Immune mediators secreted by human uterine epithelial cells confer tolerogenic properties to dendritic cells.
Keywords: female reproductive tract, endometrium, immune tolerance
Abstract
The balance between immunity and tolerance in the endometrium is governed by dynamic interactions of UEC and immune cells including DC. In this study, we tested the hypothesis that soluble immune mediators secreted by UEC modulate the differentiation and functions of human DC. We found that DC differentiated with CM from polarized UEC (i.e., CM-DC) expressed significantly lower surface CD86. Upon activation with LPS or PIC, the expression of CD80, CD86, and CD83 was decreased significantly on CM-DC relative to Con-DC. Further, mRNA for TLR3, TLR4, and TLR5 was decreased significantly in CM-DC relative to Con-DC. As a functional read-out of the effect of CM on DC, we determined the following parameters: First, analysis of cytokine production showed that when compared with Con-DC, CM-DC responded to LPS or PIC stimulation with enhanced IL-10 production but undetectable IL-12p70 secretion. Second, RT-PCR analysis showed that CM-DC significantly expressed higher mRNA for IDO, an immune tolerance-promoting enzyme. Lastly, in a MLR assay, CM-DC induced significantly lower allogeneic proliferative responses compared with Con-DC. These findings indicate collectively that epithelial cells confer a tolerogenic phenotype to DC in the endometrium. Our results suggest novel cellular and molecular mechanisms for the regulation of adaptive immunity within the FRT.
Introduction
The mechanisms that regulate immune responses within the uterine endometrium are not well defined. Although the tissue maintains a state of immunotolerance to fetal allograft and allogeneic sperm, it nevertheless is prepared to mount an effective immune response to invading pathogens [1]. The underlying molecular basis for this functional paradox is unclear. It may be dependent on the dynamic interactions of cells that are resident in the endometrium or nonresident cells that migrate from peripheral circulation. DC may be critical players of the regulatory paradigm in the endometrium by virtue of their functional plasticity, promoting immunity or induction of immunotolerance [2]. In this regard, a basic understanding of the mechanisms that influence the function of DC may provide insight into the regulation of immune responses in the endometrium.
DC are professional APC that link innate and adaptive immune responses (reviewed in refs. [3, 4]). More recent evidence indicates that DC display unique phenotypic and functional characteristics depending on the tissue location [5, 6]. Functionally, DC are educated to adopt immunogenic or tolerogenic features by the cell-cell interactions in the tissue microenvironments [7, 8]. The signatures of tolerogenic DC include lower expression of CD80, CD86, and MHC II, ability to secrete IL-10 preferentially as compared with IL-12 in response to maturation-inducing stimuli, enhanced expression of IDO, and decreased capacity to induce autologous T cell proliferation [9, 10].
The functional plasticity of DC is especially critical in diverse mucosal sites, where a delicate balance between tolerance and immunity is a desirable outcome [11]. In this regard, mucosal DC have now been described as a unique subset, whose phenotypic and functional characteristics reflect the specific local immune peculiarities of different tissues [12]. Few studies have shown that the development and functions of DC in the gut [7, 13] and the respiratory tract [8] are critically influenced by the soluble immune factors produced by sentinel cells including epithelial cells in the mucosa. However, much remains unknown about mechanisms that regulate the differentiation and functions of DC in the FRT.
Within the nonpregnant human endometrium, DC are mainly distributed in close proximity to endometrial glands [1, 14]. Further, endometrial DC express CD1a, CD11c, CD83, and HLA-DR, consistent with a myeloid lineage [15,16,17]. However, the cellular and molecular mechanisms that regulate the functions of endometrial DC are unknown. We have shown previously that primary UEC and ECC-1, a well-differentiated UEC line that is responsive to sex hormones [18], constitutively secrete a number of cytokines and chemokines, including IL-6, GM-CSF, G-CSF, TNF-α, MIF, CCL2, CCL4, CXCL8, and HBD1 and HBD2 [19,20,21,22]. Whereas there is a preferential secretion of these immune mediators into the apical compartment, significant quantities are also secreted basolaterally and may thus influence the differentiation and function of myeloid cells downstream of the epithelial layer [19,20,21]. To define the cell-cell interactions that regulate the functions of endometrial DC, we hypothesized that resident DC localized in the endometrium are modulated phenotypically and functionally by specific soluble immune mediators secreted by UEC.
The objectives of this study were to determine the effect of UEC secretions on the differentiation of DC from monocyte precursors and to determine the capacity of DC generated in the presence of UEC secretions to respond to proinflammatory stimulation with TLR ligands. Our study shows that UEC secretions inhibit the differentiation of DC and attenuate their response to TLR ligands. The results indicate that soluble mediators from epithelial cells condition DC to maintain a tolerogenic phenotype in the endometrium. This phenotype may be critical for optimal reproductive function of the endometrium and for the sustained presence of DC within the uterine environment during the menstrual cycle. Collectively, our findings suggest a novel mechanism for the regulation of adaptive and innate immunity within the FRT.
MATERIALS AND METHODS
Uterine tissues
Uterine tissue specimens were obtained from women undergoing hysterectomies at DHMC (Lebanon, NH, USA). All tissue specimens used in this study were distal to the sites of pathology and were ascertained to be unaffected by disease by a trained pathologist. Approval to use these tissues was obtained previously from the Committee for the Protection of Human Subjects (DHMC), and informed consent was obtained from the patients before surgery.
Isolation and culture of primary UEC
UEC were isolated as described previously [21]. Briefly, tissues were minced under sterile conditions into 1- to 2-mm fragments and subjected to digestion with a cocktail containing final concentrations of 3.4 mg/mL pancreatin (Invitrogen Life Technologies, Carlsbad, CA, USA), 0.1 mg/mL hyaluronidase (Worthington Biochemical Corp., Freehold, NJ, USA), 1.6 mg/mL collagenase (Worthington Biochemical Corp.), and 2 mg/mL D-glucose in 1× HBSS (Invitrogen Life Technologies). Following incubation in the enzyme cocktail for 1 h at 37°C, cells were dispersed sequentially through a 250-μm and a 40-μm mesh screen, washed, resuspended in complete DMEM/F12 medium without phenol red (Invitrogen Life Technologies), and supplemented with 20 mM HEPES, 2 mM L-glutamine (Invitrogen Life Technologies), 50 μg/mL primocin (InvivoGen, San Diego, CA, USA), and 10% heat-inactivated, defined FBS (Hyclone, Logan, UT, USA). Cells were then analyzed for cell number and viability. On average, isolated, primary UEC are cultured on inserts for 2 weeks to ensure tight junction formation, which typically occurs in 7–10 days [23]. Upon attainment of high TER, the apical and basolateral compartments were washed four times with complete DMEM/F12 medium. Cells with high TER were cultured further in complete DMEM/F12 medium for 24 h prior to the collection of apical and basolateral CM. The collected media were centrifuged for 5 min at 10,000 g and stored at −80°C. Basolateral CM harvested from polarized, primary UEC, after 24 h of culture, was used in all DC cultures. CM from primary UEC were tested and ascertained to be free of LPS contamination using the LAL (Lonza, Walkersville, MD, USA) with the lowest detection limit of 0.1 EU/mL.
Culture of UEC line (ECC-1)
ECC-1, a well-differentiated UEC line [18] (a gift from Dr. George Olt, Penn State College of Medicine, Milton S. Hershey Medical Center, State College, PA, USA), was cultured as described previously to establish cellular polarity with apical and basolateral compartments [19]. The formation of tight junctions by the epithelial cell monolayer was monitored routinely by measurement of TER [23]. Apical and basolateral CM were collected from polarized ECC-1 cells following 24 h culture in complete DMEM/F12 medium. The collected media (i.e., CM) was centrifuged for 5 min at 10,000 g and stored at −80°C. Basolateral CM harvested from polarized ECC-1 after 24 h of culture was used in all DC cultures. CM from ECC-1 were tested and ascertained to be free of LPS contamination using the LAL assay (Lonza).
