Abstract
Thiopurines were examined for their ability to produce singlet oxygen (1O2) with UVA light. The target compounds were three thiopurine prodrugs, azathioprine (Aza), 6-mercaptopurine (6-MP) and 6-thioguanine (6-TG), and their S-methylated derivatives of 6-methylmercaptopurine (me6-MP) and 6-methylthioguanine (me6-TG). Our results showed that these thiopurines were efficient 1O2 sensitizers under UVA irradiation but rapidly lost their photoactivities for 1O2 production over time by a self-sensitized photooxidation of sulfur atoms in the presence of oxygen and UVA light. The initial quantum yields of 1O2 production were determined to be in the range of 0.30–0.6 in aqueous solutions. Substitution of a hydrogen atom with a nitroimidazole or methyl group at S decreased the efficacy of photosensitized 1O2 production as found for Aza, me6-MP and me6-TG. 1O2-induced formation of 8-oxo-7,8-dihydro-2’-dexyguanosine (8-oxodGuo) was assessed by incubation of 6-methylthiopurine/UVA-treated calf thymus DNA with human repair enzyme 8-oxodGuo DNA glycosylase (hOGG1), followed by apurinic (AP) site determination. Because more 8-oxodGuo was formed in Tris D2O than in Tris H2O, 1O2 is implicated as a key species in the reaction. These findings provided quantitative information on the photosensitization efficacy of thiopurines and to some extent revealed the correlations between photoactivity and phototoxicity.
Keywords: singlet oxygen, thiopurine, UVA, guanine
1. Introduction
Although thiopurine prodrugs, such as azathioprine (Aza), 6-mercaptopurine (6-MP) and 6-thioguanine (6-TG), have been widely used in the treatment of cancer and inflammatory conditions and in the therapy of organ transplant patients for five decades,[1, 2] the long-term use of thiopurines is frequently associated with malignancy, such as acute myeloid leukemia and skin cancer.[3–5] This adverse effect is known to be phototoxic, often manifested as a severe sunburn[6] and associated with thiopurine/UVA-initiated production of reactive oxygen species (ROS).[3, 7–9] However, it has been difficult to quantitatively correlate the photoactivity of thiopurines to oxidative DNA damage due to the limited information regarding their photosensitization efficacy.
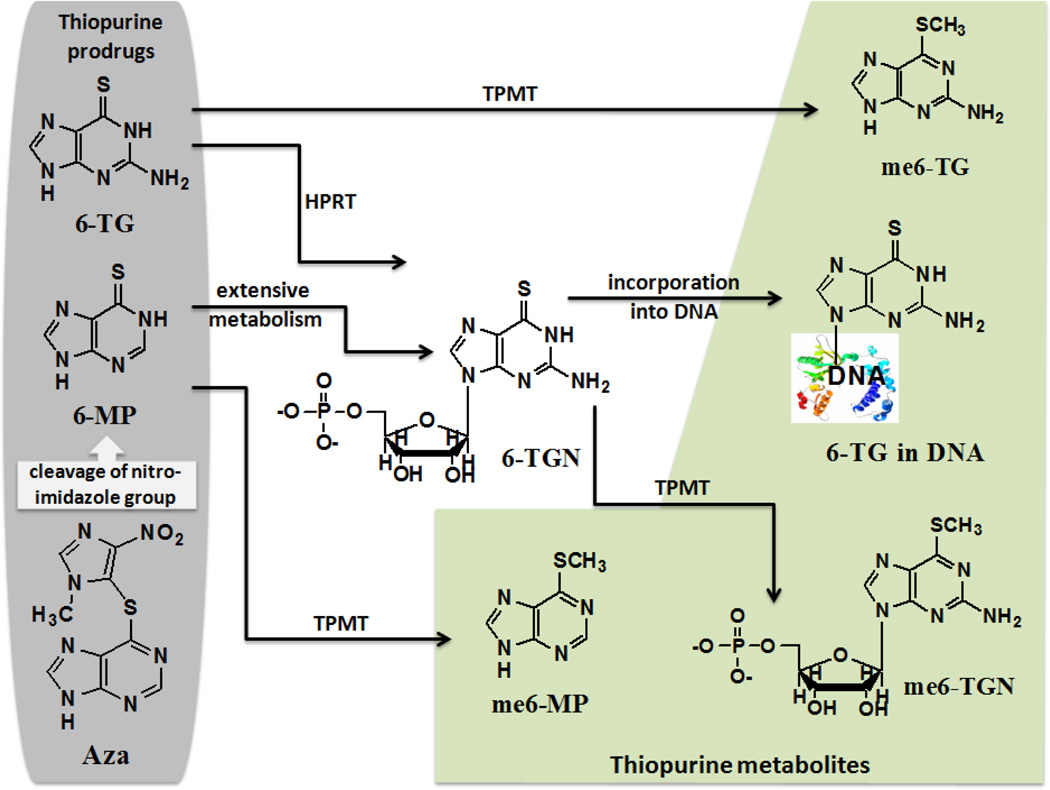
As prodrugs, Aza is cleaved to 6-MP, which in turn is metabolized to 6-thioguanine nucleotides (6-TGN) that can be incorporated into DNA of patients taking Aza.[8, 9] Thiopurines undergo enzymatic metabolism as well. One of the major pathways is initiated by thiopurine methyltransferase (TPMT), which converts 6-MP to 6-methylmercaptopurine (me6-MP) and 6-TG to 6-methylthioguanine (me6-TG). The structures and metabolism of these compounds are shown in Scheme 1. Unlike normal DNA bases, thiopurine DNA bases are strong UVA (315–400 nm, covering 90% of solar UV irradiation) chromophores. The less energetic UVA radiation can induces DNA damage through the absorption of light by sensitizers. A sensitizer may then react with DNA via electron or hydrogen abstraction to generate radicals (type I) or by energy transfer with oxygen (type II) to produce singlet oxygen (1O2). The oxidative damage of DNA by UVA radiation in cells and human skin has been reviewed,[10] indicating 1O2-initiated formation of 8-oxo-7,8-dihydro-2’-dexyguanosine (8-oxodGuo). It was reported that Aza-treated DNA contained 6-TG that was both the production source[11] and target site[12] of reactive oxygen species (ROS) including 1O2.[13] Cooke and co-workers demonstrated in vivo formation of 8-oxodGuo and alkali-labile sites in cells treated with biologically relevant doses of Aza and UVA, indicating the involvement of 1O2 in oxidative DNA damage.[14] Very recently we reported the direct observation of 1O2 production upon UVA irradiation of 6-thioguanines in aqueous solutions with quantum yield values ranging from 0.49 to 0.58.[15] Obviously, 6-TG as well as other thiopurine metabolites such as 6-methylthiopurines can be an endogenous source of ROS in a biological system under UVA irradiation. An abrupt increase in ROS could cause oxidative stress and produce mutagenic DNA lesions.[16, 17] Currently, the knowledge regarding the photosensitization efficacy of thiopurines is limited, which contrasts with the extensive studies of sulfide oxidation by 1O2[18, 19] and self-photosensitized oxidation of thioketones.[20, 21]
Scheme 1.
Structures and metabolism of thiopurine prodrugs. Azathioprine (Aza) can convert to 6-mercaptopurine (6-MP) by cleavage of nitroimidazole group. Thiopurine prodrugs undergo extensive metabolism to 6-thioguanine (6-TG) nucleotides (6-TGN). 6-TG is also directly converted to 6-TGN by hypoxanthine phosphoribosyltransferase (HPRT). 6-TGN becomes incorporated into DNA. Thiopurine methyltransferase (TPMT) can convert 6-MP to 6-methylmercaptopurine (me6-MP) and 6-TG to 6-methylthioguanine (me6-TG).
The fact that thiopurines may be efficient endogenous sources for 1O2 production in biological systems prompted us to determine systematically their photosensitization efficacy and the role of 1O2 in 6-methylthiopurine/UVA-mediated oxidative DNA damage. Our results showed that three thiopurine prodrugs (Aza, 6-MP and 6-TG) and two S-methyl derivatives (me6-MP and me6-TG) were efficient 1O2 sensitizers in vitro under UVA irradiation but rapidly lost their photoactivities for 1O2 production over time by a self-sensitized photooxidation of sulfur atoms in the presence of oxygen and UVA light. DNA damage was quantified by using an aldehyde reactive probe (ARP, N’-aminooxymethylcarbonylhydrazino-D-biotin) that reacted with aldehyde groups present in the open ring form of apurinic or apyrimidinic (AP) sites. 1O2-associated guanine oxidation was identified by incubation of treated DNA samples with 8-oxodGuo DNA glycosylase (hOGG1) prior to AP site determination. hOGG1 acts both as an N-glycosylase and an AP-lyase to release oxidative guanines from DNA to generate AP sites. We demonstrated that under our experimental conditions 6-methylthiopurine/UVA-induced DNA guanine base oxidation was mainly through type II (1O2) mechanism. Those findings provide a primary basis for the quantitative understanding of phototoxicity of thiopurines in a biological system.
