorinated PCB congeners.
In this report we tested the efficacy of using bioaugmentation with the dehalorespiring bacterium D. chlorocoercia DF-1 to stimulate the reductive dechlorination of weathered Aroclor 1260 in sediment mesocosms that simulate in situ conditions. Specifically we examined the effects of bioaugmentation on Aroclor transformation over the course of 120 days in the presence of activated carbon to assess any potential effects of bioavailability and monitored the microbial population to assess the sustainability of DF-1 within the background of the indigenous bacterial community.
MATERIALS AND METHODS
Media and growth conditions
“Dehalobium chlorocoercia” DF1 was grown anaerobically in estuarine mineral medium (ECl) as described previously 20, 22. Sodium formate (10 mM) was added as the electron donor and PCB 61 (2,3,4,5-PCB) was added in acetone (0.1% v/v) at a final concentration of 173 µM. Desulfovibrio sp. extract (1% v/v) was added as a growth factor and titanium(III) nitrilotriacetate (0.5 mM) was added as a chemical reductant 20, 23. D. chlorocoercia was routinely grown in 50 ml of medium in 160-ml serum bottles sealed with 20-mm Teflon-coated butyl stoppers (West Pharmaceutical, Inc.). All cultures were incubated statically at 30°C in the dark. Growth was monitored by gas chromatographic analysis of PCB 61 dechlorination to PCB 23 (2,3,5-PCB) and by quantitative RT-PCR of 16S rRNA gene copies (described below).
Mesocosm experiments
Mesocosms were prepared in glass 2.2 liter TLC tanks (Fisher Scientific). PCB impacted sediments were sampled on 14 May 2009 from the Northwest Branch of Baltimore Harbor (BH) with a petite Ponar grab sampler at 39°16.8_N, 76°36.2_W and stored in the dark under nitrogen for 19 days at 4 °C prior to use. Sediment was pooled and homogenized anaerobically by stirring in an anaerobic glove bag. Two liters of sediment were added to each mesocosm tank with 2 cm of indigenous water above the sediment surface. A glass plate covered each mesocosm to minimize evaporation with a 1 cm gap on one end for air exchange. Water lost due to evaporation was periodically replenished with deionized water to maintain the osmolarity of the harbor water.
The bioaugmentation inoculum was prepared using ten 50 ml cultures of DF1 grown until 50% of PCB 61 was dechlorinated. The cultures were transferred into 250 ml Oak Ridge bottles in an anaerobic glove box and sealed under nitrogen-carbon dioxide (4:1). The bottles were centrifuged at 22,000 × g for 30 min, decanted, and the pellets were pooled in 50 ml of sterile ECl medium. The concentration of pooled DF1 was approximately 5×107 16S rRNA gene copies per ml. Spent medium supernatant was prepared by passing DF-1 culture supernatant through a 0.22 micron filter (Millipore, www.millipore.com) to remove residual cells. Mesocosms were amended with one of four treatments in an anaerobic glove box: (1) 20 ml of concentrated DF1 (5 × 105 cells gram−1 sediment); (2) 20 ml of spent cell-free growth medium; (3) 20 ml of concentrated DF1 adsorbed to 25 g granulated activated carbon for 1 hour; or (4) 20 ml of spent cell-free growth medium adsorbed to 25 g activated carbon (CAS# 7440-44-0, type TOG-NDS 80×325, Calgon Carbon Corp.) for 1 hour. The total GAC added in an individual mesocosm (4.4 % sediment dry weight) was similar to concentrations (3–5%) used in situ to sequester PCBs from benthic organisms 24. No exogenous electron donor was added. Mesocosms were homogenized after addition of the amendments by stirring with a Teflon spoon, then removed from the anaerobic glove box and incubated at 23°C in the dark. Mesocosms were sampled by taking six cm deep cores using a five ml syringe barrel with the end cut off. Triplicate cores were sampled for each timepoint using a random sampling grid and homogenized prior to analysis for PCBs and DNA as described below.
DNA extraction
DNA was extracted by adding 0.25 g of sediment from each sample core to a PowerBead microfuge tube of a Power Soil DNA Isolation Kit (MOBIO Laboratories, Inc.). The PowerBead tubes were mixed by hand prior to 30 s of bead beating at speed “4.5” using a FastPrep120 (Q-Biogene, 8 CA). Total DNA was then isolated from the PowerBead tubes according to the manufacturer’s directions. DNA was eluted in 100 µl of TE buffer and quantified with a NanoDrop 1000 Spectrophotometer (ThermoScientific). Extracted DNA samples had an A260/280 ratio of ≥ 1.6 and an A260/230 ratio of ≥ 2.0. All DNA samples were diluted to 2 ng/µl in TE buffer.
Enumeration of PCB dehalorespiring bacteria by quantitative PCR
The quantification of putative dechlorinating Chloroflexi in each subcore was performed by quantitative PCR (qPCR) using iQ SYBR green supermix (Bio-Rad Laboratories) and primers specific for the 16S rRNA gene of a deep branching, putative dechlorinating clade within the Chloroflexi (348F/884R) 13. Each 25-µl reaction volume contained 1× iQ SYBR green supermix, 500 nM forward and reverse primers and 1 µl of sample DNA. PCR amplification and detection were conducted in an iCycler (Bio-Rad Laboratories). qPCR conditions were as follows: initial denaturation for 15 min at 95°C followed by 35 cycles of denaturation for 30 s at 95 °C, annealing for 30 s at 61 °C, elongation for 30 s at 72 °C, with a final extension step of 5 min at 72 °C. One copy of the gene per genome was assumed based on the genomes of Dehalococcoides ethenogenes strain 195 and Dehalococcoides sp, strain CBDB125–26. qPCR data were analyzed with MJ Opticon Monitor Analysis Software v3.1 and compared to a standard curve of purified DF1 348F/884R 16S rRNA gene product. The standard curve consisted of duplicate dilutions over 6 orders of magnitude. The specificity of qPCR amplification was verified by melting curve analysis followed by gel electrophoresis. Amplification efficiencies of dilutions of gel purified DF1 16S rRNA gene PCR product used as standards were 87% ± 7% (r2 = 0.99), and amplification efficiencies of DF1 cells and environmental samples were almost identical to those of the standard curve (86 ± 7%). The observed departure of amplification efficiencies from 100% was possibly due to the consensus nature of primer 884R, which has 1 mismatch with the DF-1 16S rRNA gene combined with the stringency of the PCR reaction conditions.