Isolation of monocytes and preparation of MDDC
PBMC were obtained from residues of platelet pheresis as described previously [24]. Monocytes were subsequently enriched with Rosette Sep reagent (StemCell, Vancouver, BC, Canada). Blood specimens were from healthy adult males at the Dartmouth-Hitchcock Blood Donor Program. Immature DC were generated from monocytes in vitro with GM-CSF (50 ng/ml) and IL-4 (50 ng/ml; PeproTech, Rocky Hill, NJ, USA), as described previously [25]. Immature DC were differentiated for 7 days in the presence or absence of 24 h CM (1:1 dilution), obtained from ECC-1 or from primary UEC. Following 7 days of culture, immature DC were harvested, washed, and used subsequently for flow cytometry analyses. In some experiments, immature DC were stimulated with LPS (100 ng/mL, InvivoGen) or PIC (25 μg/mL, InvivoGen) for 48 h prior to flow cytometry analyses. Approval to use the samples from the platelet donors was obtained previously from the Committee for the Protection of Human Subjects (DHMC), and informed consent was obtained from the patients before sample collection.
Flow cytometry
Following 7 days of in vitro differentiation, surface staining of MDDC was performed with the following flourochrome-labeled mAb: CD1a (clone HI149), CD14 (clone 61D3), CD80 (2D10.4), CD86 (IT2.2), CD83 (HB15e), and HLA-DR (TU36) from eBioscience (San Diego, CA, USA) and CD163 (clone 215927) from R&D Systems (Minneapolis, MN, USA). Matched isotype controls for the antibodies were used to control for nonspecific binding. Following antibody staining, cells were washed with staining buffer and fixed with 2% methanol-free paraformaldehyde in 1× PBS (2% paraformaldehyde) and then analyzed subsequently on a FACSCalibur (Becton Dickinson, San Jose, CA, USA). Ad hoc analyses of the acquired FACS data were performed by CellQuest™ (Becton Dickinson).
Real-time RT-PCR
Real-time RT-PCR was performed with a two-step protocol as described previously [21]. Total RNA was isolated from cells using TRIzol reagent, according to the manufacturer’s recommendations (Invitrogen Life Technologies) and purified with RNeasy columns (Qiagen, Valencia, CA, USA). Further RNA purification was achieved with on-column DNase digestion using the RNase-free DNase set (Qiagen). For each specimen, 400 ng total RNA was reverse-transcribed using the iScript cDNA synthesis kit, according to the manufacturer’s recommendations (Bio-Rad, Hercules, CA, USA), in a 20-μl volume and resulting cDNA used as a template for Taqman real-time RT-PCR on an ABI 7700 Prism real-time PCR instrument (Applied Biosystems, Foster City, CA, USA). Relative expression levels of respective costimulatory molecule genes were performed with the following primer/probe sets: TLR2 (Hs00152932_ml), TLR3 (Hs00152933_ml), TLR4 (Hs00152939_ml), TLR5 (Hs00152825_ml), and IDO (Hs00158032_ml; Applied Biosystems). Amplification of human β-actin (4333762; Applied Biosystems) served as an endogenous control to normalize loading of cDNA samples. Data were compared by subtracting the β-actin CT value from the experimental gene CT for each sample and expressed as fold-change in mRNA expression between control and treatment groups determined by the 2–ΔΔct method [21].
Cytokine ELISA
The concentrations of IL-10, IL-12p70, and IL-8 in the culture supernatants of stimulated (LPS or PIC) and unstimulated DC were determined using commercial ELISA kits (R&D Systems) as per the manufacturer’s specifications.
MLR
MLR were performed using a modification of a protocol described previously [26]. Briefly, responder PBMC were cocultured with Con-DC, CM-DC, or TGF-β1 DC (DC generated in the presence of 10 ng/mL TGF-β1) in 96-well round-bottom plates in a total volume of 250 μl complete RPMI-1640 medium. Ratios of PBMC:DC ranged from 4:1 to 64:1 in triplicate wells. Controls included PBMC and DC cultured alone. Following 3 days of culture, wells were pulsed with 1 μCi [3H] thymidine (Dupont NEN, Boston, MA, USA) and harvested 8 h later onto unifilter 96 GF/C plates for assessment of thymidine incorporation by scintillation counting (Packard MicroSant NXT Counter, PerkinElmer, Waltham, MA, USA). After background correction, results are reported as mean ± sem cpm for thymidine incorporation.
Statistical analyses
Statistical analysis was performed using Mann-Whitney test (GraphPad Prism, Version 5.0, GraphPad Software, San Diego, CA, USA), assuming non-normal data distribution. A value of P < 0.05 was considered significant.
RESULTS
CM modulates the differentiation of DC on monocytes
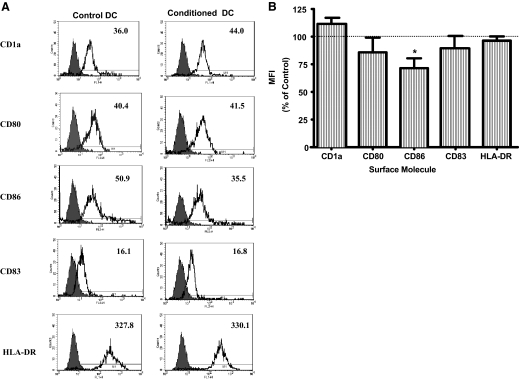
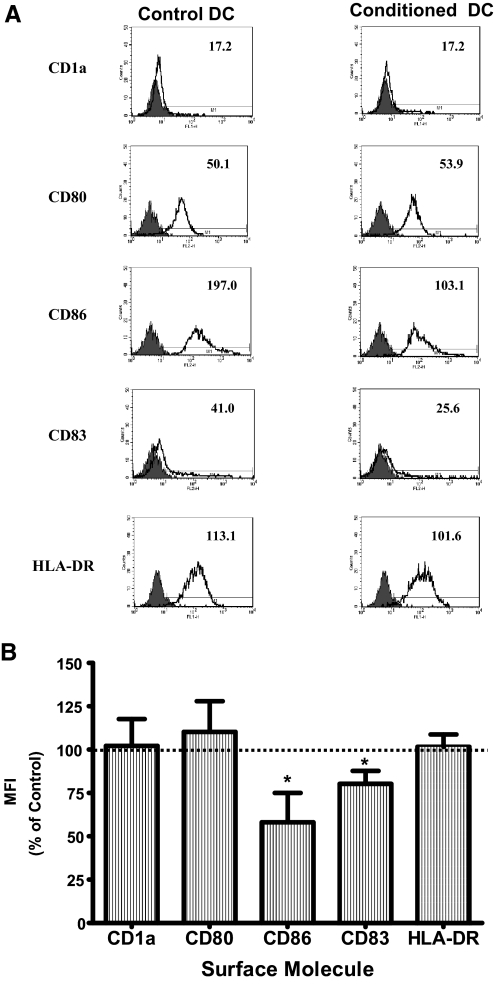
Monocytes can be differentiated in vitro into immature DC with IL-4 and GM-CSF using a well-established protocol [25]. The differentiation process results in the down-regulation of surface molecules (e.g., CD14), expressed specifically on monocytes, and the expression of surface molecules unique to immature DC (e.g., CD1a) [25]. We determined the effects of CM obtained from ECC-1 or primary UEC on the differentiation of DC from monocytes. ECC-1 CM decreased the expression of CD86 on immature DC (Fig. 1A). This effect was seen consistently with immature DC derived from monocytes obtained from multiple donors (P<0.05; n=5; Fig. 1B). Similarly, primary UEC CM decreased CD86 on immature DC and in addition, decreased CD83 expression (Fig. 2A). The changes exerted by primary UEC CM were reproduced consistently with DC from multiple donors in five independent experiments (P<0.05; Fig. 2B). In contrast, the expression of CD1a (Figs. 1 and 2), as well as CD14 and CD163 (data not shown), was not altered, demonstrating the specificity of the effects of CM on the differentiation of monocytes to immature DC. In all experiments, CM from ECC-1 and primary UEC were used at a 1:1 dilution with fresh complete RPMI medium to avoid nutritional deprivation to the cells during the differentiation process. These findings indicate that primary UEC secrete soluble mediators that selectively affect the differentiation of DC from monocyte precursors by lowering the expression of costimulatory molecules (CD83 and CD86).