2. Experimental Section
Materials and Instrumentation
Reagents and solvents were obtained commercially and used without further purification. meso-tetra(4-carboxylphenyl) porphine (TCPP) was purchased from Frontier Scientific, Inc. [2-(dicyclohexyl phosphino) ethyl]trimethyl ammonium chloride (> 98%) was purchased from Strem Chemicals Inc. Azathioprine (Aza), 6-mercaptopurine (6-MP), 6-thioguanine (6-TG), S-methylmercaptopurine (me6-MP), S-methylthioguanine (me6-TG), sodium hydroxide, sodium azide (NaN3), deuterium acetonitrile-d3 (CD3CN, 99.8% of D), deuterium oxide (D2O, 99% of D), calf thymus DNA (D1501) and Tris(hydroxymethyl)aminomethine (> 99.8%), were purchased from Sigma-Aldrich. Colorimetric Assay Kits for DNA Damage Quantification were purchased from Oxford Biomedical Research (Produc No. FR 09) or Dojindo (Product Code: DK02-12). The 8-oxodGuo DNA glycosylase (hOGG1, 1600 units/mL) was purchased from New England Biolabs Inc. Deionized water was obtained from a Nanopure Water System (Barnsted System, USA). A Q-switched Nd:YAG laser with pulse duration of 3–4 ns and a maximum energy of 7 mJ at 355 nm (Polaris II, Electro Scientific Industries, Inc.), equipped with a liquid N2-cooled germanium photodetector (Applied Detector Corporation) was used for time-resolved 1O2 luminescence measurements. Steady-state photooxidation was conducted in oxygen-saturated solution using a 150 W Xenon lamp (6255 Xenon lamp housed in 66907 Arc Lamp Source, Newport Oriel Instruments) equipped with an IR blocking filter (59042, Newport Oriel Instruments) and a monochromator with primary wavelength region 450–2000 nm (77250 1/8 m Monochromator and 77305 Grating, Newport Oriel Instruments), where the intensities in UVA range is below 15 W. A BioMate 3 UV-Vis spectrophotometer (Thermo Scientific) and a Cary 300 UV-Vis spectrophotometer (Varian, Inc.) were used for the measurements of absorbance and spectra. The determination of photooxidation products was performed using either a 300 MHz Bruker Spectrospin FT-NMR or a Varian Vnmrs 500 MHz NMR. All of the measurements were carried out at ambient temperature. Samples were protected from light when not being irradiated. Thermo Labsystems Multiskan Ascent 354 from Labrecyclers Inc. was used for absorbance measurements for the microplate colorimetric assay. The production of 1O2 was tested in CD3CN and pH 10 NaOH/D2O solutions. The measurements of absorption spectra and the oxidative DNA damage analyses were carried out in TE (50 mM Tris-HCl, 1 mM EDTA, pH 7.4) D2O or H2O buffer solutions. The stock solutions of 0.10 M H2O2, 0.10 M FeCl2·4H2O and 10K Units of SOD were prepared in TE buffer. DNA samples were also prepared using TE buffer at a final concentration of 0.10 mg/mL for oxidative DNA damage assays.
Direct observation of 1O2 upon 355 nm irradiation of thiopurines
Kinetics of 1O2 phosphorescence was monitored at 1270 nm, as previously described.[15, 22] Thiopurines were dissolved in either CD3CN or pH 10 NaOH/D2O solutions under dark to avoid light-induced oxidation. The absorbance of the samples was controlled to be in the range of 0.1–0.4 at an excitation wavelength of 355 nm, depending on the solubility of each thiopurine compound. First-order kinetic fitting of 1O2 decay was calculated using Origin 6.1 program. 1O2 decay curves were corrected from control experiments by using the same but N2-saturated sample for pH 10 NaOH/D2O solutions or air-saturated sample in the presence of 1.5 mM NaN3 for CD3CN solutions. Data points of the initial ~ 5 µs were not used due to electronic interference signals from the detector.
ΦΔ measurement
ΦΔ was determined in O2-saturated pH 10 NaOH/D2O solutions on a relative basis by steady-state trapping experiment using TCPP as a reference (ΦΔ = 0.53 in weak alkaline solutions),[23] as previously reported.[15] A water-soluble phosphine, [2-(dicyclohexylphosphino)ethyl]trimethylammonium chloride, was used as an 1O2 trap for both thiopurine and TCPP samples. The OD readings of thiopurines and TCPP at an excitation wavelength of 350 nm were matched. The ΦΔ values of thiopurines were calculated based on the comparison of phosphine oxidation yields by thiopurines to those by TCPP, a reference sensitizer with known ΦΔ.[23] An internal standard was not needed in determining the percent yield of phosphine oxides because the identical 1O2 trapping conditions were applied to both thiopurines and TCPP samples. Control experiments in the dark and in the absence of thiopurines were also conducted to correct for any phosphine oxidation by heat or by ground-state oxygen molecules (Figure S1 in Supporting Information). Thiopurines lose their photoactivities rapidly upon UVA irradiation in the presence of oxygen molecules. A 20 minute-irradiation time led to a complete inhibition of thiopurine photoactivity while the photosensitization efficacy of TCPP was stable with irradiation time. The ΦΔ measurements were based on the phosphine oxidation in a 20-minute-irradiation period. Taking this into consideration, the initial ΦΔ values from thiopurines were approximated by multiplication of an empirical factor of 2 (eq. 2). ΦΔ, thiopurine and ΦΔ, TCPP in eq. 2 are the ΦΔ from thiopurine and TCPP, respectively; and %phosphine oxide by thiopurine and %phosphine oxide by TCPP are the conversion yields of phosphine oxidation in the presence of thiopurine and TCPP, respectively.
 |
(1) |
| (2) |
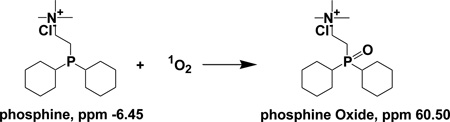
A brief description of trapping experiments is as the following. A mixture of 3.00 mL of 3.0–5.0 mM phosphine and 0.05–0.10 mM thiopurine with OD readings of 0.1–1.0 at a wavelength of 350 nm was added into a 1-cm quartz cuvette and irradiated using UVA light of 350 nm for 20 minutes followed by an immediate measurement of phosphine oxidation by 31P NMR using a delay time of 3 seconds between pulses.[23] 1O2 photooxidation of phosphine trap leads to the formation of a sole product of phosphine oxide (eq. 1). The peaks at δ −6.45 (s, 1P) and δ 60.50 (s, 1P) represent phosphine and phosphine oxide, respectively. The percent yields of phosphine oxide were controlled below 20% and calculated by comparison of the integrated 31P NMR peaks of phosphine with those of phosphine oxide. The same trapping conditions were applied to the reference sensitizer TCPP. Control experiments in the dark and in the absence of thiopurines were also conducted to correct for any phosphine oxidation by heat or by ground-state oxygen molecules.
Preparation of 6-methylthiopurine/UVA-treated DNA samples
6-methylthiopurine/UVA-treated DNA samples were prepared to test 1O2-induced DNA damage, the absorbance of 6-methylthiopurines including me6-TG and me6-MP was controlled between 0.6 and 0.7 at a wavelength of 320 nm. 6-methylthiopurines and 1.00 mL of 0.10 mg/mL calf thymus DNA were irradiated at a wavelength of 320 nm in O2-saturated TE/D2O (50 mM Tris-HCl, 1 mM EDTA, pH 7.4) or TE/H2O buffer solutions for 20 minutes, followed by incubation with hOGG1 and quantification of the number of AP sites generated in DNA.
Incubation with hOGG1 to quantify 1O2-induced guanine oxidation
To identify 1O2-induced oxidative DNA damage, treated DNA samples prepared above were incubated with hOGG1 prior to the quantification of AP sites using manufacture’s protocol (New England Biolabs Inc). Briefly, a 7.70 µL reaction containing 5.00 µL of 0.10 mg/mL DNA, 0.70 µL NEBuffer (10×) and 2.00 µL of hOGG1 (3.2 units) was incubated at 37 °C for 16 hours to release oxidized guanines from double stranded DNA to generate AP sites. Next, 10.00 µL of ARP (aldehyde reactive probe) was added to the mixture and incubated at 37 °C for 1 hour. The purification of ARP-labeled genomic solutions was performed using either ethanol precipitation or DNA filtration tubes.
Quantification of AP sites using a Colorimetric Assay
The biotin-avidin-peroxidase assays were performed according to the manufacturer’s protocols (Dojindo or Oxford Biomedical Research). Briefly, an aliquot of the diluted ARP-labeled DNA (60.00 µL) was added with 100 µL DNA binding solution to each well of a microwell plate. The samples were incubated overnight at room temperature. After the solution was discarded, the microplate was washed five times with diluted washing buffer. An aliquot of freshly diluted streptavidin-HRP solution (100–150 µL depending on the protocol used) was added and the microplate was placed on a plate shaker at 100 rpm for 1 hour. After the excess streptavidin-HRP solution was discarded, the plate was washed five times with diluted wash buffer. An aliquot of substrate solution (100.00 µL) was added and incubated for 1 hour at 37 °C for color development. The absorbance was taken at 650 nm on a microplate spectrophotometer. The number of AP sites in samples was quantified by comparing the standard ARP-tagged DNA conjugates with 0–40 ARP/105 bp DNA standard.