Community analysis of PCB dechlorinating bacteria by denaturing HPLC
Denaturing HPLC (DHPLC) analyses were performed using a WAVE 3500 HT system (Transgenomic, Inc.) as described previously 27 except that the instrument was equipped with a florescence detector (excitation 490 nm, emission 520 nm). The primer set 348F/884R was used for PCR amplification of 16S rRNA genes from bacteria within the Chloroflexi 13. DHPLC fractions were sequenced as described previously 27. A phylogenetic tree was created with Tree Builder (http://rdp.cme.msu.edu/index.jsp).
PCB extraction
Sediment samples were extracted using an Accelerated Solvent Extractor (Dionex) following EPA method 3545. Approximately 5 grams wet weight sediment was dried with pelletized diatomaceous earth (Dionex) at room temperature in a desiccator containing CaCl2 · 2H2O. The dried sediment (1 g) was transferred to an 11 ml stainless steel extraction cell containing 0.6 g Cu and 2.4 g fluorosil between two cellulose filters on the bottom of the cell and the remaining cell volume was filled with anhydrous Na2SO4. To correct for extraction efficiency, 10 µl of a 400 µg l−1 solution of PCB 166 in hexane was pipetted on top of the Na2SO4. The sample containing the surrogate was extracted with approximately 20 ml of pesticide grade hexane (Acros Organics) at 100°C and purged with 13.8 MPa nitrogen. The sample was evaporated to a final volume of 1 ml at 30°C under nitrogen using a N-EVAP 111 nitrogen evaporator (Organomation Associates). Before PCB analysis, 10 µl of PCB 30 and PCB 204 (400 µg l−1 each in acetone) was added to the sample as internal standards.
PCB analysis
PCB congeners were analyzed using a Hewlett-Packard 6890 series II gas chromatograph (GC) with a DB-1 capillary column (60 m by 0.25 mm by 0.25 µm; JW Scientific) and a 63Ni electron capture detector by a modified method of EPA 8082. PCB congeners in a mixture containing 250 µg l−1 Aroclor 1232, 180 µg l−1 Aroclor 1248 and 180 µg l−1 Aroclor 1262 were quantified with a 10-point calibration curve using PCB 30 and PCB 204 as internal standards. Individual congeners and respective concentrations were obtained from Mullins et al 28. Fifty-five additional congeners not present in the Aroclor mixture that were potential dechlorination products were added to the calibration table containing the Aroclor congeners. The additional congeners were quantified with 10-point calibration curves at concentrations of 2, 5, 10, 20, and 40 µg l−1 (in duplicate) for the low range calibration and 40, 100, 200, 400, and 800 µg l−1 (in duplicate) for the high range calibration. Using this protocol 173 congeners were resolved in 130 individual peaks (excluding internal standards PCB 30 and PCB 204 and surrogate PCB166) (Table S1). The final concentration of individual congeners in samples was corrected for the recovery efficiency of the surrogate (82 ± 6%), which was reduced below 100% by the high content of carbon black in Baltimore Harbor sediments 30 and the activated carbon amendment. Co-eluting peaks were indicated as multiple congeners. Total chlorines per biphenyl was calculated as the product of the average number of chlorines and molar concentration of each congener divided by the sum of the total molar concentration of all congeners 29. When co-eluting peaks were different homologs, equal amounts of each homolog were assumed 29. All PCBs (99% purity) were purchased from AccuStandard.
RESULTS
Characteristics of mesoscosm sediment
Sediment used in mesocosms was black in color with a slightly gelatinous texture. Relatively high amounts of PAHs, trans-nonachlor and heavy metals have been reported near this site as recently as 1997 31. Total PCB concentration in the pooled sediment samples was 1.3 ± 0.15 ppm with a mean of 4.76 chlorines per biphenyl. The congener profile was consistent with the first reported survey of PCBs in BH by Morgan and Sommer 32 showing that Aroclor 1260 occurred predominantly with smaller amounts of Aroclor 1254 (Table S1). However, less chlorinated congeners were detected that are potential weathered products of Aroclor 1260 (Table S1).
After bioaugmentation the sediments appeared to be anaerobic 1–2 cm below the surface based on black coloration and the distinct odor of sulfide. In mesocosms without GAC the sediment surface developed a grey coloration; with GAC the sediment surface developed a distinct orange-brown coloration. The surface of the mesocosm was disrupted within 1 week by holes (about 0.2 cm in diameter) caused by the activity of benthic worms but the activity was less apparent by day 60 (Figure S1).