Figure 1.
Effect of ECC-1 CM on the differentiation of DC. (A) Representative FACS histograms of DC differentiated in the presence (Conditioned DC) or absence (Control DC) of ECC-1 CM. ECC-1 were cultured in cell-inserts to confluence and functional polarization, as assessed by high TER. CM was then harvested from the basolateral compartment following 24 h of culture. Unfilled histograms are isotype control antibodies, and the filled histograms indicate specific staining for respective surface antigens. Numbers indicate MFI of a representative experiment (five different experiments). FL1/2-H, Fluorescence 1/2-height. (B) Summarized MFI data for specific antigens expressed by DC from five different donors. Horizontal dotted lines indicate MFI for the expression of surface molecules by Con-DC; values set at 100%. Results are expressed as percent MFI ratio = (MFI of CM-DC÷MFI of Con-DC×100) ± sem; *, P < 0.05.
Figure 2.
Effect of primary UEC CM on the differentiation of DC. (A) Representative FACS histograms of DC differentiated in the presence (Conditioned DC) or absence (Control DC) of primary UEC CM. Primary UEC CM were from epithelial cells isolated from endometrial tissue specimens. Purified epithelial cells were grown to confluence, and CM was harvested from the basolateral compartment following 24 h culture. Unfilled histograms are isotype control antibodies, and the filled histograms indicate specific staining for respective surface antigens. Numbers indicate MFI of a representative experiment (representative of five different experiments). (B) Averaged MFI data for specific antigens expressed by DC from five different donors. Horizontal dotted lines indicate MFI for the expression of surface molecules by Con-DC; values set at 100%. Results are expressed as percent MFI ratio = (MFI of CM-DC÷MFI of Con-DC×100) ± sem; *, P < 0.05.
Reduced responses of CM-DC to TLR-mediated maturation
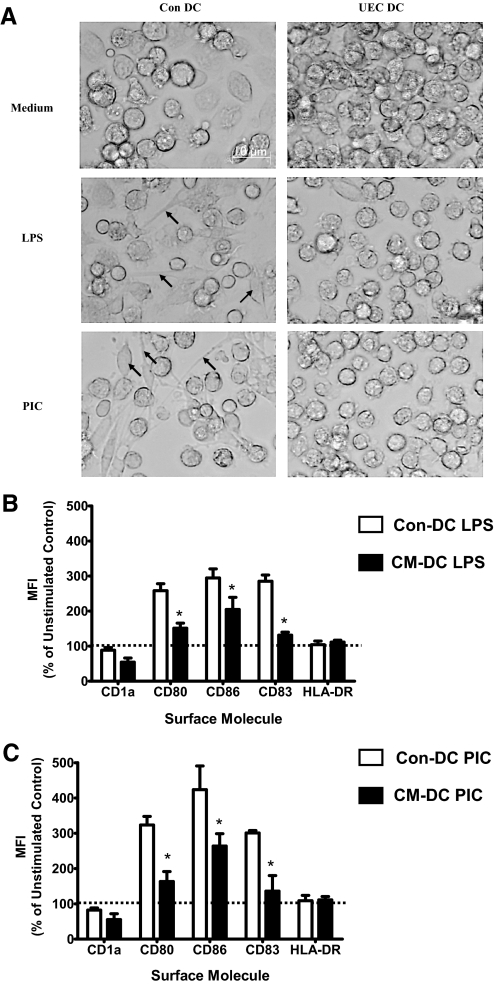
Upon stimulation with TLR ligands, immature DC derived from monocytes undergo a maturation process that is characterized by enhanced expression of MHC class II, costimulatory molecules, and expression of CD83 and a functional change from antigen capture to antigen presentation [27]. Having found that CM affects the differentiation of immature DC from monocytes, we sought to determine the capacity of CM-DC to mature in response to TLR ligands. For these experiments, DC were differentiated from monocytes as described above. Following 7 days of differentiation, immature Con-DC or CM-DC were stimulated with LPS (TLR4) or PIC (TLR3) for 48 h. Microscopic analyses showed that Con-DC responded to LPS or PIC stimulation by distinct morphological changes, assuming a spindle-shape appearance with extensive cytoplasmic extensions (Fig. 3A). These changes were absent in CM-DC stimulated with LPS or PIC. Instead, CM-DC retained a round shape that is characteristic of immature DC (Fig. 3A). FACS was performed to determine the effect of CM on the phenotype of immature and mature DC. Stimulation with LPS or PIC resulted in enhanced expression of CD80, CD86, and CD83 on mature DC when compared with immature, unstimulated DC. However, DC differentiated in the presence of UEC CM had significantly reduced surface expression of CD80, CD86, and CD83 following stimulation with LPS (Fig. 3B) or PIC (Fig. 3C; P<0.05) relative to Con-DC. Similar decreases in response to LPS and PIC stimulation were obtained with DC differentiated with ECC-1 CM (data not shown). These findings suggest that soluble mediators secreted by UEC alter the capacity of DC to respond to maturation stimuli and may thus serve to maintain DC in a less-mature state in the endometrium.
Figure 3.
Primary UEC CM suppresses TLR-induced maturation of DC. DC differentiated in the presence (CM-DC) or in the absence (Con-DC) of primary UEC CM were stimulated for 48 h with LPS (100 ng/mL) or PIC (25 μg/mL). (A) Morphological characteristics of DC. Con-DC or CM-DC (UEC DC) were unstimulated or stimulated for 48 h with LPS (100 ng/mL) or PIC (25 μg/mL). Images of live cells were obtained with bright field microscopy using a Ziess IM35 microscope (FLD-20/0.25 objective lens). The arrows show cytoplasmic processes and spindle-shaped appearances of LPS- or PIC-stimulated Con-DC. (B and C) Phenotypic changes of CM-DC following stimulation with LPS or PIC, respectively. The MFI of Con-DC or CM-DC stimulated with LPS or PIC is expressed as a percentage of the MFI of unstimulated Con-DC (value set at 100%, horizontal, dotted lines). *, P < 0.05, for activated CM-DC relative to activated Con-DC.
We explored the role of TGF-β as a candidate molecule that mediates the effects of UEC CM on the differentiation and maturation of DC. In separate HIV-1-related studies, we found that CM from ECC-1 and primary UEC decreased DC-specific ICAM-grabbing nonintegrin expression and simultaneously increased CXCR4 and CCR5 expression by DC (D. O. Ochiel et al., manuscript submitted for publication). Specific blockade of TGF-β activity using TGF-β type 1 receptor 1 inhibitor (SB-431542) [28] abolished the effects of CM on DC expression of the three HIV-1 receptors. Building on these observations, we investigated whether TGF-β played a significant role in regulating the expression of CD80, CD86, and CD83 on DC. Blockade of TGF-β activity had no effect on the observed, CM-induced down-regulation of CD80, CD86, and CD83 on DC (data not shown). These findings indicate that the effects of UEC CM on the expression of these costimulatory molecules by DC may not be directly dependent on TGF-β.