3. Results
3.1. Spectroscopic properties of thiopurine drugs
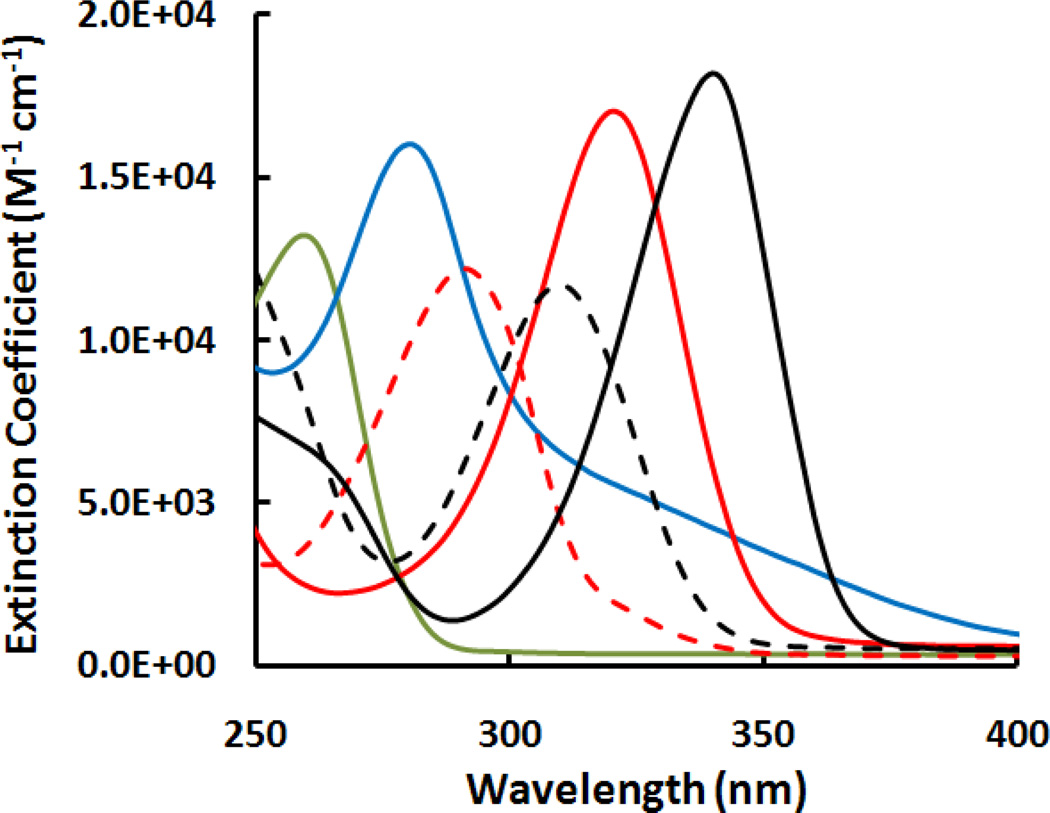
The absorption spectra of thiopurines were collected pH 7.4 TE buffer. Figure 1 shows the changes in extinction coefficients. Unlike normal DNA bases (e.g., adenine in Figure 1) which absorb little at wavelength longer than 300 nm, thiopurines absorb in the UVA region although their absorption maximum are often in the UVB. The extinction coefficient at maximum wavelengths for the thiopurines studied here were determined (Table 1). Our data are comparable to those from the literature, e.g., 1.34×104 M−1 cm−1 for adenine at 261 nm,[24] 1.73×104 M−1 cm−1 at 280.5 nm for Aza and 2.07×104 M−1 cm−1 at 321.6 nm for 6-MP in phosphate buffer at pH 7.4.[25]
Figure 1.
Extinction coefficient spectra measured at ambient temperature in 50 mM TE buffer (pH 7.4) solutions for adenine (green solid line), Aza (blue solid line), 6-MP (red solid line), me6-MP (red dot line), 6-TG (black solid line) and me6-TG (black dot line)
Table 1.
Wavelength maximum (λmax) and extinction coefficient maximum (εmax at λmax) determined from electronic absorption spectra of adenine and thiopurines at ambient temperature in pH 10 NaOH solutions.
| Compound | λmax, nm | εmax, M−1 cm−1 |
|---|---|---|
| Adenine | 260.0 | 1.3×104 |
| Aza | 274.0 | 1.5×104 |
| Me6-MP | 292.0 | 1.2×104 |
| Me6-TG | 310.0 | 1.2×104 |
| 6-MP | 321.0 | 1.7×104 |
| 6-TG | 340.0 | 2.1×104[15] |
3.2. Production of 1O2 upon UVA irradiation of thiopurines
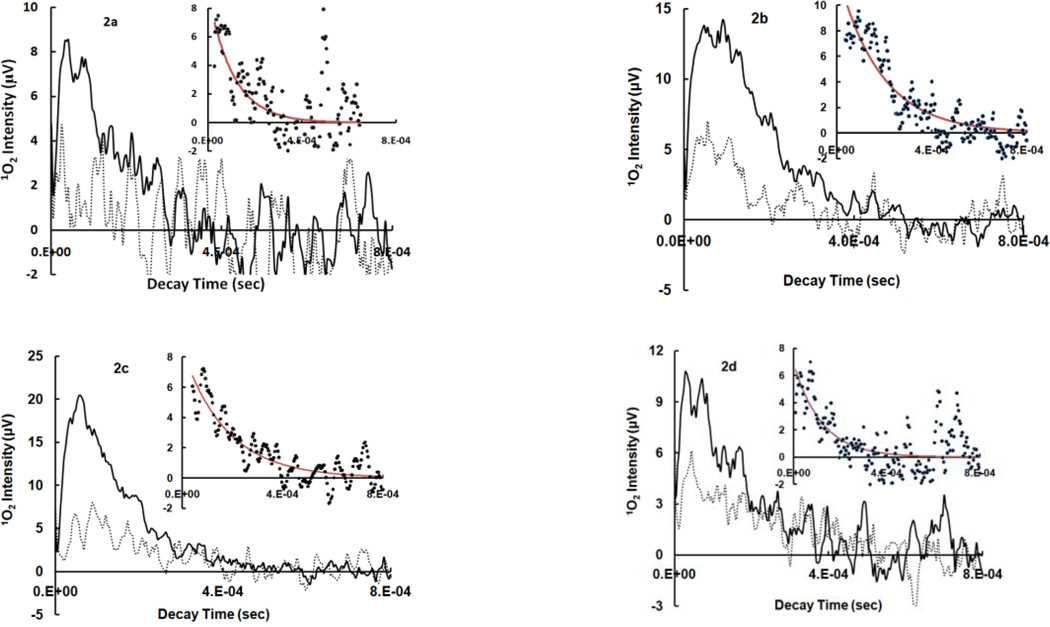
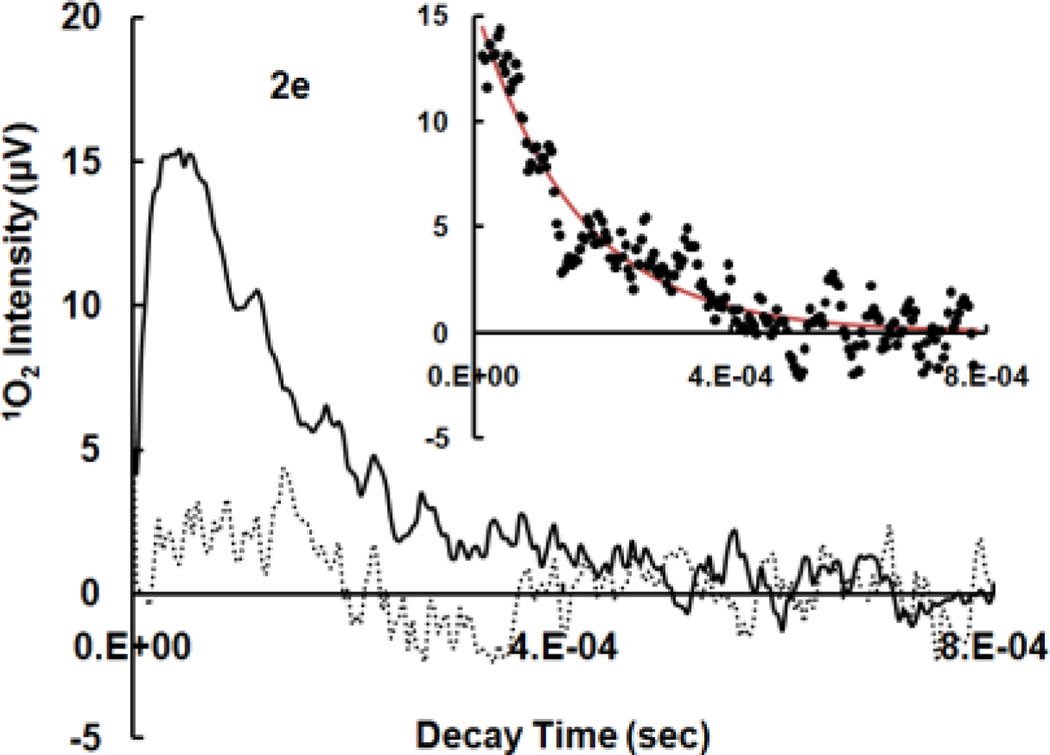
Thiopurines were examined for their ability to produce 1O2 by time-resolved laser and steady-state trapping experiments. In general, 1O2 detection at 1270 nm can be challenging due to the weak luminescence signal. Variations such as quenching, limited solubility, aggregation, formation of particles and electronic interference from the detector may adversely affect the measurement. Thiopurines are fairly soluble in polar organic solvents such as CH3CN and aqueous solutions at basic pH due to the possible deprotonation of thiols or amines. The pKa values for thiopurine drugs were reported to be in the range of 7.7–8.5.[26, 27] We have shown that upon UVA irradiation of thioguanines, no remarkable difference in ΦΔ was observed in pH 7.4 TE/D2O buffer and pH 10 NaOH/D2O solutions although thiol deprotonation may occur at pH 10.[15] Kinetics of 1O2 luminescence as well as trapping experiments were therefore conducted in either air-saturated CD3CN or pH 10 NaOH/D2O solutions for accurate results. The data in Figure 2 were assigned to 1O2 phosphorescence because both kinetics and intensities of 1O2 signals were sensitive to the concentrations of NaN3 in the solution. NaN3 is a well-known efficient 1O2 quencher that physically reacts with 1O2 at a rate constant of 5.0×108 M−1 s−1 in water.[28] Figures 2a–2e indicate that azide ions quench not only the lifetime of 1O2 but also the initial intensity of 1O2 luminescence. A decrease in initial 1O2 intensity could be attributed to the reactions of azide ions with excited triplet states of a sensitizer, although the quenching of triplet states might be far less efficient than that of 1O2.[29, 30] For comparison, the quenching rate constants by NaN3 are 1.3×107 M−1 s−1 for triplet states of aluminum tetrasulphonated phthalocyanine and 4.4×108 M−1 s−1 for 1O2.[30] The kinetic decay was exponential with observed 1st-order solvent deactivation rate constants of 1O2 (kd) in CD3CN equal to 9.3×103 s−1 for Aza, 5.5×103 s−1 for 6-MP, 5.6×103 s−1 for 6-TG,[15] 8.2×103 s−1 for me6-MP and 9.3×103 s−1 for me6-TG, which are comparable to literature values ranging from 2.3×103 s−1[31] to 9.3×103 s−1[32] in CD3CN. The kinetic simulation is shown in the insertions of Figures 2a–2e. For accurate calculations, decay traces were corrected for the background from other rapid events synchronized with laser pulses such as electronic interference from the detector, by using the same sample but in the presence of NaN3 as a control. The production of 1O2 was further confirmed by steady-state trapping experiments using a phosphine, [2-(dicyclohexylphosphino)ethyl]trimethylammonium chloride, as an 1O2 trap (see ΦΔ measurements).