Effects of treatments on reductive dechlorination of weathered PCBs
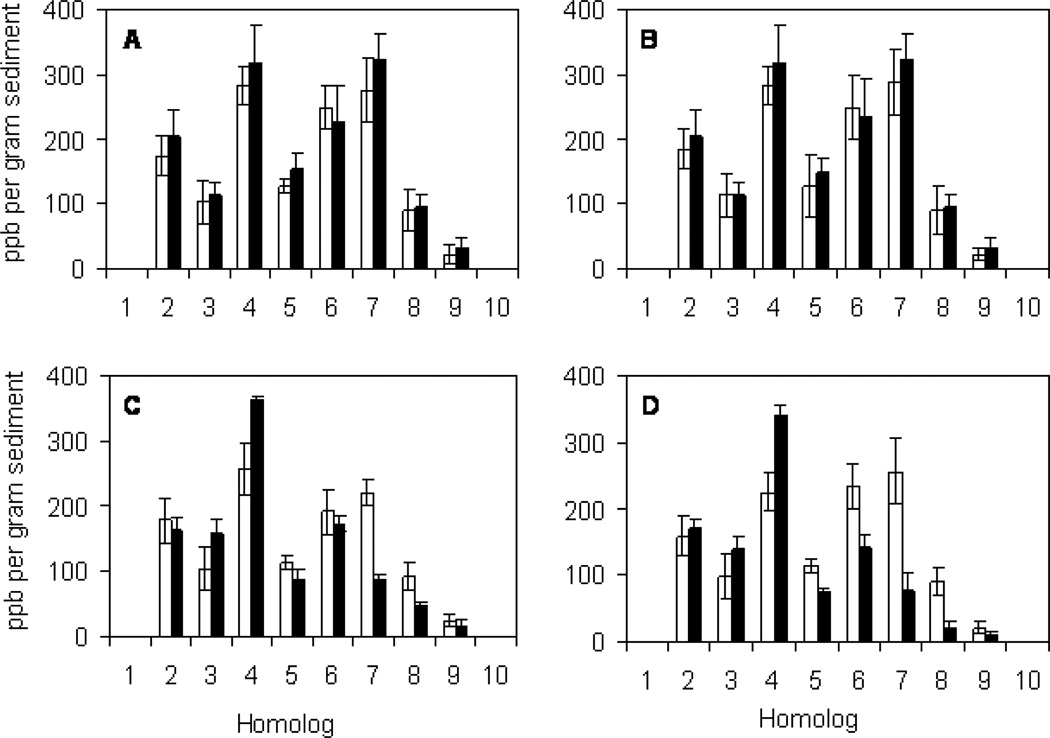
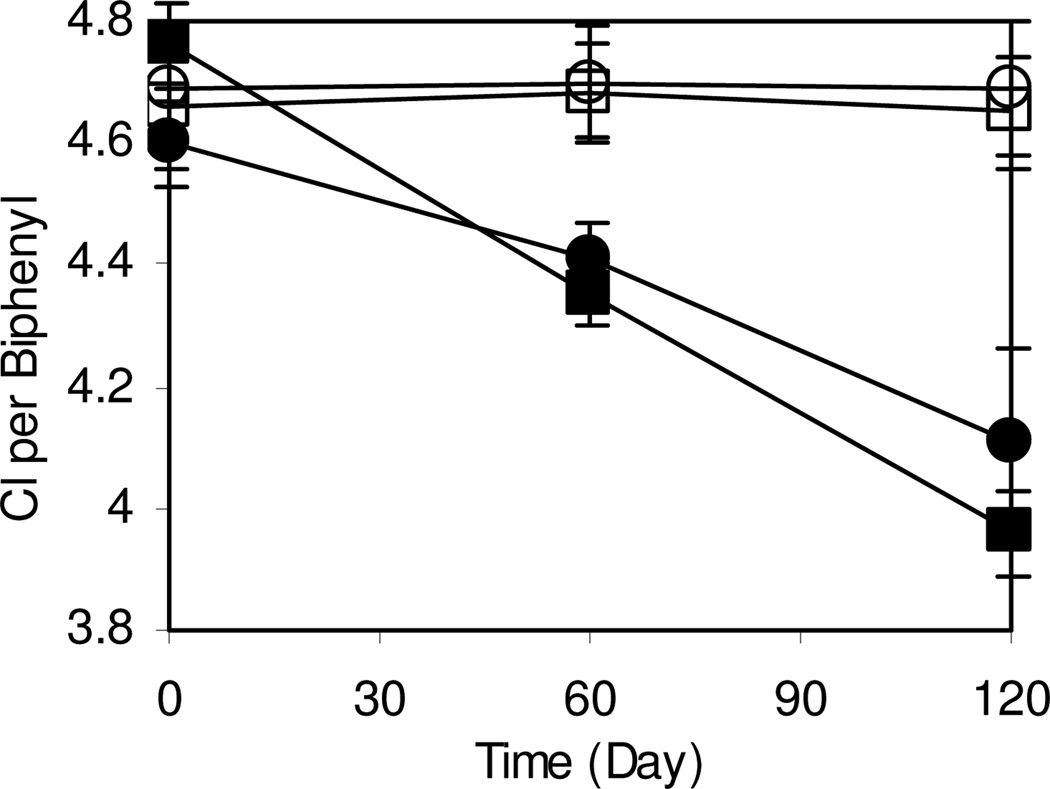
BH sediment mesocosms were bioaugmented with dehalorespiring D. chlorocoercia DF-1 to a final concentration of approximately 5×105 cells g−1 sediment and PCBs were monitored over 120 days. In mesocosms bioaugmented directly with DF1, there was a net decrease in the total amounts of septa- and octa-PCBs (Figure 1C). In mesocosms bioaugmented with DF-1 with GAC, which reduces the bioavaiability of PCBs 24, there was a net decrease in the total amounts of penta- through octa- PCBs and a net increase in the total amount of tetra- and tri-PCBs over the course of 120 days (Figure 1D). In contrast, there was no obvious change in homolog distribution in non-bioaugmented mesocoms treated with spent cell-free medium with or without GAC. Bioaugmentation both directly and with GAC as an adsorption substrate resulted in 0.6 to 0.7 mol Cl per biphenyl dechlorinated, respectively, after 120 days (Figure 2). A greater rate of dechlorination (0.0067 Cl/biphenyl/day) was observed after bioaugmentation with GAC compared with direct injection (0.0041 Cl/biphenyl/day). A t-Test (two-sample assuming unequal variances, α=0.05) showed a significant difference in dechlorination rates between the bioaugmentation treatments with a P-value of 0.041 (df = 4).
Figure 1.
PCB analysis by homolog at day 0 (white bars) and day 120 (black bars) after treatment with filter sterilized spent growth medium (A), sterilized spent growth medium and GAC (B), concentrated DF1 in growth medium inoculated directly into the sediment (C) and concentrated DF1 adsorbed onto GAC (D). Each bar represents the mean and standard deviation of three replicates samples.
Figure 2.
Changes in Cl per biphenyl in mesocosms over time after treatment with sterilized spent growth medium (○), sterilized spent growth medium and GAC (□),DF1 inoculated directly into sediment (●), and DF1 adsorbed onto GAC inoculated into sediment (■). Each datum point represents the mean and standard deviation of three replicates samples.