Conditioning of DC with UEC CM decreases TLR mRNA expression
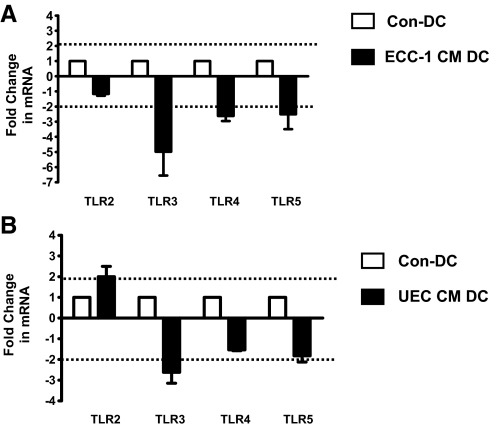
To further define the mechanisms underlying the reduced capacity of CM-DC to respond to TLR ligands, we analyzed the relative mRNA expression of TLR2, TLR3, TLR4, and TLR5 by quantitative Taqman RT-PCR. For these experiments, RNA was isolated from Con-DC or CM-DC following 3 days of differentiation in the presence of IL-4 and GM-CSF. Purified RNA was then reverse-transcribed into cDNA for subsequent analysis with Taqman RT-PCR with specific primers for the TLRs. The mean fold-change in mRNA expression of TLRs by CM-DC compared with Con-DC, normalized to endogenous β-actin gene expression, is shown. TLR mRNA expression was analyzed on DC derived from monocytes (n=5 donors) in the presence or absence of ECC-1 CM or CM from primary UEC. As shown in Figure 4, DC conditioned with ECC-1 CM (Fig. 4A) or primary UEC CM (Fig. 4B) lowered extensively the expression of TLR3 (five out of five donors) with a fold-change of two considered significant. In addition, ECC-1 CM decreased the expression of TLR4 and TLR5 significantly (five out of five donors; Fig. 4A). The expression of TLR2 was not altered significantly by ECC-1 CM but was enhanced modestly by primary UEC CM (Fig. 4B). These findings suggest that by regulating the pattern of DC TLR expression, soluble mediators secreted by UEC influence the capacity of DC in the endometrium to respond to pathogen-derived products.
Figure 4.
UEC CM modulates the expression of mRNA of TLRs by DC. DC were differentiated from monocytes in the presence or absence of ECC-1 CM (A) or in the presence or absence of primary UEC CM (B). Taqman real-time RT-PCR was performed to measure relative expression of TLRs, which was normalized to endogenous human β-actin gene. Data are presented as fold-change in gene expression by CM-DC relative to Con-DC (value set at 1). Horizontal, dotted lines indicate a twofold-change. Data are shown as mean fold-change in TLR mRNA for five donors ± sem.
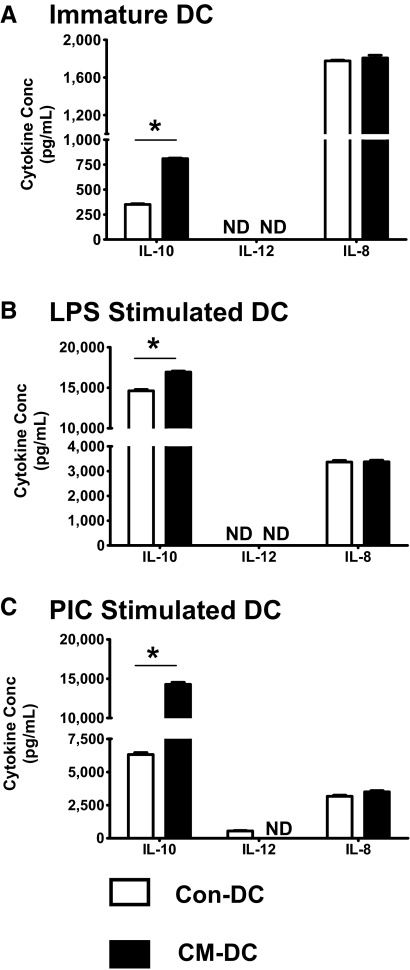
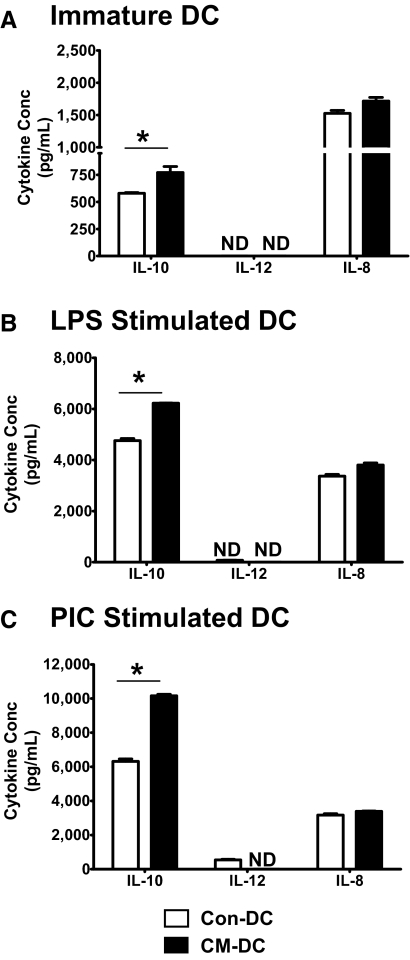
UEC CM alters the profile of cytokine secretion by DC
As a functional assessment of the effect of UEC CM on DC, we examined the profile of cytokine production by unstimulated or stimulated (LPS, PIC) DC using ELISA. For these experiments, three cytokines were analyzed, namely IL-12, a key cytokine for DC-mediated polarization of Th1 responses [29]; IL-10, an immunoregulatory cytokine that promotes Th2 responses [30]; and IL-8, a proinflammatory chemokine that promotes neutrophil chemotaxis [31]. Higher IL-10 production was observed consistently for DC generated with ECC-1 CM (Fig. 5) or with primary UEC CM (Fig. 6). This CM-dependent increase in IL-10 production was observed for immature DC (Figs. 5A and 6A) as well as for DC stimulated with LPS (Figs. 5B and 6B) or PIC (Figs. 5C and 6C). Analysis of IL-12 production showed that the cytokine was undetectable in the culture supernatants of immature, unstimulated Con-DC or CM-DC (Figs. 5A and 6A). However, PIC stimulation induced IL-12 secretion by Con-DC and not by CM-DC (Figs. 5C and 6C). With regards to IL-8, immature Con-DC and CM-DC secreted IL-8 constitutively (Figs. 5A and 6A), and secretion was enhanced further with LPS (Figs. 5B and 6B) or PIC (Figs. 5C and 6C) stimulation. There was, however, no significant effect of CM on the levels of IL-8 secreted by unstimulated Con-DC or following stimulation with LPS or PIC (Figs. 5 and 6).
Figure 5.
Effect of ECC-1 CM on cytokine production by DC. DC were differentiated in the presence (CM-DC) or absence (Con-DC) of primary UEC CM for 7 days. The cells were stimulated further for 48 h with LPS (100 ng/mL) or PIC (25 μg/mL). Secretion of IL-10, IL-12 p70, and IL-8 in the culture supernatants of unstimulated, immature DC (A) or DC stimulated with LPS (B) or PIC (C) was determined by ELISA. Samples and cytokine standards were analyzed in triplicate. ND indicates undetectable levels of cytokine. Presented is a representative of five different experiments. *, P < 0.05.
Figure 6.
Effect of primary UEC CM on cytokine production by DC. DC were differentiated in the presence (CM-DC) or absence (Con-DC) of primary UEC CM for 7 days. The cells were stimulated further for 48 h with LPS (100 ng/mL) or PIC (25 μg/mL). Secretion of IL-10, IL-12 p70, and IL-8 in the culture supernatants of unstimulated, immature DC (A) or DC stimulated with LPS (B) or PIC (C) was determined by ELISA. Samples and cytokine standards were analyzed in triplicate. Presented is a representative of five different experiments. *, P < 0.05.