Figure 2.
Time-resolved 1O2 phosphorescence recorded at 1270 nm upon pulsed-irradiation of thiopurines at 355 nm. 2a–2e: 1O2 decay in air-saturated CD3CN solutions in the absence (solid line) and presence of 1.5 mM NaN3 (dot line), insertion: 1st-order kinetic fitting of 1O2 decay after correction with the same sample but in the presence of NaN3 as a control, dots: experimental data and red line: theoretical simulation. Each decay curve is an average of data points obtained from 2–5 laser pulses. 2a: Aza at OD355 nm = 0.21, 2b: 6-MP at OD355 nm = 0.19, 2c: 6-TG at OD355 nm = 0.19, 2d: me6-MP at OD355 nm = 0.15, 2e: me6-TG at OD355 nm = 0.07
3.3. ΦΔ upon UVA irradiation of thiopurine drugs
ΦΔ was determined according to previously established method using TCPP as a reference[15] and a water-soluble phosphine, [2-(dicyclohexylphosphino)ethyl]trimethylammonium chloride as a 1O2 acceptor. Phosphine oxidation was monitored by 31P NMR and calculated by comparison of the integrated 31P NMR peaks of phosphine with those of phosphine oxides.[23] The ΦΔ values from thiopurine compounds were determined after irradiation for 20 minutes at an excitation wavelength of 350 nm. The phosphine oxidation was insignificant after 20 minutes irradiation due to the rapid deactivation of thiopurine photoactivity. Examples of NMR spectra are shown in Supporting Information. The values of ΦΔ were calculated according to eq. 2 and summarized in Table 2. ΦΔ values for all of the thiopurines studied in this work fall in the range of 0.3 to 0.6.
Table 2.
ΦΔ obtained upon UVA irradiation of thiopurines
| Compound | λirradiation, nm | ΦΔ |
|---|---|---|
| Aza | 350 | 0.30 ± 0.03 |
| 6-MP | 350 | 0.52 ± 0.05 |
| 6-TG | 334 | 0.58 ± 0.08[15] |
| me6-MP | 350 | 0.36 ± 0.05 |
| me6-TG | 350 | 0.46 ± 0.05 |
3.4. Oxidation of guanines and formation of AP Sites in calf thymus DNA
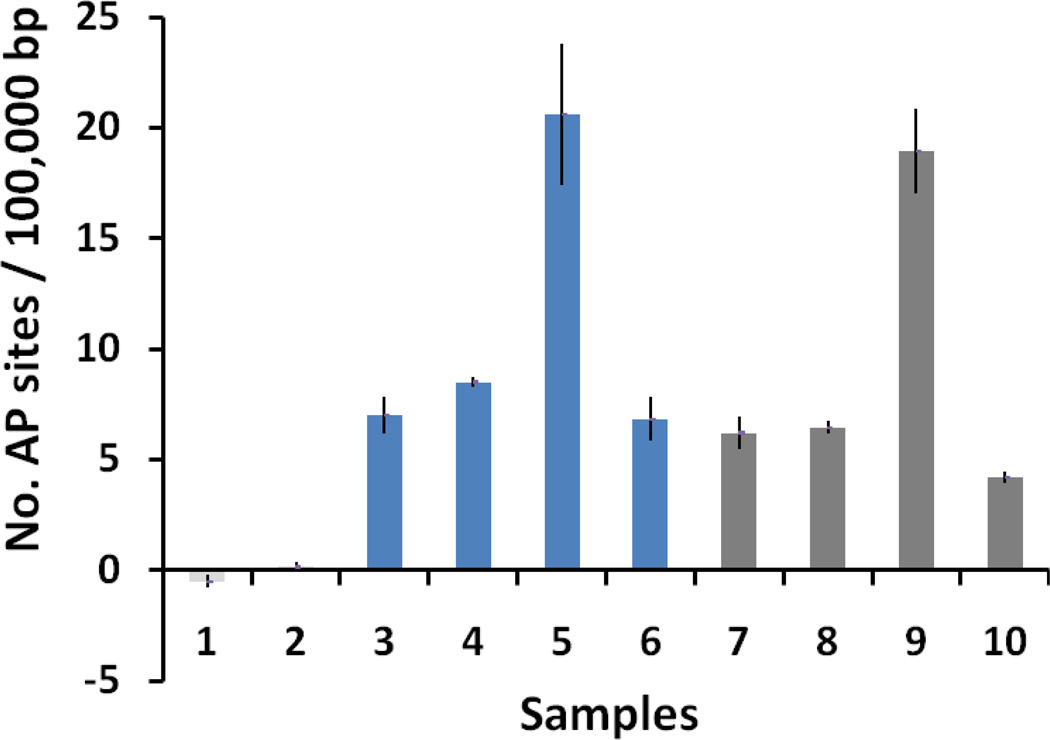
Studies have shown that 1O2 can react with guanine nucleobase to form 8-oxodGuo.[16, 33–37] The hOGG1, an 8-oxodGuo DNA glycosylase that acts both as a N-glycosylase and an AP-lyase, was employed to release oxidative guanines from double stranded DNA to generate the AP sites. The AP-lyase activity cleaves 3′ to the AP site leaving a 5′ phosphate and a 3′-phospho-α, β-unsaturated aldehyde.[38] The dependence of AP site formation on 1O2 was studied in both H2O and D2O of 50 mM TE buffer (pH 7.4) solutions where 1O2 has different lifetimes, e.g., 67 µs in D2O[39] and 4 µs in H2O.[40] Examples are shown in Figure 3. There was a 3–4 fold higher generation of AP sites for me6-TG in D2O as compared to me6-TG in H2O (columns 5 and 6 in Figure 3). Similar results were obtained for me6-MP with a higher generation of AP sites for me6-MP in D2O as compared to me6-MP in H2O (columns 9 and 10 in Figure 3). Control experiments for DNA/me6-TG and DNA/me6-MP without hOGG1 incubation showed similar results for the direct generation of AP sites for D2O (columns 3 for me6-TG and 7 for me6-MP in Figure 3) and H2O (columns 4 for me6-TG and 8 for me6-MP in Figure 3), which was likely due to strand breakage directly induced by ROS.[41] Our results are in agreement with literature reports showing 1O2 reaction with DNA could form strand breaks at guanine residues.[10, 42] No oxidative DNA damage was observed from the control DNA samples in the absence of 6-methylthiopurines (Columns 1 and 2 in Figure 3). These data clearly indicated that 1O2 was the dominate species in 6-methylthiopurine/UVA-induced guanine oxidation.
Figure 3.