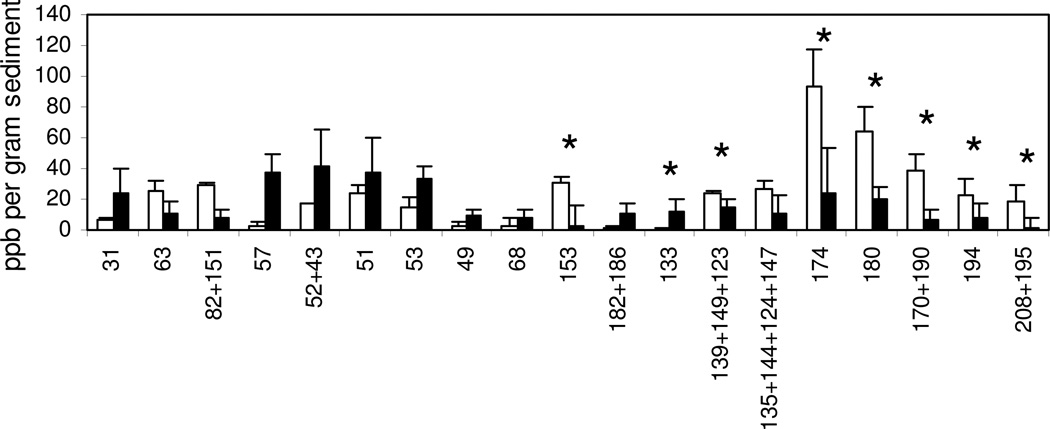
Single congener analysis of the mesocosm bioaugmented with DF1 adsorbed to GAC resulted in a significant decrease in higher chlorinated congeners and corresponding increase in lesser chlorinated congeners (Figure 3 and Table S1). The total sum of predicted substrates and products (Figure 3) was 1.26 ± 0.307 ×10−9 mol per gram sediment at day 0 and 1.02 ± 0.639×10−9 mol g−1 sediment at day 120. Predicted substrate congeners decreased from 1.00 ± 0.228 to 0.291 ± 0.282×10−9 mol per gram sediment and product congeners increased from 0.256 ± 0.0786 to 0.729 ± 0.356×10−9 mol per gram sediment. The inability to obtain a zero mass balance might have resulted from the absence of possible dechlorination products in the GC method (e.g., PCBs 187, 109, 92, 96, and 84 among others, Table S1 and Table S2), volatilization of less chlorinated congeners or aerobic degradation by indigenous microorganisms. Strikingly, PCB 194 decreased from about 23 to 8 ppb and PCB 133, the predicted product of sequential double flanked para reductive dechlorination of PCB 194 (via PCB 172) was found to increase from about 1 to 12 ppb over 120 days (Figure 3 and Table S2). Potential dechlorination products of double flanked reductive dechlorination of PCB 208 (PCB 179 or PCB 136), PCB 195 (PCB 187), PCB 170 (PCB 87), PCB 190 (PCB 163), PCB 180 (PCB153 or PCB 141) and PCB 174 (PCB135 or PCB 149) were detected after 120 days; however, these products did not accumulate to substantial amounts. Furthermore, the less chlorinated congeners that did accumulate were not products resulting from dechlorination of double flanked chlorines (PCB 51, PCB 53, PCB 52, PCB 49, PCB 32, and PCB 57, Table S2). Since DF-1 is reported to reductively dechlorinate only doubly flanked chlorines 20 the dechlorination products likely resulted from enhanced activity by the indigenous population of dehalorespiring bacteria. Addition of cell-free medium used to grow DF-1 did not stimulate PCB dechlorination, indicating that the enhanced activity by indigenous microorganisms did not result from “priming” by residual PCBs or biostimulation by the medium.
Figure 3.
Congeners showing greatest change in mesocosms receiving both GAC and DF1 at day 0 (□) and day 120 (■). An asterisk (*) indicates congeners that could be dechlorination substrates of DF1, or products from double flanked dechlorination by DF-1. Each bar represents the mean and standard deviation of three replicates samples.
Sustainability of D. chlorocoercia DF1 after bioaugmentation
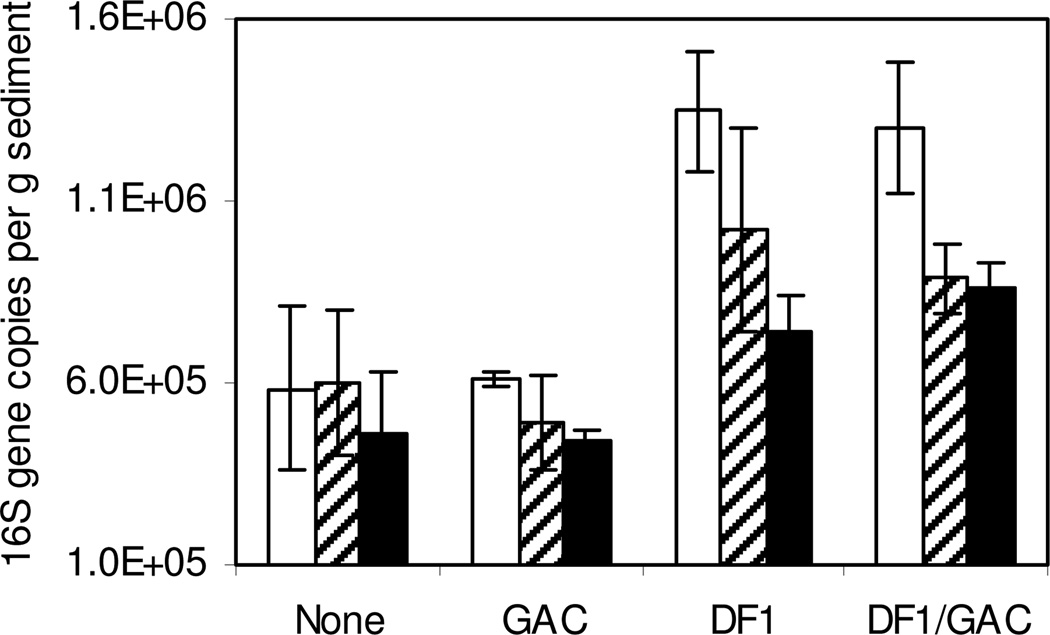
To determine whether DF-1 was sustainable in the presence of relatively low PCB concentrations and the indigenous microbial community, putative dehalorespiring microorganisms were enumerated during the experiment based on the number of 16S rRNA gene copies g−1 dry sediment. In both bioaugmented mesocosms the numbers of putative dehalorespiring bacteria was initially about 2-fold higher than in untreated mesocosms (1.3×106 compared t o about 6.0×105 copies per gram, respectively) indicating that added DF-1 accounted for approximately half the total population of putative dehalorespiring bacteria in those mesocosms. A Student’s t-Test (α=0.05) showed a significant difference between initial 16S rRNA gene copy numbers between treatments with a two-tail P-value of 0.01 (df=3). The total 16S rRNA copy numbers in treated mesocosms decreased by 60 days before reaching an apparent steady state for the 120 day incubation period (Figure 4). However, for bioaugmentation treatments by both direct injection and on GAC substrate, the total number of putative dehalorespiring microorganisms remained nearly 2-fold higher in the bioaugmented mesocosms compared with the untreated mesocosms (8.0×105 compared to about 4.5×105 copies per gram at day 120, respectively). Detection of putative dechlorinating bacteria in untreated inactive mesocosms suggests that only a small proportion of the indigenous bacteria within this clade of the Chloroflexi were capable of dechlorinating PCBs. The results indicate that greater numbers of dehalorespiring microorganisms were maintained in bioaugmented mesocosms during active dechlorination of the weathered Aroclor compared with non-bioaugmented controls.