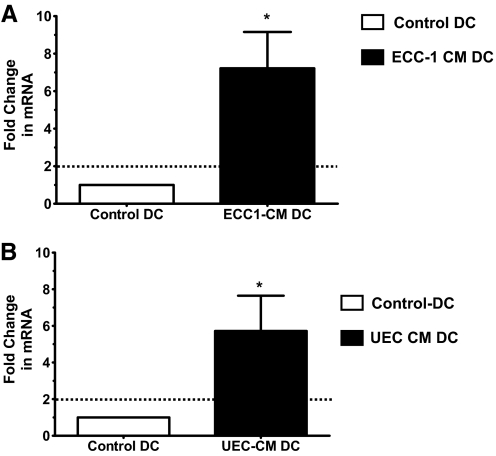
DC conditioned with UEC CM have enhanced expression of IDO
IDO is an enzyme that confers tolerogenic capacity to DC [32]. We measured the relative expression of IDO by real-time RT-PCR on RNA isolated from Con-DC or CM-DC. As shown in Figure 7, ECC-1 CM (Fig. 7A) and primary UEC CM (Fig. 7B) significantly enhanced the baseline expression of IDO by immature DC. IDO may be one of the mechanisms by which UEC-derived soluble factors confer a tolerogenic phenotype to DC in the endometrium.
Figure 7.
UEC CM increases the expression of IDO mRNA by DC. Taqman real-time RT-PCR was performed to measure the expression of IDO by DC following differentiation from monocytes in the presence or absence of ECC-1 CM (A) or primary UEC CM (B). Expression of IDO is normalized to an endogenous human β-actin gene. Data are presented as fold-change in gene expression by CM-DC relative to Con-DC (value set at 1). Horizontal, dotted lines indicates a twofold-change. Data are shown as mean fold-change in IDO mRNA for five donors ± sem; *, P < 0.05.
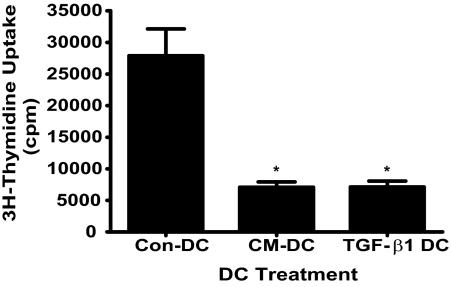
Reduced allogeneic responses with DC generated in the presence of primary UEC CM
To examine directly the effect of primary UEC CM on DC functions, we examined the ability of DC to induce allogeneic PBMC proliferation in a MLR assay. We found significantly reduced proliferative responses when PBMC were cocultured at a ratio of 4:1 with CM-DC relative to Con-DC (Fig. 8). In parallel experiments, we determined the effect of rTGF-β1 on the ability of DC to induce allogeneic PBMC proliferation. Similar to the results obtained with CM-DC, we found significantly reduced responses when PBMC were cocultured at a ratio of 4:1 with TGF-β1 DC as compared with Con-DC (Fig. 8). Similarly reduced, proliferative responses by CM-DC and TGF-β1 DC were obtained at a PBMC:DC ratio of 8:1 (data not shown).
Figure 8.
Reduced allogeneic, proliferative responses by CM-DC and TGF-β1 DC. Allogeneic PBMC (4×105) were cocultured in triplicate wells with 1 × 105 Con-DC, CM-DC, or TGF-β1 DC (i.e., 4:1 ratio) for 3 days, as described in Materials and Methods. Cells were pulsed with 1 μCi [3H] thymidine and harvested 8 h later onto unifilter 96 GF/C plates for assessment of thymidine incorporation by scintillation counting. Results are reported as mean cpm ± sem for thymidine incorporation. *, P < 0.05, compared with Con-DC.
DISCUSSION
To define the cellular interactions in the FRT, we have examined the effects of UEC on the differentiation and functions of DC. Our studies show that secretions from UEC (i.e., CM) affect the normal differentiation of DC derived from blood monocytes. When stimulated with LPS or PIC, CM-DC had an attenuated response to these TLR ligands, which was characterized by reduced expression of CD80, CD86, and CD83. In addition, LPS- or PIC-stimulated CM-DC had a skewed cytokine profile marked by enhanced IL-10 and reduced IL-12. Further, CM-DC reduced expression of the transcripts for TLR3, TLR4, and TLR5. In addition, CM-DC had a significantly elevated baseline expression of IDO mRNA, a tryptophan-depleting enzyme associated with immune tolerance. Lastly, CM-DC induced lower allogeneic PBMC responses relative to Con-DC. These findings suggest that UEC regulate immune responses in the endometrium by modulating the functions of DC, critical cellular determinants of the balance between immunity and tolerance.
The characteristics and functions of immature DC in the nonpregnant human endometrium are undefined. Numerous studies at other mucosal sites indicate that DC adopt unique phenotypic and functional characteristics as a consequence of the prevailing, local cytokine milieu [5, 12, 33,34,35]. We have reported previously that UEC secrete a number of soluble immune mediators [19,20,21,22]. In the present study, we hypothesized that instructive signals derived from UEC regulate the development and function of DC to enable a balance between immune protection and immune tolerance in the human endometrium. We report in this study that DC generated in the presence of secretions from ECC-1 or primary UEC show lower expression of the costimulatory molecule CD86. In addition, CM from primary UEC lowered CD83 expression on DC. Interestingly, the expression of CD1a, CD80, HLA-DR, CD14, and CD163 was not altered significantly on CM-DC relative to Con-DC, indicating specificity in the effect of CM on DC differentiation. Signals from costimulatory molecules are critical for optimal activation of T cell immune responses by DC during antigen presentation [36]. Several studies have reported that DC expressing lower costimulatory molecules promote tolerance in vivo (reviewed in ref. [37]). Therefore, our findings, showing reduced expression of the costimulatory molecule CD86 on DC following treatment with soluble factors secreted by UEC, suggest a potential mechanism by which epithelial cells regulate innate and adaptive immune responses in the endometrium.
DC derived from human monocytes express functional TLR1–5 [38]. Upon ligation of the TLRs with respective TLR ligands, DC undergo a process of maturation that is characterized by phenotypic alterations and production of various regulatory cytokines [27]. Maturation induced by TLR agonists alters the function of DC from antigen capturing to antigen presentation [27]. In the present study, upon stimulation with LPS (TLR4 ligand) or PIC (TLR3 ligand), CM-DC showed a decreased response to these ligands, which is manifested as lower CD80, CD86, and CD83 relative to Con-DC. The relatively lower expression of CD80, CD86, and CD83 suggests that UEC secretions maintain DC in a semi-mature, intermediate state. A semi-mature phenotype of DC has been described to promote immune tolerance [37, 39, 40]. Thus, by maintaining DC in a semi-mature state, soluble immune mediators secreted by UEC may confer functional plasticity to DC. This may facilitate the generation of immune responses to pathogens, while concomitantly maintaining a quiescent state under physiological conditions when immunity is not a desirable outcome, such as during implantation.
We found further that upon stimulation with LPS or PIC, CM-DC failed to display the characteristic morphologic features associated with maturation [41, 42]. The morphological changes induced by TLR ligands are thought to enhance the antigen capture, processing, and presentation by DC [42,43,44]. Our results showing lack of TLR-induced morphological changes in CM-DC suggest a mechanism, whereby soluble immune mediators in the secretions of polarized UEC inhibit specific steps in the maturation process of DC. Further studies should define the mechanisms by which UEC secretions influence cytoskelatal rearrangements in DC during TLR-mediated maturation.