The effect of D2O and H2O on AP site formation upon UVA (~10 W lamp) irradiation of 0.10 mg/mL DNA in the presence of 6-methylthiopurines. The data represent the mean of three to six repeating experiments in D2O or H2O of 50 mM TE buffer (pH 7.4) solutions. 1. DNA in D2O, 2. DNA in H2O, 3. DNA and me6-TG (0.05 mM) in D2O, 4. DNA and me6-TG (0.05 mM) in H2O, 5. DNA and me6-TG in D2O prior incubation with hOGG1, 6. DNA and me6-TG (0.05 mM) in H2O prior incubation with hOGG1, 7. DNA and me6-MP (0.15 mM) in D2O, 8. DNA and me6-MP (0.15 mM) in H2O, 9. DNA and me6-MP (0.15 mM) in D2O prior incubation with hOGG1, 10. DNA and me6-MP (0.15 mM) in H2O prior incubation with hOGG1.
4. Discussion
4.1. 1O2 production and detection after UVA irradiation of thiopurines
It was identified as early as 1960s that both UV- and x-ray-sensitivity of E. coli was enhanced when an appreciable proportion of normal DNA bases were substituted by thioguanines.[43] In 1980s, Moore and his coworkers demonstrated that free 6-MP and Aza were photodynamically active and underwent light-induced reactions that required molecular oxygen.[44, 45] Their observations on the formation of triplet states and reactions with the quenchers of ROS suggested the formation of 1O2 as well as O2.− from these compounds. It was only recently acknowledged that 1O2 was a major risk factor for skin cancer for patients treated with Aza.[14, 46, 47] We report herein the direct observation of 1O2 luminescence at 1270 nm upon UVA irradiation of thiopurines (Figure 2). Thiopurines are strong UVA chromophores with extinction coefficients up to 104 M−1 cm−1, as shown in Figure 1 and Table 1. The general mechanisms of thiopurine-induced 1O2 photosensitization are presented in eq. 3 and eq. 4. Upon UVA irradiation, thiopurines are activated to the excited singlet state (1Thiopurine) which undergoes an intersystem crossing (ISC) to the excited triplet state (3Thiopurine) (eq. 3). The triplet energy of thiopurines is quenched efficiently by ground state oxygen (3O2) to generate 1O2 (type II, eq. 4). Triplet thiopurines may also lose energy via electron transfer to 3O2 to produce O2.− or other ROS. This process is normally favored in the presence of electron donors (e− donor, eq. 5).
| (3) |
| (4) |
| (5) |
1O2 luminescence at 1270 nm was observed after UVA irradiation of thiopurines in air-saturated CD3CN or in pH 10 NaOH/D2O solutions. The efficient quenching of signals by NaN3 (Figures 2a–2e) indicated the formation of 1O2, although the data is murky in some cases (e.g., Aza in Figure 2a) due to the weak 1O2 emission. The rapid inhibition of thiopurine photoactivity in the presence of oxygen molecules and light was due to the efficient quenching of 1O2 by thiopurine itself, resulting in the oxidation of sulfur atoms. Thiopurines belong to the analogue of sulfides that are readily oxidized by 1O2[18] at a magnitude of rate constants 106–107 M−1 s−1.[48] The formation of 1O2 is also supported by steady-state trapping experiments using a phosphine as an 1O2 acceptor (see discussion below). Our observation confirmed that thiopurines were both the production sources and target sites of 1O2.
4.2. ΦΔ as a measure of photosensitization efficacy for thiopurines in vitro
ΦΔ is an important measure of photosensitization efficacy and usually determined on a relative basis that requires a reference. It is difficult to quantify the photosensitization ability for thiopurines using time-resolved 1O2 measurement because of the weak emission signals and the rapid deactivation of photoactivity. ΦΔ reported in this paper were determined according to previously reported method by steady-state photolysis using a phosphine as a 1O2 trap.[15] The bimolecular removal rate constants of 1O2 by both sulfides [48] and phosphines [49] are at the same magnitude of 106–107 M−1 s−1. Under our experimental conditions, the concentrations of phosphine (3–5 mM) were controlled considerably higher than those of thiopurines (0.05–0.10 mM) to ensure that the majority of 1O2 would react with the phosphine trap, while neglecting the quenching of 1O2 by thiopurines. Phosphadioxirane is an intermediate as well as a powerful oxidant in the reaction of ortho-substituted arylphosphine with 1O2.[50] A high concentration of phosphine would also ensure that intermolecular reactions occurred only between phosphadioxirane (if there is any) and phosphines but not thiopurines. The percent yields of phosphine oxides were controlled below 20% to assure an efficient/steady trapping condition for all of the thiopurines as well as the TCPP reference.
Many of the biological molecules such as dinucleotide,[51] purine and pyrimidine bases,[52, 53] were reported to be able to act as 1O2 sensitizers. Unlike these biomolecules that contain normal DNA bases, sulfur atoms in thiopurines have two lone pair electrons and can be oxidized to sulfinate and sulfonate. The oxidation of sulfur atoms leads to the inhibition of thiopurine photoactivity. ΦΔ were calculated by comparing the conversion yield of phosphine oxides induced by thiopurine/UVA to that induced by a reference TCPP sensitizer. The ΦΔ values obtained were in the range of 0.3–0.6 for all five thiopurines (Table 2), which was comparable to the literature values of some other thiocarbonyl compounds, e.g., 0.63 for 6-azauracil in CH3CN upon UVB irradiation[54] and 0.50 for 4-thiothymidine in CH3CN upon UVA irradiation.[55] Our data indicated that 6-TG (ΦΔ = 0.58)[15] and 6-MP (ΦΔ= 0.52) were the most efficient 1O2 sensitizers among the thiopurines studied in this paper. Since the thiopurine ring is the reactive site for 1O2 production, the frequency of collisions that result in the formation of 1O2 is related to the probability that an 3O2 molecule makes physical contact with the reactive site in its excited triplet state. The obviously larger steric effect of the nitroimidazole group in Aza (scheme 1) could result in reduced collision frequencies and subsequently, a lower ΦΔ when compared to those from other thiopurines. Similar explanations might also, at least partially, apply to the lower ΦΔ values from me6-MP and me6-TG than those from 6-MP and 6-TG, respectively. These findings provide a quantitative basis for understanding of molecular events in thiopurine/UVA-initiated DNA damage. Our results were supported by the triplet excitation nature of thiol-DNA or thiol-RNA bases as well. The photosensitized production of 1O2 is actually the quenching process of a sensitizer’s triplet state by ground oxygen. To some extent the formation yield of the triplet state can be a measure of 1O2 production. In general, the formation yields of the triplet state in thiocarbonyl compounds was found to be very efficient, e.g., 0.9 for thiouracils in H2O[56], 0.99 for 6-thiopurine in THF,[57] 0.7[58] and 0.6[59] for 4-thiouridine in CH3CN, an unity for 4-thiothymidine in an ionic liquid,[60] and 0.8 for thioguanosine in aqueous solution[61], and was quenched by 3O2 at a diffusing rate constant of 6.8×109 M−1 s−1.[62] These measurements are in line with our ΦΔ value of 0.58 for 6-TG.[15]
4.3. Thiopurine/UVA-induced oxidative DNA damage
Oxidative reactions within DNA commonly result in base modification. Among the four DNA bases, guanine is the most susceptible to various oxidants, with one of the major products being 8-oxodGuo[33–37] as well as others such as spiroiminodihydantoin from the reaction of guanine with 1O2 were identified.[10, 33, 63, 64] Studies by Foote’s group on an organic-soluble guanosine derivative, 2’,3’,5’-O-(tert-butyldimethylsilyl)guanosine, led to the conclusion that an unstable endoperoxide was the primary adduct produced via the [4+2] cycloaddition of 1O2 with the imidazole ring.[36, 65, 66] The chemical reaction rate constants (kR) for all nucleosides but guanosine derivatives were too low to be accurately determined.[67] The kR and total quenching rate constants (kT) for the guanosine derivatives were measured to be (4.8±0.5) × 104 M−1 s−1 and (3.0±0.2) × 106 M−1 s−1 in 1,1,2-trichlorotrifluoroethane, respectively, while kT for all other nucleoside derivatives were in the range of 6×103 to 6×104 M−1 s−1. These data indicated that guanine was the only reactive DNA base toward 1O2. Further research showed that 1O2 reacting with guanosine or deoxyguanosine part of nucleotides did not, by itself, cause DNA cleavage.[68] These results implied that the quantification of 1O2-induced DNA damage should involve the identification of guanine oxidation. Incubation of damaged DNA with various repair enzymes is therefore needed to recognize the specific base lesions and generated strand breaks.[69–72]
The histochemical and immunohistochemical methods have been reviewed to localize ROS-induced damage in tissues and cells.[73] Studies concluded that several kinds of human cancer tissues such as lung,[74] renal,[75] and colorectal carcinoma[76, 77] showed the higher levels of DNA oxidation compared to their nontumorous counterparts, based on 8-oxodGuo determination that might be induced among hydroxyl radical, 1O2 or photodynamic action[73, 78]. ˙OH is non-selective oxidant. The two main decomposition pathways of guanine moiety involve initial addition of ˙OH at C4 (~60%) and C8 (~25%).[79] The involvement of 1O2 in 8-oxodGuo formation upon UVA irradiation of 6-methylthiopurines was examined by performing the reactions in 50 mM TE (pH 7.4)/D2O buffer. The lifetime of 1O2 is ~ 17 times longer in D2O than in H2O. Consequently, enhanced guanine oxidation should occur in D2O if 1O2 was involved in the reactions. This was indeed the case. The generation of 8-oxodGuo was examined based on AP site formation from DNA/6-methylthiopurines incubated with hOGG1. The AP sites were tagged with biotin residues and quantified using a biotin-avidin-peroxidase assay. UVA irradiation of DNA in the presence of thiopurines produced 3–4 fold more AP sites in 50 mM pH 7.4 Tris/D2O than in 50 mM pH 7.4 Tris/H2O (Figure 3). These results suggest that 1O2 is the primary intermediate in 6-methylthiopurine/UVA-induced production of ROS and is responsible for the production of 8-oxodGuo. 6-methylthiopurines exist as the major metabolites of thiopurine chemotherapy in a biological system. When these photoactive compounds occur in DNA, they tend to react close to their site of formation because ROS are highly unstable, thus inducing efficient biological damage. 6-methylthiopurine/UVA-initiated guanine oxidation confirmed the efficient production of 1O2 and clarified the selective role of 1O2 in oxidative DNA damage. It should be noted that the UVA/thiopurine-induced photosensitized formation of triplet states and ROS may be largely affected by their binding to DNA. The oxidation of DNA guanine bases in cells treated with Aza/UVA has been observed.[14] A comparative study of 6-TG incorporated into DNA and its free counterpart would reveal the effect of DNA helix on photosensitization. Apparently, this is a research area that requires further investigation.