Figure 4.
Enumeration of putative dechlorinating Chloroflexi normalized to 16S rRNA gene copies/gram sediment at day 0 (□), day 60 ( ) and day 120 (■). Treatments included spent medium only, spent medium and GAC, amendment with DF-1 by direct injection, and amendment with DF-1 adsorbed to GAC. Each bar represents the mean and standard deviation of three replicates samples.
) and day 120 (■). Treatments included spent medium only, spent medium and GAC, amendment with DF-1 by direct injection, and amendment with DF-1 adsorbed to GAC. Each bar represents the mean and standard deviation of three replicates samples.
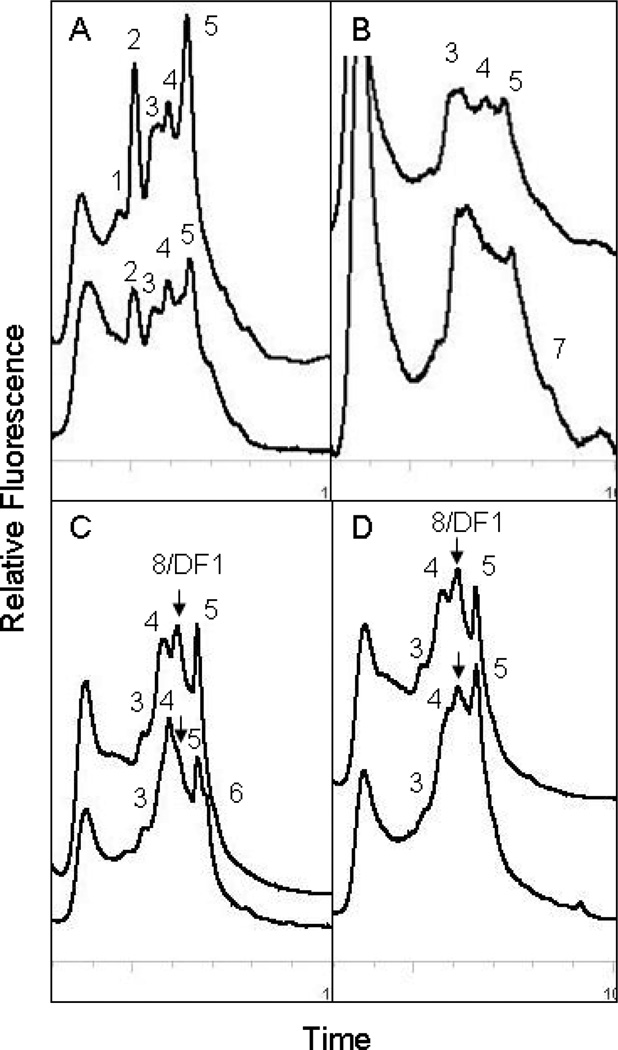
Seven predominant phylotypes were detected by DHPLC in the BH sediment mesocosms. The community phylotypes were generally similar between mesocosms over 120 days, with the exception of DF1, which was only detected in bioaugmented mesocosms (Figure 5). The putative DF1 fraction was collected, sequenced, and found to be 100% identical to DF1 (Figure S2 and Table S3.). One phylotype present at time zero (BH 4) was 100% identical to phylotype DEH10, a PCB dechlorinating bacterium previously detected BH sediment microcosms 13, 15, but no other previously reported BH phylotypes were detected.
Figure 5.
DHPLC community analysis of putative dechlorinating Chloroflexi 16S rRNA genes in mesocosms at day 0 (bottom trace) and day 120 (top trace). A, addition of spent growth media alone; B, addition of spent growth media and GAC; C, addition of DF1; D, addition of DF1and GAC. Peak eluting at about 6.3 minutes (labeled 8/DF1 with arrow) in C and D confirmed DF1 16S rRNA gene by sequencing. Other phylotypes of putative dechlorinating Chloroflexi labeled 1, 2, 3, 4, 5, 6, or 7 are described in Table S2.
DISCUSSION
There have been several reports on aerobic bioremediation by microbes and plants, but these approaches are only effective for treatment of less chlorinated congeners 7. Although highly chlorinated congeners commonly associated with commercial Aroclors such as Aroclor 1254, 1258, 1260 and 1262 can be reductively dechlorinated to less chlorinated congeners that serve as substrates for complete degradation by aerobic bacteria, concerns over several factors have called into question whether bioaugmentation by dehalorespiring bacteria is an effective strategy for in situ treatment of PCB impacted sediments.
Bioavailability
Previous studies on the kinetics of reductive dechlorination in sediment microcosms spiked with Aroclor 1242 18 or 1248 19 reported that activity was limited at concentrations below 37–40 ppm. However, Magar et al. 33 reported evidence of reductive dechlorination at PCB concentrations as low as 1–2 ppm in Lake Hartwell over a period of 11 years, which suggested that dehalorespiring bacteria slowly dechlorinated low levels of weathered Arcolor over time. In the current report we show unequivocally that bioaugmentation with the dehalorespiring bacterium DF-1 successfully stimulates the reductive dechlorination of sediment containing 1.3 ppm of weathered Aroclor 1260. Furthermore, addition of GAC, which increases the partition coefficient of PCBs between the sediment matrix and aqueous phase, had no inhibitory effect on dechlorination activity in bioaugmented mesocosms. GAC has been reported to reduce the bioavailability of PCBs to macroorganisms that ingest the particles 34, however, the slight stimulatory effect of GAC observed in the bioaugmented mesocosm suggests that PCB dehalorespiring bacteria have mechanisms that enable them to utilize weathered PCBs sequestered in the inorganic carbon fraction. The true threshold of bioavailability for PCB dechlorinating bacteria is not currently known, however, the results clearly demonstrate that bioaugmentation can stimulate reductive dechlorination of low levels of weathered PCBs in the 1–2 ppm range even with activated carbon present to sequester PCBs from any rapid desorption pool of sediment-associated PCB. Although the less chlorinated PCBs generated by bioaugmentation are more bioavailable to benthic organisms, they are generally less toxic because they are non-coplanar or have at least two adjacent unsubstituted carbon atoms that would make them subject to rapid metabolic degradation 35.