A potential mechanism by which secretions produced by UEC could attenuate the responses to TLR ligands is by regulating the expression of the respective TLRs. In our study, CM from UEC selectively inhibited the expression of TLR3, TLR4, and TLR5 mRNA. Thus, the attenuated response to LPS and PIC by CM-DC may be a result of the reduced expression of TLR3 and TLR4, respectively. Others have shown that decreased TLR expression results in decreased capacity of DC to respond to cognate TLR ligands [45, 46]. Our studies extend these findings by showing that secreted products of polarized UEC decrease DC expression of mRNA encoding selected TLRs (TLR3, TLR4, and TLR5). This decrease in TLR expression may account for the observed decrease in the capacity of CM-DC to undergo maturation in response to the ligands for TLR3 (LPS) and TLR4 (PIC). The underlying molecular mechanisms by which UEC regulate the expression of TLRs on DC remain to be elucidated. Previous reports indicate that LC, a subset of DC associated with mucosal epithelia, have impaired expression of TLR2, TLR4, and TLR5 [45, 47]. This pattern of TLR expression may have functional relevance for LC given their exclusive presence in tissue sites colonized with commensal bacteria [48]. We found that primary UEC CM modestly increased DC expression of TLR2, and ECC-1 CM had no effect on its expression. It is unclear whether this increase is a result of a specific factor that is secreted by primary UEC and not by ECC-1. We have shown previously that primary UEC and ECC-1 generally secrete similar, soluble immune mediators in culture, including IL-6, GM-CSF, G-CSF, TNF-α, MIF, CCL2, CCL4, CXCL8, HBD1, and HBD2 [19,20,21,22]. Further studies are needed to identify the specific secreted molecules from UEC that regulate the pattern of expression of TLR on DC.
DC derived from monocytes typically respond to LPS or PIC stimulation by preferentially secreting IL-12 and very low IL-10 [49]. Although IL-12 primes T cell responses toward Th1 [29, 50], IL-10, on the other hand, is an immunoregulatory cytokine promoting Th2 responses [30]. We found that CM-DC responded to TLR-mediated activation by secreting higher amounts of IL-10 and decreased IL-12 significantly. By tilting the balance of cytokine production of maturing DC, secretions from UEC may skew immune responses in the endometrium toward Th2. These findings support the predominance of Th2 over Th1 responses in the endometrium described already [1].
To define further the mechanisms by which UEC modulate DC function, we analyzed the relative expression of IDO transcripts using RNA isolated from Con-DC and CM-DC. IDO is the rate-limiting enzyme in the catabolism of tryptophan via the kynerenine pathway [51]. The enzyme is induced during the differentiation of monocytes to DC [32, 52], and its activity correlates with reduced T cell responses [32]. This enzyme has been proposed as one of the mechanisms by which DC confer immune tolerance to T cells [32, 52,53,54]. Our findings indicate that CM-DC had higher baseline expression of IDO than Con-DC. Based on the ascribed role of IDO on immune tolerance, it suggests that in the human endometrium, soluble mediators constitutively produced by UEC create a milieu within which immune cells are primed to promote tolerogenic responses. Whether this is an explanation for the reduced CTL activity, observed previously by us during the secretory stage of the menstrual cycle, remains to be determined [55]. What is clear is that DC, under the control of UEC, have an increased potential for inactivating tryptophan, which may lead to decreased uterine CTL activity.
Our study has further provided direct evidence for regulation of DC functions by secretions produced by endometrial epithelial cells. In an in vitro allogeneic proliferation assay, we found that CM-DC were relatively less efficient at inducing PBMC proliferation as compared with Con-DC. Although the in vivo functions of DC conditioned with endometrial epithelia cell secretions remain to be determined, our findings suggest that specific factors produced by epithelial cells may lower the capacity of DC to induce T cell responses in the human endometrium.
The identity of soluble factor(s) in the UEC CM that mediate its effects on the functions of DC remains to be determined. We investigated the putative role of TGF-β as a candidate molecule that mediates the effects of UEC CM on DC functions. TGF-β is an immunoregulatory cytokine that influences the functions of immune cells including DC (reviewed in ref. [56]). TGF-β and its receptors are expressed by epithelial cells and stromal cells of the human endometrium [57,58,59]. We asked whether TGF-β played a significant role in the regulation of CD80, CD86, and CD83 on DC by UEC CM. We blocked TGF-β activity specifically in the CM with SB-431542 [28] and determined the expression of these surface molecules on DC generated in the presence or absence of UEC CM. Blockade of TGF-β activity had no effect on UEC regulation of CD80, CD86, and CD83. These findings indicate that the effects of UEC CM on these costimulatory molecules may not be directly dependent on TGF-β. Interestingly, we found that DC generated in the presence of TGF-β1 induced significantly lower allogeneic PMBC responses. Our findings are consistent with previously published work indicating that TGF-β inhibits the APC potency of DC [60]. Further studies are, however, needed to clearly define the molecular mechanisms by which soluble factors present in UEC CM influence the differentiation and functions of human DC.
In conclusion, we have shown multiple mechanisms by which UEC secretions modulate human DC. Significant changes induced by UEC include: decreased expression of CD86 and CD83 on immature DC, decreased maturation of DC in response to LPS and PIC, decreased expression of mRNA encoding TLR3, TLR4, and TLR5, increased secretion of IL-10, enhanced expression of mRNA for IDO, and reduced allogeneic proliferative responses. These characteristics are consistent with those described for tolerogenic DC. Our findings collectively suggest a model in which UEC skew DC immune responses in the endometrium toward tolerance by specifically influencing the functions of DC. Transient uterine immune tolerance in the nonpregnant woman is an essential outcome in the endometrium and most likely a prerequisite for successful fertilization and implantation. Our results suggest that the local mucosal microenvironment milieu, comprised of secretions from epithelial cells and other sentinel cells, is a critical determinant of the nature of immune response in distinct mucosal tissue sites.
AUTHORSHIP
D.O.O., M.G., J.V.F., P.M.G., and C.R.W. deigned research; D.O.O. and C.R.W. performed research; D.O.O., P.M.G., and C.R.W. analyzed data; and D.O.O., M.G., J.V.F., P.M.G., and C.R.W. wrote the manuscript.
ACKNOWLEDGMENTS
This work was supported by a National Institutes of Health grants (AI51877 and AI-071761 to C. R. W.). We thank all of the study participants. We are grateful to Richard M. Russoll and Zheng Shen for excellent technical support. We thank Dr. William R. Green, Dr. Wen Li, and Kathy A. Green (Department of Microbiology and Immunology, DHMC) for technical assistance with the MLR assay. We acknowledge support from Dr. Zbigniew M. Szczepiorkowski and Ms. Sue Braley of the DHMC Blood Donor Program. We also thank the Department of Pathology, the Obstetrics and Gynecology surgeons, O.R. nurses, and support personnel at DHMC.
DISCLOSURE
The authors have declared no conflicts of interest.