It is important to note that various factors may affect the behavior of thiopurine drugs in vitro or in a biological system. Persulfoxide is the primary intermediate in the reactions between 1O2 and sulfides.[18] This weakly bound species can behave as an oxidant to undergo various inter- and intra-molecular reactions, and has a tendency to convert to secondary intermediates or transfer an oxygen atom to a nucleophilic trap. The involvement of subsequent reactions between the persulfoxides and DNA is yet unknown. Moreover, levels of thiopurines in vivo can be kept constantly in reduced forms in the presence of proper reductases. The possible regulation of thiopurine DNA bases in vivo may provide a constant photoactivity site. 8-oxodGuo is readily subjected to further oxidation as well, which has become a point of interest.[64, 80] Except for 8-oxodGuo, 1O2 also reacts with DNA to form strand breaks.[42] Type I pathway may become efficient especially in the presence of electron donors. These factors should be taken into considerations in elucidation of thiopurine/UVA-induced biological damage.
5. Conclusion
In conclusion, by using photochemical techniques, 1O2 luminescence at 1270 nm was observed directly upon UVA irradiation of thiopurine prodrugs and their S-methylated metabolites. The photoactivity of these compounds toward UVA light and molecular oxygen was systematically evaluated by ΦΔ in vitro. Our results show that thiopurines are efficient 1O2 sensitizers with the initial ΦΔ values ranging from 0.3 to 0.6. S-methylation somewhat inhibits photosensitized production of 1O2. Methylthiopurine/UVA-associated formation of 8-oxodGuo gave rise to the most oxidative DNA damage, subsequently indicating 1O2 as the major ROS. The specific pathways through which thiopurine levels are regulated in vivo and transformed into products by light require further investigations. Our data provides primary basis for a better understanding of thiopurine photoactivity and its correlation to phototoxicity in a biological system.
Highlights.
Photosensitized singlet oxygen production was directly observed upon UVA irradiation of thiopurines.
Thiopurines possess high quantum yields of singlet oxygen production.
Substitution of a hydrogen atom with methyl group at sulfur position decreases the efficacy of singlet oxygen production.
Oxidative DNA damage by thiopurines induces guanine base oxidation.
Phototoxicity of thiopurines via type II energy transfer mechanism was quantitatively confirmed.
Supplementary Material
Acknowledgement
We thank the support from National Science Foundation (NSF-PREM DMR-0611539). This work was also partially supported by National Institutes of Health (NIH-RCMI G12RR013459 and NIH-RTRN U54RR022762) and NSF HRD-1008708. Any opinions, findings, and conclusions or recommendations are those of the authors and do not reflect the views of NSF or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary data: Supplementary data of 31P NMR spectra for ΦΔ measurements can be found at doi:.
References
- 1.Aarbakke J, Janka-Schaub G, Elion GB. Thiopurine biology and pharmacology. Trends in Pharmacological Sciences. 1997;18:3–8. doi: 10.1016/s0165-6147(96)01007-3. [DOI] [PubMed] [Google Scholar]
- 2.Relling MV, Dervieux T. Pharmacogenetics and cancer therapy. Nature Reviews Cancer. 2001;1:99–108. doi: 10.1038/35101056. [DOI] [PubMed] [Google Scholar]
- 3.Karran P. Thiopurines, DNA damage, DNA repair and therapy-related cancer. Br. Med. Bull. 2006;79–80:153–170. doi: 10.1093/bmb/ldl020. [DOI] [PubMed] [Google Scholar]
- 4.Penn I. Tumour incidence in human allograft recipients. Transpl. Proc. 1979;XI:1047–1051. [PubMed] [Google Scholar]
- 5.Kinlen LJ, Sheil AGR, Peto J, Doll R. Collaborative United Kingdom-Australian study of cancer in patients treated with immunosuppressive drugs. Br. Med. J. 1979;8:1461–1466. doi: 10.1136/bmj.2.6203.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murphy CJ, Gole AM, Stone JW, Sisco PN, Alkilany AM, Goldsmith EC, Baxter SC. Gold Nanoparticles in Biology: Beyond Toxicity to Cellular Imaging. Acc. Chem. Res. 2008;41:1721–1730. doi: 10.1021/ar800035u. [DOI] [PubMed] [Google Scholar]
- 7.Coulthard SA, Hogarth LA. Old Drugs-Current Perspectives. Current Pharmacogenomics. 2004;2:163–173. [Google Scholar]
- 8.Warren DJ, Andersen A, Slordal L. Quantitation of 6-thioguanine residues in peripheral blood leukocyte DNA obtained from patients receiving 6-mercaptopurine-based maintenance therapy. Cancer Res. 1995;55:1670–1674. [PubMed] [Google Scholar]
- 9.Cuffari C, Seidmen EG, Latour S, Theoret Y. Quantitation of 6-thioguanine in peripheral blood leukocyte DNA in Crohn’s disease patients on maintenance 6-mercaptopurine therapy. Can J Physiol Pharmacol. 1996;74:580–585. [PubMed] [Google Scholar]
- 10.Cadet J, Douki T. Oxidatively Generated Damage to DNA by UVA Radiation in Cells and Human Skin. Journal of Investigative Dermatology. 2011;131:1005–1007. doi: 10.1038/jid.2011.51. [DOI] [PubMed] [Google Scholar]
- 11.Brem R, Li F, Karran P. Reactive oxygen species generated by thiopurine UVA cause irreparable transcription-blocking DNA lesions. Nucleic Acids Research. 2009;37:1951–1961. doi: 10.1093/nar/gkp070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daehn I, Karran P. Immune Effector Cells Produce Lethal DNA Damage in Cells Treated with a Thiopurine. Cancer Res. 2009;69:2393–2399. doi: 10.1158/0008-5472.CAN-08-4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Jeffs G, Ren X, O’Donovan P, Montaner B, Perrett CM, Karran P, Xu Y-Z. Novel DNA lesions generated by the interaction between therapeutic thiopurines and UVA light. DNA Repair. 2007;6:344–354. doi: 10.1016/j.dnarep.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Cooke MS, Duarte TL, Cooper D, Chen J, Nandagopal S, Evans MD. Combination of azathioprine and UVA irradiation is a major source of cellular 8-oxo-7,8-dihydro-2’-deoxyguanosine. DNA Repair. 2008;7:1982–1989. doi: 10.1016/j.dnarep.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Zhu X, Smith J, Haygood MT, Gao R. Direct Observation and Quantitative Characterization of Singlet Oxygen in Aqueous Solution upon UVA Excitation of 6-Thioguanines. J. Phys. Chem. B. 2011;115:1889–1894. doi: 10.1021/jp109590t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cadet J, Douki T, Gasparutto D, Ravanat JL. Oxidative damage to DNA: formation, measurement and biochemical features. Mutat. Res. 2003;531:5–23. doi: 10.1016/j.mrfmmm.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Barnes DE, Lindahl T. Repair and Genetic Consequences of Endogenous DNA Base Damage in Mammalian Cells. Annu. Rev. Genet. 2004;38:445–476. doi: 10.1146/annurev.genet.38.072902.092448. [DOI] [PubMed] [Google Scholar]
- 18.Clennan EL. Persulfoxide: Key Intermediate in Reactions of Singlet Oxygen with Sulfides. Acc. Chem. Res. 2001;34:875–884. doi: 10.1021/ar0100879. [DOI] [PubMed] [Google Scholar]
- 19.Liang JJ, Gu CL, Kacher ML, Foote CS. Chemistry of Singlet Oxygen. 45. Mechanism of the Photooxidation of Sulfides. J. Am. Chem. Soc. 1989;111:4717–4721. [Google Scholar]