Sustainability
One factor that is critical for successful bioaugmentation of weathered PCB-impacted sediments is the ability of the inoculated biocatalyst to compete with the indigenous community of non-dehalorespiring bacteria for electron donors and nutrients. In the current study DF-1 was detected in bioaugmented sediment mesocosms after 120 days indicating that non-native DF1 could coexist with the indigenous sediment community. The total numbers of putative dehalorespiring bacteria decreased by approximately half after 90 days to a steady state of 7–8 × 105 cells per gram of sediment, but community analysis showed that DF-1 was sustained as a predominant member of the putative dehalorespiring community. Although the total numbers of putative dehalorespiring phylotypes was 1–2 orders of magnitude lower than observed in prior reports of Aroclor 1260 dechlorinating microcosms11, 15 neither exogenous electron donors nor electron acceptors were added in our study; therefore the lower steady state numbers likely reflect lowers concentrations of indigenous electron donors or acceptors available in the sediment. The observation that dehalorespiring bacteria were sustained in the sediment mesocosms without an exogenous electron donor is consistent with the ability of dehalorespiring bacteria to outcompete hydrogenotrophic sulfate reducers, acetogens, and methanogens in the presence of limited hydrogen concentration 36–37. Interestingly, the indigenous phylotypes of putative dehalorespiring bacteria, with the exception of phylotype DEH10 had not been reported previously in Baltimore Harbor sediment Aroclor 1260 enrichment microcosms 15. One possible explanation is that a different population of dehalorespiring bacteria was enriched with high concentrations of spiked Aroclor 1260 in the prior studies and the population in the current study is more relevant at in situ concentrations. The results show that DF-1 was able to coexist successfully with the indigenous microbial population even with the lower background concentrations of electron donor.
Another significant observation was the detection of PCB congeners that did not result from dechlorination of doubly flanked chlorines. These dechlorination reactions were not observed in control mesocosms with spent cell-free medium indicating that the activity observed was neither due to a “priming” effect from residual PCB 61 or 23 in the inoculum nor a nutrient effect from carryover of formate or cell byproducts in the medium. This phenomenon has been reported previously. May et al. 20 observed enhanced dechlorination of congeners without flanked chorines in Aroclor 1260 impacted soil microcosms inoculated with DF-1. Krumins et al. 21 observed in PCB-impacted Anacostia River sediments bioaugmented with D. ethenogenes strain 195 stimulated PCB dechlorination and although this species was not sustained enhanced activity continued, which was attributed to the indigenous dehalorespiring community. One possible explanation for this effect is “priming” by the accumulation of dechlorination products from the initial activity of high numbers of DF-1 inoculated into the sediments. Addition of PCB congeners and analogs is known to stimulate the reductive dechlorination of PCBs in lab studies 21, 38–39 and in field tests 40. Overall the results indicate that in addition to initiating and directly dechlorinating weathered PCBs, bioaugmentation with DF-1 had a synergistic effect on the indigenous dehalorespiring community by an as yet unknown mechanism that contributed further to the dechlorination process.
Bioaugmentation
There have been recent attempts to test the effects of bioaugmentation with pure cultures of dehalorespiring bacteria. Krumins et al. 21 reported enhanced reductive dechlorination of weathered PCBs (ca. 2 ppm) in sediment microcosms after bioaugmentation with D. ethenogenes strain 195 and May et al. 20 reported enhanced dechlorination of Aroclor impacted soil (4.6 ppm) after bioaugmentation with DF-1. In contrast to the prior studies where bioaugmentation stimulated the reductive dechlorination of PCB by 0.2 Cl/biphenyl after 415 days and 0.35 Cl/biphenyl after 145 days, respectively, bioaugmentation in the current study stimulated the reductive dechlorination by 0.7 Cl/biphenyl after only 120 days. Furthermore, bioaugmentation results in the current study stimulated 56% by mass reduction of penta- through nona-chlorobiphenyls to lesser-chlorinated congeners (primarily tetrachlorobiphenyls), which are susceptible to aerobic degradation, with no detectable activity in untreated controls. The discrepancies in the rates and extent of dechlorination could possibly occur due to a number of factors including available nutrients, presence of inhibitory co-contaminants, the dehalorespiring strain used and the growth state and numbers of cells used for bioaugmentation. Distribution of cells in the current study was effective either by direct injection or on GAC particles. The ability to use a solid substrate such as GAC for inoculation of cells offers a possible solution for dispersing cells in the field.
The results of this study support the potential feasibility of using bioaugmentation for treatment of PCB impacted sediments. We showed that bioavailability does not prevent bioaugmentation from treating low levels of weathered PCBs in sediment mesocosms and that GAC actually enhanced the overall process. Furthermore, DF-1, a non-indigenous species, was sustained throughout the dechlorination process and had a positive synergistic effect on the indigenous dehalorespiring population that contributed to the process. Although DF-1 was used successfully to bioaugment reductive dechlorination of weathered A roclor 1260 in Baltimore Harbor sediments, discrepancies between the current and prior studies highlight the importance of testing inoculum with sediments from each site. All PCB bioaugmentation studies including the current study have been conducted on a laboratory scale and field studies will be required ultimately to validate the approach for in situ treatment of contaminated sites. However, the overall results of this mesocosm-based study provide compelling evidence to support further testing and development of bioaugmentation with dehalorespiring bacteria as an environmentally less invasive and lower cost alternative for in situ treatment of PCB impacted sediments.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the National Institute of Environmental Health Science Superfund Research Program (5R01ES016197-02) and US Department of Defense, Strategic Environmental Research and Development Program (ER-1502).