Footnotes
Abbreviations: CM=conditioned medium, CM-DC=conditioned dendritic cell(s), Con-DC=control dendritic cell(s), CT=cycle threshold, DC=dendritic cell(s), DHMC=Dartmouth-Hitchcock Medical Center, ECC-1=epithelial cell cluster 1, FRT=female reproductive tract, HBD1/2=human β-defensin 1/2, IDO=indoleamine 2,3-dioxygenase, LAL=Limulus amoebocyte lysate, LC=Langerhans cell(s), MDDC=monocyte-derived dendritic cell(s), MFI=mean fluorescent intensity, PIC=polyinosinic:polycytidylic acid, TER=transepithelial resistance, UEC=uterine epithelial cell(s)
References
- Kammerer U., von Wolff M., Markert U. R. Immunology of human endometrium. Immunobiology. 2004;209:569–574. doi: 10.1016/j.imbio.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Ardavin C. Dendritic cell heterogeneity: developmental plasticity and functional diversity. Semin Immunol. 2005;17:251–252. doi: 10.1016/j.smim.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Adams S., O'Neill D. W., Bhardwaj N. Recent advances in dendritic cell biology. J Clin Immunol. 2005;25:177–188. doi: 10.1007/s10875-005-4086-2. [DOI] [PubMed] [Google Scholar]
- Rossi M., Young J. W. Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J Immunol. 2005;175:1373–1381. doi: 10.4049/jimmunol.175.3.1373. [DOI] [PubMed] [Google Scholar]
- Von Garnier C., Filgueira L., Wikstrom M., Smith M., Thomas J. A., Strickland D. H., Holt P. G., Stumbles P. A. Anatomical location determines the distribution and function of dendritic cells and other APCs in the respiratory tract. J Immunol. 2005;175:1609–1618. doi: 10.4049/jimmunol.175.3.1609. [DOI] [PubMed] [Google Scholar]
- Belkaid Y., Oldenhove G. Tuning microenvironments: induction of regulatory T cells by dendritic cells. Immunity. 2008;29:362–371. doi: 10.1016/j.immuni.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler M., Ng C. Y., van Heel D. A., Lombardi G., Lechler R., Playford R. J., Ghosh S. Modulation of dendritic cell phenotype and function in an in vitro model of the intestinal epithelium. Eur J Immunol. 2006;36:864–874. doi: 10.1002/eji.200535497. [DOI] [PubMed] [Google Scholar]
- Mayer A. K., Bartz H., Fey F., Schmidt L. M., Dalpke A. H. Airway epithelial cells modify immune responses by inducing an anti-inflammatory microenvironment. Eur J Immunol. 2008;38:1689–1699. doi: 10.1002/eji.200737936. [DOI] [PubMed] [Google Scholar]
- Steinman R. M., Hawiger D., Nussenzweig M. C. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Morelli A. E., Thomson A. W. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–621. doi: 10.1038/nri2132. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Czerkinsky C. Mucosal immunity and vaccines. Nat Med. 2005;11:S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol. 2007;25:381–418. doi: 10.1146/annurev.immunol.25.022106.141634. [DOI] [PubMed] [Google Scholar]
- Rimoldi M., Chieppa M., Salucci V., Avogadri F., Sonzogni A., Sampietro G. M., Nespoli A., Viale G., Allavena P., Rescigno M. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- Kamat B. R., Isaacson P. G. The immunocytochemical distribution of leukocytic subpopulations in human endometrium. Am J Pathol. 1987;127:66–73. [PMC free article] [PubMed] [Google Scholar]
- Rieger L., Honig A., Sutterlin M., Kapp M., Dietl J., Ruck P., Kammerer U. Antigen-presenting cells in human endometrium during the menstrual cycle compared to early pregnancy. J Soc Gynecol Investig. 2004;11:488–493. doi: 10.1016/j.jsgi.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Ivanova E., Kyurkchiev D., Altankova I., Dimitrov J., Binakova E., Kyurkchiev S. CD83 monocyte-derived dendritic cells are present in human decidua and progesterone induces their differentiation in vitro. Am J Reprod Immunol. 2005;53:199–205. doi: 10.1111/j.1600-0897.2005.00266.x. [DOI] [PubMed] [Google Scholar]
- Schulke L., Manconi F., Markham R., Fraser I. S. Endometrial dendritic cell populations during the normal menstrual cycle. Hum Reprod. 2008;23:1574–1580. doi: 10.1093/humrep/den030. [DOI] [PubMed] [Google Scholar]
- Mo B., Vendrov A. E., Palomino W. A., DuPont B. R., Apparao K. B., Lessey B. A. ECC-1 cells: a well-differentiated steroid-responsive endometrial cell line with characteristics of luminal epithelium. Biol Reprod. 2006;75:387–394. doi: 10.1095/biolreprod.106.051870. [DOI] [PubMed] [Google Scholar]
- Schaefer T. M., Desouza K., Fahey J. V., Beagley K. W., Wira C. R. Toll-like receptor (TLR) expression and TLR-mediated cytokine/chemokine production by human uterine epithelial cells. Immunology. 2004;112:428–436. doi: 10.1111/j.1365-2567.2004.01898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahey J. V., Schaefer T. M., Channon J. Y., Wira C. R. Secretion of cytokines and chemokines by polarized human epithelial cells from the female reproductive tract. Hum Reprod. 2005;20:1439–1446. doi: 10.1093/humrep/deh806. [DOI] [PubMed] [Google Scholar]
- Schaefer T. M., Fahey J. V., Wright J. A., Wira C. R. Innate immunity in the human female reproductive tract: antiviral response of uterine epithelial cells to the TLR3 agonist poly(I:C) J Immunol. 2005;174:992–1002. doi: 10.4049/jimmunol.174.2.992. [DOI] [PubMed] [Google Scholar]
- Schaefer T. M., Fahey J. V., Wright J. A., Wira C. R. Migration inhibitory factor secretion by polarized uterine epithelial cells is enhanced in response to the TLR3 agonist poly (I:C) Am J Reprod Immunol. 2005;54:193–202. doi: 10.1111/j.1600-0897.2005.00298.x. [DOI] [PubMed] [Google Scholar]
- Richardson J. M., Kaushic C., Wira C. R. Polymeric immunoglobin (Ig) receptor production and IgA transcytosis in polarized primary cultures of mature rat uterine epithelial cells. Biol Reprod. 1995;53:488–498. doi: 10.1095/biolreprod53.3.488. [DOI] [PubMed] [Google Scholar]
- Dietz A. B., Bulur P. A., Emery R. L., Winters J. L., Epps D. E., Zubair A. C., Vuk-Pavlovic S. A novel source of viable peripheral blood mononuclear cells from leukoreduction system chambers. Transfusion. 2006;46:2083–2089. doi: 10.1111/j.1537-2995.2006.01033.x. [DOI] [PubMed] [Google Scholar]
- Sallusto F., Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor α. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhala R. H., Fahey J. V., Humphrey S. L., Edkins R. D., Stern J. E., Wira C. R. Regulation by human uterine cells of PBMC proliferation: influence of the phase of the menstrual cycle and menopause. J Reprod Immunol. 1998;40:25–45. doi: 10.1016/s0165-0378(98)00034-5. [DOI] [PubMed] [Google Scholar]
- Reis e Sousa C. Dendritic cells in a mature age. Nat Rev Immunol. 2006;6:476–483. doi: 10.1038/nri1845. [DOI] [PubMed] [Google Scholar]
- Inman G. J., Nicolas F. J., Callahan J. F., Harling J. D., Gaster L. M., Reith A. D., Laping N. J., Hill C. S. SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol Pharmacol. 2002;62:65–74. doi: 10.1124/mol.62.1.65. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- Couper K. N., Blount D. G., Riley E. M. IL-10: the master regulator of immunity to infection. J Immunol. 2008;180:5771–5777. doi: 10.4049/jimmunol.180.9.5771. [DOI] [PubMed] [Google Scholar]