- 20.Jayaraj N, Maddipatla MVSN, Prabhakar R, Jockusch S, Turro NJ, Ramamurthy V. Closed Nanocontainer Enables Thioketones to Phosphoresce at Room Temperature in Aqueous Solution. J. Phys. Chem. B. 2010;114:14320–14328. doi: 10.1021/jp911698s. [DOI] [PubMed] [Google Scholar]
- 21.Ramnath N, Ramesh V, Ramamurthy V. Photochemical Oxidation of Thioketones: Steric and Electronic Aspects. J. Org. Chem. 1983;48:214–222. [Google Scholar]
- 22.Li W, Gandra N, Courtney SN, Gao R. Singlet Oxygen Production upon Two-Photon Excitation of TiO2 in Chloroform. ChemPhysChem. 2009;10:1789–1793. doi: 10.1002/cphc.200900155. [DOI] [PubMed] [Google Scholar]
- 23.Li W, Gandra N, Ellis E, Cartney S, Gao R. A pH responsive recoverable sensitizer for singlet oxygen production in aqueous solution. ACS Appl. Mater. & Interface. 2009;1:1778–1784. doi: 10.1021/am9003039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fasman GD. Handbook of Biochemistry and Molecular Biology: Nucleic Acids. Cleveland OH: CRC Press; 1975. pp. 65–215. [Google Scholar]
- 25.Chrzanowska M, Halas A, Kuehn M. Comparative Kinetics of Azathioprine and Metazathioprine Mercaptolysis in presence of Physiological Thiols. Acta Poloniac Pharmaccutica - Drug Research. 2003;60:269–273. [PubMed] [Google Scholar]
- 26.Szeghalmi AV, Leopold L, Pînzaru S, Chis V, Silaghi-Dumitrescu I, Schmitt M, Popp J, Kiefer W. Adsorption of 6-mercaptopurine and 6-mercaptopurine-ribosideon silver colloid: A pH-dependent surface-enhanced Raman spectroscopy and density functional theory study. II. 6-mercaptopurine-riboside. Biopolymers. 2005;78:298–310. doi: 10.1002/bip.20280. [DOI] [PubMed] [Google Scholar]
- 27.Chrzanowska M, Sobiak J, Kuehn M, Dorawa E, Hermann T. Partition coefficients of some purine derivatives and its application to pharmacokinetics. Pharmazie. 2009;64:804–806. [PubMed] [Google Scholar]
- 28.Haag WR, Mill T. Rate constants for interaction of 1O2 with azide ion in water. Photochemistry and Photobiology. 1987;45:317–321. [Google Scholar]
- 29.Hall RD, Chignell CF. Steady-State Near-Infrared Detection of Singlet Molecular Oxygen: A Stern-Volmer Quenching Experiment with Sodium Azide. Photochem. Photobiol. 1987;45:459–464. doi: 10.1111/j.1751-1097.1987.tb05403.x. [DOI] [PubMed] [Google Scholar]
- 30.Niedre M, Patterson MS, Wilson BC. Direct Near-infrared Luminescence Detection of Singlet Oxygen Generated by Photodynamic Therapy in Cells in Vitro and Tissues in Vivo. Photochem. Photobiol. 2002;75:382–391. doi: 10.1562/0031-8655(2002)075<0382:DNILDO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 31.Hurst JR, Schuster GB. Nonradiative relaxation of singlet oxygen in solution. J. Am. Chem. Soc. 1983;105:5756–5760. [Google Scholar]
- 32.Rossbroich G, Garcia NA, Braslavsky SE. Thermal-Leansing Measurements of Singlet Molecular Oxygen: Quantum Yields of Formation and Lifetimes. J. Photochem. 1985;31:37–48. [Google Scholar]
- 33.McCallum JEB, Kuniyoshi CY, Foote CS. Characterization of 5-Hydroxy-8-Oxo-7,8-Dihydroguanosine in the Photosensitized Oxidation of 8-Oxo-7,8-Dihydroguanosine and Its Rearrangement to Spiroiminodihydantoin. J. Am. Chem. Soc. 2004;126:16777–16782. doi: 10.1021/ja030678p. [DOI] [PubMed] [Google Scholar]
- 34.Hickerson RP, Prat F, Muller JG, Foote CS, Burrows CJ. Sequence and Stacking Dependence of 8-Oxoguanine Oxidation: Comparison of One-Electron vs Singlet Oxygen Mechanisms. J. Am. Chem. Soc. 1999;121:9423–9428. [Google Scholar]
- 35.Sheu C, Foote CS. Reactivity toward Singlet Oxygen of a 7,8-Dihydro-8-oxoguanosine(“8-Hydroxyguanosine”) Formed by Photooxidation of a Guanosine Derivative. J. Am. Chem. Soc. 1995;117:6439–6442. [Google Scholar]
- 36.Sheu C, Kang P, Khan S, Foote CS. Low-Temperature Photosensitized Oxidation of a Guanosine Derivative and Formation of an Imidazole Ring-Opened Product. J. Am. Chem. Soc. 2002;124:3905–3913. doi: 10.1021/ja011696e. [DOI] [PubMed] [Google Scholar]
- 37.Ravanat JL, Cadet J. Reaction of singlet oxygen with 2¢-deoxyguanosine and DNA. Isolation and characterization of the main oxidation products. Chem. Res. Toxicol. 1995;8:379–388. doi: 10.1021/tx00045a009. [DOI] [PubMed] [Google Scholar]
- 38.Bjøras M, Luna L, Johnsen B, Hoff E, Haug T, Rognes T, Seeberg E. Opposite base-dependent reactions of a human base excision repair enzyme on DNA containing 7,8-dihydro-8-oxoguanine and abasic sites. EMBO J. 1997;16:6314–6322. doi: 10.1093/emboj/16.20.6314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ogilby PR, Foote CS. Chemistry of singlet oxygen. 36. Singlet molecular oxygen luminescence in solution following pulsed laser excitation. Solvent deuterium isotope effects on the lifetime of singlet oxygen. J. Am. Chem. Soc. 1982;104:2069–2070. [Google Scholar]
- 40.Egorov S. Yu, Kamalov VF, Koroteev NI, Krasnovsky JAA, Toleutaev BN, Zinukov SV. Rise and decay kinetics of photosensitized singlet oxygen luminescence in water. Measurements with nanosecond time-correlated single photon counting technique. Chemical Physics Letters. 1989;163:421–424. [Google Scholar]
- 41.Wiseman H, Halliwell B. Damage to DNA by reactive oxygen and nitrogen species : role in inflammatory disease and progression to cancer. Biochem. J. 1996;313:17–29. doi: 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Devasagayam TPA, Steenken S, Obendorf MSW, Schulz WA, Sies H. Formation of 8-hydroxy(deoxy)- guanosine and generation of strand breaks at guanine residues in DNA by singlet oxygen. Biochemistry. 1991;30:6283–6289. doi: 10.1021/bi00239a029. [DOI] [PubMed] [Google Scholar]
- 43.Kaplan HS, Smith KC, Tomlin P. Radiosensitization Of E. Coli by Purine and Pyrimidine Analogues Incorporated in Deoxyribonucleic Acid. Nature. 1961;190:794–496. doi: 10.1038/190794a0. [DOI] [PubMed] [Google Scholar]
- 44.Hemmens VJ, Moore DE. Photochemical Sensitization by Azathioprine and Its Metabolites-I. 6-Mercatopurine. Photochemistry and Photobiology. 1986;43:247–255. doi: 10.1111/j.1751-1097.1986.tb05601.x. [DOI] [PubMed] [Google Scholar]
- 45.Hemmens VJ, Moore DE. Photo-oxidation of 6-mercaptopurine in aqueous solution. J. Chem. Soc., Perkin Trans. 1984;2(2):209–211. [Google Scholar]
- 46.O’Donovan P, Perrett CM, Zhang X, Montaner B, Xu Y, Harwood CA, McGregor JM, Walker SL, Hanaoka F, Karran P. Azathioprine and UVA Light Generate Mutagenic Oxidative DNA Damage. Science. 2005;309:1871–1874. doi: 10.1126/science.1114233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Perrett CM, Harwood CA, McGregor JM, Karran P. Skin Cancer after Organ Transplantation. Cancer Treatment and Research, Springer Science+Business Media, LLC; 2009. Carcinogenic Mechanisms Related to Immunosuppressive Therapy; pp. 123–132. [DOI] [PubMed] [Google Scholar]
- 48.Wilkinson F, Phillip Helman W, Ross AB. Rate Constants for the Decay and Reactions of the Lowest Electronically Excited Singlet State of Molecular Oxygen in Solution. An Expanded and Revised Compilation. J. Phys. Chem. Ref. Data. 1995;24:663–1021. [Google Scholar]
- 49.Gao R, Ho DG, Dong T, Khuu D, Franco N, Sezer O, Selke M. Reaction of Arylphosphines with Singlet Oxygen: Intra- vs Intermolecular Oxidation. Org. Lett. 2001;3:3719–3722. doi: 10.1021/ol010195v. [DOI] [PubMed] [Google Scholar]