Footnotes
SUPPORTING INFORMATION AVAILABLE
Image of laboratory mesocosms, phylogenetic tree of Chloroflexi identified in mesocosms, congeners detected in mesocosms, inferred pathways of PCB dechlorination in bioaugmented mesocosm and identification of phylotypes in mesocosms. This information is available free of charge via the Internet at http://pubs.acs.org/.
LITERATURE CITED
- 1.Alder AC, Häggblom MM, Oppenheimer SR, Young LY. Reductive dechlorination of polychlorinated biphenyls in anaerobic sediments. Environ. Sci. Technol. 1993;27:530–538. [Google Scholar]
- 2.Quensen JF, III, Boyd SA, Tiedje JM. Dechlorination of four commercial polychlorinated biphenyl mixtures (Aroclors) by anaerobic microorganisms from sediments. Appl. Environ. Microbiol. 1990;56:2360–2369. doi: 10.1128/aem.56.8.2360-2369.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Versar I. PCBs in the United States: industrial use and environmental distribution. 1976 EPA report 560/6-76-005. [Google Scholar]
- 4.Crisp TM, Clegg ED, Cooper RL, Wood WP, Anderson DG, Baetcke KP, Hoffmann JL, Morrow MS, Rodier DJ, Schaeffer JE, Touart LW, Zeeman MG, Patel YM. Environmental endocrine disruption: an effects assessment and analysis. Environ. Health Perspect. 1998;106:11–56. doi: 10.1289/ehp.98106s111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Safe S. Toxicology, structure-function relationship, and human and environmental health impacts of polychlorinated biphenyls: progress and problems. Environ. Health Perspect. 1993;100:259–268. doi: 10.1289/ehp.93100259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.EPA. Contaminated sediment remediation guidance for hazardous waste sites. 2005 EPA-540-R-05-012. Final Report. [Google Scholar]
- 7.Abraham W-R, Nogales B, Golyshin PN, Pieper DH, Timmis KN. Polychlorinated biphenyl-degrading microbial communities in soils and sediments. Curr. Opin. Microbiol. 2002;5:246–253. doi: 10.1016/s1369-5274(02)00323-5. [DOI] [PubMed] [Google Scholar]
- 8.Wu Q, Wiegel J. Two anaerobic polychlorinated biphenyl-dehalogenating enrichments that exhibit different para-dechlorination specificities. Appl. Environ. Microbiol. 1997;63(12):4826–4832. doi: 10.1128/aem.63.12.4826-4832.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bedard DL, VanDort HM, May RJ, Smullen LA. Enrichment of microorganisms that sequentially meta, para-dechlorinate the residue of Aroclor 1260 in Housatonic River sediment. Environ. Sci. Technol. 1997;31(11):3308–3313. [Google Scholar]
- 10.Natarajan MR, Nye J, Wu W-M, Wang H, Jain MK. Reductive dechlorination of PCB contaminated Raisin River sediments by anaerobic microbial granules. Biotechnol. Bioeng. 1997;55:181–190. doi: 10.1002/(SICI)1097-0290(19970705)55:1<182::AID-BIT19>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 11.Bedard DL, Ritalahti KM, Loffler FE. The Dehalococcoides population in sediment-free mixed cultures metabolically dechlorinates the commercial polychlorinated biphenyl mixture Aroclor 1260. Appl. Environ. Microbiol. 2007;73(8):2513–2521. doi: 10.1128/AEM.02909-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cutter LA, Watts JEM, Sowers KR, May HD. Identification of a microorganism that links its growth to the reductive dechlorination of 2,3,5,6-chlorobiphenyl. Environ. Microbiol. 2001;3(11):699–709. doi: 10.1046/j.1462-2920.2001.00246.x. [DOI] [PubMed] [Google Scholar]
- 13.Fagervold SK, Watts JEM, May HD, Sowers KR. Sequential reductive dechlorination of meta-chlorinated polychlorinated biphenyl congeners in sediment microcosms by two different Chloroflexi phylotypes. Appl. Environ. Microbiol. 2005;71(12):8085–8090. doi: 10.1128/AEM.71.12.8085-8090.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Q, Watts JEM, Sowers KR, May HD. Identification of a bacterium that specifically catalyzes the reductive dechlorination of polychlorinated biphenyls with doubly flanked chlorines. Appl. Environ. Microbiol. 2002;68:807–812. doi: 10.1128/AEM.68.2.807-812.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fagervold SK, May HD, Sowers KR. Microbial reductive dechlorination of Aroclor 1260 in Baltimore Harbor sediment microcosms is catalyzed by three phylotypes within the phylum Chloroflexi. Appl. Environ. Microbiol. 2007;73(9):3009–3018. doi: 10.1128/AEM.02958-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adrian L, Dudkova V, Demnerova K, Bedard DL. “Dehalococcoides” sp. strain CBDB1 extensively dechlorinates the commercial polychlorinated biphenyl mixture Aroclor 1260. Appl. Environ. Microbiol. 2009;75(13):4516–4524. doi: 10.1128/AEM.00102-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fagervold SK, Watts JEM, May HD, Sowers KR. Effects of bioaugmentation on indigenous PCB dechlorinating activity in sediment microcosms. Wat. Res. 2011;45:3899–3907. doi: 10.1016/j.watres.2011.04.048. [DOI] [PubMed] [Google Scholar]
- 18.Fish KM. Influence of Aroclor 1242 concentration on polychlorinated biphenyl biotransformations in Hudson River test tube microcosms. Appl. Environ. Microbiol. 1996;62(8):3014–3016. doi: 10.1128/aem.62.8.3014-3016.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rhee GY, Sokol RC, Bethoney CM, Cho YC, Frohnhoefer RC, Erkkila T. Kinetics of polychlorinated biphenyl dechlorination and growth of dechlorinating microorganisms. Environ. Toxicol. Chem. 2001;20:721–726. [PubMed] [Google Scholar]