- Baggiolini M., Dewald B., Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- Mellor A. L., Munn D. H. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- Akbari O., DeKruyff R. H., Umetsu D. T. Pulmonary dendritic cells producing IL-10 mediate tolerance induced by respiratory exposure to antigen. Nat Immunol. 2001;2:725–731. doi: 10.1038/90667. [DOI] [PubMed] [Google Scholar]
- Bilsborough J., Viney J. L. Gastrointestinal dendritic cells play a role in immunity, tolerance, and disease. Gastroenterology. 2004;127:300–309. doi: 10.1053/j.gastro.2004.01.028. [DOI] [PubMed] [Google Scholar]
- Iijima N., Thompson J. M., Iwasaki A. Dendritic cells and macrophages in the genitourinary tract. Mucosal Immunol. 2008;1:451–459. doi: 10.1038/mi.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroczek R. A., Mages H. W., Hutloff A. Emerging paradigms of T-cell co-stimulation. Curr Opin Immunol. 2004;16:321–327. doi: 10.1016/j.coi.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Lutz M. B., Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- Visintin A., Mazzoni A., Spitzer J. H., Wyllie D. H., Dower S. K., Segal D. M. Regulation of Toll-like receptors in human monocytes and dendritic cells. J Immunol. 2001;166:249–255. doi: 10.4049/jimmunol.166.1.249. [DOI] [PubMed] [Google Scholar]
- Braun D., Galibert L., Nakajima T., Saito H., Quang V. V., Rubio M., Sarfati M. Semimature stage: a checkpoint in a dendritic cell maturation program that allows for functional reversion after signal-regulatory protein-α ligation and maturation signals. J Immunol. 2006;177:8550–8559. doi: 10.4049/jimmunol.177.12.8550. [DOI] [PubMed] [Google Scholar]
- Rutella S., Danese S., Leone G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood. 2006;108:1435–1440. doi: 10.1182/blood-2006-03-006403. [DOI] [PubMed] [Google Scholar]
- Granucci F., Ferrero E., Foti M., Aggujaro D., Vettoretto K., Ricciardi-Castagnoli P. Early events in dendritic cell maturation induced by LPS. Microbes Infect. 1999;1:1079–1084. doi: 10.1016/s1286-4579(99)00209-9. [DOI] [PubMed] [Google Scholar]
- West M. A., Wallin R. P., Matthews S. P., Svensson H. G., Zaru R., Ljunggren H. G., Prescott A. R., Watts C. Enhanced dendritic cell antigen capture via Toll-like receptor-induced actin remodeling. Science. 2004;305:1153–1157. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- Watts C., Zaru R., Prescott A. R., Wallin R. P., West M. A. Proximal effects of Toll-like receptor activation in dendritic cells. Curr Opin Immunol. 2007;19:73–78. doi: 10.1016/j.coi.2006.11.014. [DOI] [PubMed] [Google Scholar]
- West M. A., Prescott A. R., Chan K. M., Zhou Z., Rose-John S., Scheller J., Watts C. TLR ligand-induced podosome disassembly in dendritic cells is ADAM17 dependent. J Cell Biol. 2008;182:993–1005. doi: 10.1083/jcb.200801022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi J., Watari E., Shinya E., Norose Y., Matsumoto M., Seya T., Sugita M., Kawana S., Takahashi H. Down-regulation of Toll-like receptor expression in monocyte-derived Langerhans cell-like cells: implications of low-responsiveness to bacterial components in the epidermal Langerhans cells. Biochem Biophys Res Commun. 2003;306:674–679. doi: 10.1016/s0006-291x(03)01022-2. [DOI] [PubMed] [Google Scholar]
- Flacher V., Bouschbacher M., Verronese E., Massacrier C., Sisirak V., Berthier-Vergnes O., de Saint-Vis B., Caux C., Dezutter-Dambuyant C., Lebecque S., Valladeau J. Human Langerhans cells express a specific TLR profile and differentially respond to viruses and Gram-positive bacteria. J Immunol. 2006;177:7959–7967. doi: 10.4049/jimmunol.177.11.7959. [DOI] [PubMed] [Google Scholar]
- Van der Aar A. M., Sylva-Steenland R. M., Bos J. D., Kapsenberg M. L., de Jong E. C., Teunissen M. B. Loss of TLR2, TLR4, and TLR5 on Langerhans cells abolishes bacterial recognition. J Immunol. 2007;178:1986–1990. doi: 10.4049/jimmunol.178.4.1986. [DOI] [PubMed] [Google Scholar]
- Merad M., Ginhoux F., Collin M. Origin, homeostasis and function of Langerhans cells and other Langerin-expressing dendritic cells. Nat Rev Immunol. 2008;8:935–947. doi: 10.1038/nri2455. [DOI] [PubMed] [Google Scholar]
- Re F., Strominger J. L. Heterogeneity of TLR-induced responses in dendritic cells: from innate to adaptive immunity. Immunobiology. 2004;209:191–198. doi: 10.1016/j.imbio.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Hilkens C. M., Kalinski P., de Boer M., Kapsenberg M. L. Human dendritic cells require exogenous interleukin-12-inducing factors to direct the development of naive T-helper cells toward the Th1 phenotype. Blood. 1997;90:1920–1926. [PubMed] [Google Scholar]
- Takikawa O., Kuroiwa T., Yamazaki F., Kido R. Mechanism of interferon-γ action Characterization of indoleamine 2,3-dioxygenase in cultured human cells induced by interferon-γ and evaluation of the enzyme-mediated tryptophan degradation in its anticellular activity. J Biol Chem. 1988;263:2041–2048. [PubMed] [Google Scholar]
- Lopez A. S., Alegre E., LeMaoult J., Carosella E., Gonzalez A. Regulatory role of tryptophan degradation pathway in HLA-G expression by human monocyte-derived dendritic cells. Mol Immunol. 2006;43:2151–2160. doi: 10.1016/j.molimm.2006.01.007. [DOI] [PubMed] [Google Scholar]
- Hwu P., Du M. X., Lapointe R., Do M., Taylor M. W., Young H. A. Indoleamine 2,3-dioxygenase production by human dendritic cells results in the inhibition of T cell proliferation. J Immunol. 2000;164:3596–3599. doi: 10.4049/jimmunol.164.7.3596. [DOI] [PubMed] [Google Scholar]
- Chung D. J., Rossi M., Romano E., Ghith J., Yuan J., Munn D. H., Young J. W. Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood. 2009;114:555–563. doi: 10.1182/blood-2008-11-191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White H. D., Crassi K. M., Givan A. L., Stern J. E., Gonzalez J. L., Memoli V. A., Green W. R., Wira C. R. CD3+ CD8+ CTL activity within the human female reproductive tract: influence of stage of the menstrual cycle and menopause. J Immunol. 1997;158:3017–3027. [PubMed] [Google Scholar]
- Li M. O., Wan Y. Y., Sanjabi S., Robertson A. K., Flavell R. A. Transforming growth factor-β regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- Chegini N., Zhao Y., Williams R. S., Flanders K. C. Human uterine tissue throughout the menstrual cycle expresses transforming growth factor-β 1 (TGF β 1), TGF β 2, TGF β 3, and TGF β type II receptor messenger ribonucleic acid and protein and contains [125I]TGF β 1-binding sites. Endocrinology. 1994;135:439–449. doi: 10.1210/endo.135.1.8013382. [DOI] [PubMed] [Google Scholar]
- Godkin J. D., Dore J. J. Transforming growth factor β and the endometrium. Rev Reprod. 1998;3:1–6. doi: 10.1530/ror.0.0030001. [DOI] [PubMed] [Google Scholar]
- Kim M. R., Park D. W., Lee J. H., Choi D. S., Hwang K. J., Ryu H. S., Min C. K. Progesterone-dependent release of transforming growth factor-β1 from epithelial cells enhances the endometrial decidualization by turning on the Smad signaling in stromal cells. Mol Hum Reprod. 2005;11:801–808. doi: 10.1093/molehr/gah240. [DOI] [PubMed] [Google Scholar]
- Lyakh L. A., Sanford M., Chekol S., Young H. A., Roberts A. B. TGF-β and vitamin D3 utilize distinct pathways to suppress IL-12 production and modulate rapid differentiation of human monocytes into CD83+ dendritic cells. J Immunol. 2005;174:2061–2070. doi: 10.4049/jimmunol.174.4.2061. [DOI] [PubMed] [Google Scholar]