- 50.Ho DG, Gao R, Celaje J, Chung H, Selke M. Phosphadioxirane: A Peroxide from an Ortho-Substituted Arylphosphine and Singlet Dioxygen. Science. 2003;302:259–262. doi: 10.1126/science.1089145. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka M, Ohkubo K, Fukuzumi S. DNA Cleavage by UVA Irradiation of NADH with Dioxygen via Radical Chain Processes. J. Phys. Chem. A. 2006;110:11214–11218. doi: 10.1021/jp064130r. [DOI] [PubMed] [Google Scholar]
- 52.Bishop SM, Malone M, Phillips D, Parker AW, Symonsd MCR. Singlet Oxygen Sensitisation by Excited State DNA. J. Chem. Soc., Chem. Commun. 1994:871–872. [Google Scholar]
- 53.Mohammad T, Morrison H. Evidence for the photosensitized formation of singlet oxygen by UVB irradiation of 2'-deoxyguanosine 5'-monophosphate. J. Am. Chem. Soc. 1996;118:1221–1222. [Google Scholar]
- 54.Kobayashi T, Harada Y, Suzuki T, Ichimura T. Excited State Characteristics of 6-Azauracil in Acetonitrile: Drastically Different Relaxation Mechanism from Uracil. J. Phys. Chem. A. 2008;112:13308–13315. doi: 10.1021/jp803096j. [DOI] [PubMed] [Google Scholar]
- 55.Harada Y, Suzuki T, Ichimura T, Xu Y-Z. Triplet Formation of 4-Thiothymidine and Its Photosensitization to Oxygen Studied by Time-Resolved Thermal Lensing Technique. J. Phys. Chem. B. 2007;111:5518–5524. doi: 10.1021/jp0678094. [DOI] [PubMed] [Google Scholar]
- 56.Milder SJ, Kliger DS. Spectroscopy and photochemistry of thiouracils: implications for the mechanism of photocrosslinking in tRNA. J. Am. Chem. Soc. 1985;107:7365–7373. [Google Scholar]
- 57.Alam MM, Fujitsuka M, Watanabe OIA. Photochemical Properties of Excited Triplet State of 6H-Purine-6-thione Investigated by Laser Flash Photolysis. J. Phys. Chem. A. 1998;102:1338–1344. [Google Scholar]
- 58.Foote CS, Dobrowolski DC. Singlet Oxygen Production from Photodynamic Sensitizers. In: Bors W, Saran M, Tait D, editors. Oxygen Radicals Chem. Biol. Berlin, Germany: Walter de Gruyter, Inc; 1984. pp. 465–472. [Google Scholar]
- 59.Heihoff K, Redmond RW, Braslavsky SE, Rougee M, Salet C, Favre A, Bensasson RV. Photochem. Photobiol. 1990;51:634–641. [PubMed] [Google Scholar]
- 60.Reichardt C, Crespo-Hernández CE. Ultrafast Spin Crossover in 4-Thiothymidine in an Ionic Liquid. Chem. Commun. 2010;46:5963–5965. doi: 10.1039/c0cc01181a. [DOI] [PubMed] [Google Scholar]
- 61.Reichardt C, Guo C, Crespo-Hernández CE. Excited-State Dynamics in 6-Thioguanosine from Femtosecond to Microsecond Time Scale. J. Phys. Chem. B. 2011;115:3263–3270. doi: 10.1021/jp112018u. [DOI] [PubMed] [Google Scholar]
- 62.Euvrard S, Kanitakis J, Claudy A. Skin Cancers after Organ Transplantation. N. Engl. J. Med. 2003;348:1681–1691. doi: 10.1056/NEJMra022137. [DOI] [PubMed] [Google Scholar]
- 63.DeFedericis H, Patrzyc HB, Rajecki MJ, Budzinski EE, Iijima H, Dawidzik JB, Evans MS, Greene KF, Box HC. Singlet Oxygen-Induced DNA Damage. Radiat. Res. 2006;165:445–451. doi: 10.1667/rr3533.1. [DOI] [PubMed] [Google Scholar]
- 64.Duarte V, Gasparutto D, Jaquinod M, Ravanat J.-Luc, Cadet J. Repair and Mutagenic Potential of Oxaluric Acid, a Major Product of Singlet Oxygen-Mediated Oxidation of 8-Oxo-7,8-dihydroguanine. Chem. Res. Toxicol. 2001;14:46–53. doi: 10.1021/tx0001629. [DOI] [PubMed] [Google Scholar]
- 65.Sheu C, Foote CS. Photosensitized Oxygenation of a 7,8-Dihydro-8-oxoguanosine Derivative. Formation of Dioxetane and Hydroperoxide Intermediates. J. Am. Chem. Soc. 1995;117:474–477. [Google Scholar]
- 66.Sheu C, Foote CS. Endoperoxide Formation in a Guanosine Derivative. J. Am. Chem. Soc. 1993;115:10446–10447. [Google Scholar]
- 67.Prat F, Hou C, Foote CS. Determination of the Quenching Rate Constants of Singlet Oxygen by Derivatized Nucleosides in Nonaqueous Solution. J. Am. Chem. Soc. 1997;119:5051–5052. [Google Scholar]
- 68.Chanon M, Julliard M, Mehta G, Maiya BG. Is 1O2 alone sufficient for DNA cleavage? Possible involvement of paramagnetic intermediates. Research on Chemical Intermediates. 1999;25:633–644. [Google Scholar]
- 69.Leipold MD, Muller JG, Burrows CJ, David SS. Removal of hydantoin products of 8-oxoguanine oxidation by the Escherichia coli DNA repair enzyme. FPG, Biochemistry. 2000;39:14984–14992. doi: 10.1021/bi0017982. [DOI] [PubMed] [Google Scholar]
- 70.Leipold MD, Workman H, Muller JG, Burrows CJ, David SS. Recognition and removal of oxidized guanines in duplex DNA by the base excision repair enzymes hOGG1, yOGG1, and yOGG2. Biochemistry. 2003;42:11373–11381. doi: 10.1021/bi034951b. [DOI] [PubMed] [Google Scholar]
- 71.Schulz I, Mahler H-C, Boiteux S, Epe B. Oxidative DNA base damage induced by singlet oxygen and photosensitization: recognition by repair endonucleases and mutagenicity. Mutat. Res. 2000;46:145–156. doi: 10.1016/s0921-8777(00)00049-5. [DOI] [PubMed] [Google Scholar]
- 72.Ravanat J.-Luc, Sauvaigo S, Caillat S, Martinez GR, Medeiros MHG, Mascio PD, Favier A, Cadet J. Singlet oxygen-mediated damage to cellular DNA determined by the comet assay associated with DNA repair enzymes. Biol. Chem. 2004;385:17–20. doi: 10.1515/BC.2004.003. [DOI] [PubMed] [Google Scholar]
- 73.Toyokuni S. Reactive Oxygen Species-Induced Molecular Damage and Its Application in Pathology. Pathology International. 1999;49:91–102. doi: 10.1046/j.1440-1827.1999.00829.x. [DOI] [PubMed] [Google Scholar]
- 74.Jaruga P, Zastawny TH, Skokowski J, Dizdaroglu M, Olinski R. Oxidative DNA base damage and antioxidant enzyme activities in human lung cancer. FEBS Lett. 1994;341:59–64. doi: 10.1016/0014-5793(94)80240-8. [DOI] [PubMed] [Google Scholar]
- 75.Okamoto K, Toyokuni S, Uchida K, Ogawa O, Takenawa J, Kakehi Y, Kinoshita H, Hattori-Nakakuki Y, Hiai H, Yoshida O. Formation of 8-hydroxy-2′-deoxyguanosine and 4-hydroxy-2-nonenal-modified proteins in human renal-cell carcinoma. Int. J. Cancer. 1994;58:825–829. doi: 10.1002/ijc.2910580613. [DOI] [PubMed] [Google Scholar]
- 76.Kondo S, Toyokuni S, Iwasa Y, Tanaka T, Onodera H, Hiai H. Imamura M. Persistent oxidative stress in human colorectal carcinoma, but not in adenoma. Free Radical Biol. Med. 1999;27:401–410. doi: 10.1016/s0891-5849(99)00087-8. [DOI] [PubMed] [Google Scholar]
- 77.Oliva MR, Ripoll F, Muniz P, Iradi A, Trullenque R, Valls V, Drehmer E, Saez GT. Genetic alterations and oxidative metabolism in sporadic colorectal tumors from a Spanish community. Mol. Carcinog. 1997;18:232–243. [PubMed] [Google Scholar]
- 78.Kasai H. Analysis of a form of oxidative DNA damage, 8-hydroxy-2′-deoxyguanosine, as a marker of cellular oxidative stress during carcinogenesis. Mutat. Res. 1997;387:147–163. doi: 10.1016/s1383-5742(97)00035-5. [DOI] [PubMed] [Google Scholar]
- 79.Cadet J, Delatour T, Douki T, Gasparutto D, Pouget E, Ravanat J.-Luc, Sauvaigo S. Hydroxyl radicals and DNA base damage. Mutation Research. 1999;424:9–21. doi: 10.1016/s0027-5107(99)00004-4. [DOI] [PubMed] [Google Scholar]
- 80.Misiaszek R, Uvaydov Y, Crean C, Geacintov NE, Shafirovich V. Combination Reactions of Superoxide with 8-Oxo-7,8-dihydroguanine Radicals in DNA. J. Biol. Chem. 2005;280:6293–6300. doi: 10.1074/jbc.M412253200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.