- 20.May HD, Miller GS, Kjellerup BV, Sowers KR. Dehalorespiration with polychlorinated biphenyls by an anaerobic ultramicrobacterium. Appl. Environ. Microbiol. 2008;74(7):2089–2094. doi: 10.1128/AEM.01450-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krumins V, Park JW, Son EK, Rodenburg LA, Kerkhof LJ, Haggblom MM, Fennell DE. PCB dechlorination enhancement in Anacostia River sediment microcosms. Wat. Res. 2009;43(18):4549–4558. doi: 10.1016/j.watres.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 22.Berkaw M, Sowers KR, May HD. Anaerobic ortho dechlorination of polychlorinated biphenyls by estuarine sediments from Baltimore Harbor. Appl. Environ. Microbiol. 1996;62(7):2534–2539. doi: 10.1128/aem.62.7.2534-2539.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moench TT, Zeikus JG. An improved preparation method for a titanium(III) media reductant. J. Microbiol. Methods. 1983;1:199–202. [Google Scholar]
- 24.Ghosh U, Luthy RG, Cornelissen G, Werner D, Manzie CA. In-situ Sorbent Amendments: A New Direction in Contaminated Sediment Management. Environ. Sci. Technol. 2011;45(4):1163–1168. doi: 10.1021/es102694h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Seshadri R, Adrian L, Fouts DE, Eisen JA, Phillippy AM, Methe B, Ward NL, Nelson WC, Deboy RT, Khoudry HM, Kolonay JF, Dodson RJ, Daugherty SC, Brinkac LM, Sullivan SA, Madupu R, Nelson KE, Kang KH, Impraim M, Tran K, Robinson JM, Forberger HA, Fraser CM, Zinder SH, Heidelberg JF. Genome sequence of the PCE-dechlorinating bacterium Dehalococcoides etheneogenes. Sci. 2005;307:105–108. doi: 10.1126/science.1102226. [DOI] [PubMed] [Google Scholar]
- 26.Kube M, Beck A, Zinder SH, Kuhl H, Reinhardt R, Adrian L. Genome sequence of the chlorinated compound-respiring bacterium Dehalococcoides species strain CBDB1. Nat. Biotechnol. 2005;23:1269–1273. doi: 10.1038/nbt1131. [DOI] [PubMed] [Google Scholar]
- 27.Kjellerup BV, Sun X, Ghosh U, May HD, Sowers KR. Site-specific microbial communities in three PCB-impacted sediments are associated with different in situ dechlorinating activities. Environ. Microbiol. 2008;10:1296–1309. doi: 10.1111/j.1462-2920.2007.01543.x. [DOI] [PubMed] [Google Scholar]
- 28.Mullin MD, Pochini CM, McCrindle S, Romkes M, Safe SH, Safe LM. High resolution PCB analysis: synthesis and chromatographic properties of all 209 PCB congeners. Environ. Sci. Technol. 1984;18:468–476. doi: 10.1021/es00124a014. [DOI] [PubMed] [Google Scholar]
- 29.Cho YC, Kwon OS, Sokol RC, Bethoney CM, Rhee GY. Microbial PCB dechlorination in dredged sediments and the effect of moisture. Chemosphere. 2001;43(8):1119–1126. doi: 10.1016/s0045-6535(00)00193-4. [DOI] [PubMed] [Google Scholar]
- 30.Grossman A, Ghosh U. Measurement of activated carbon and other black carbons in sediments. Chemosphere. 2009;75(4):469–475. doi: 10.1016/j.chemosphere.2008.12.054. [DOI] [PubMed] [Google Scholar]
- 31.Baker J, Mason R, Cornwell J, Ashley J, Halka J, Hill J. UMCES CBL Reference Series, 97-142. Solomons, MD: University of Maryland Center for Environmental Science, Chesapeake Biological Laboratory; 1997. Aug, Spatial Mapping of Sedimentary Contaminants in the Baltimore Harbor/Patapsco River/Back River System. [Google Scholar]
- 32.Morgan WP, Sommer SE. Polychlorinated biphenyls in Baltimore Harbor sediments. Bull. Environ. Cont. Toxicol. 1979;22:413–419. doi: 10.1007/BF02026964. [DOI] [PubMed] [Google Scholar]
- 33.Magar VS, Brenner RC, Johnson GW, Quensen JF., 3rd Long-term recovery of PCB-contaminated sediments at the Lake Hartwell superfund site: PCB dechlorination. 2. Rates and extent. Environ. Sci. Technol. 2005;39(10):3548–3554. doi: 10.1021/es0486216. [DOI] [PubMed] [Google Scholar]
- 34.Sun X, Ghosh U. The effect of activated carbon on partitioning, desorption, and biouptake of native PCBs in four freshwater sediments. Environ. Toxicol. Chem. 2008;27:2287–2295. doi: 10.1897/08-020.1. [DOI] [PubMed] [Google Scholar]
- 35.Safe S. Polyhalogenated aromatics: uptake, disposition and metabolism. In: Kimbrough RD, Jensen AA, editors. Halogenated Biphenyls, Naphthalenes, Dibenzodioxins and Related Products. 2nd ed. Elsevier-North Holland: Amsterdam; 1989. pp. 51–69. [Google Scholar]
- 36.Fennell DE, Gossett JM. Modeling the production of and competition for hydrogen in a dechlorinating culture. Environ. Sci. Technol. 1998;32(16):2450–2460. [Google Scholar]
- 37.Loffler FE, Tiedje JM, Sanford RA. Fraction of electrons consumed in electron acceptor reduction and hydrogen thresholds as indicators of halorespiratory physiology. Appl. Environ. Microbiol. 1999;65(9):4049–4056. doi: 10.1128/aem.65.9.4049-4056.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bedard DL, VanDort H, Deweerd KA. Brominated biphenyls prime extensive microbial reductive dehalogenation of Aroclor 1260 in Housatonic River sediment. Appl. Environ. Microbiol. 1998;64(5):1786–1795. doi: 10.1128/aem.64.5.1786-1795.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deweerd KA, Bedard DL. Use of halogenated be nzoates and other halogenated aromatic compounds to stimulate the microbial dechlorination of PCBs. Environ. Sci. Technol. 1999;33(12):2057–2063. [Google Scholar]
- 40.Bedard DL, Quensen JF. Microbial reductive dechlorination of polychlorinated biphenyls. In: Young LY, Cerniglia CE, editors. Microbial transformation and degradation of toxic organic chemicals. New York: A. John Wiley; 1995. pp. 127–216. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.