Abstract
It has been well established that aging is the most prominent risk factor for PD. In the MPTP mouse model which has been widely used to study PD, studies have shown that MPTP exhibits its neurotoxic effects on the dopaminergic system in an age-dependent manner. Although it is recognized the serotonergic system is impacted in PD, how aging influences serotonergic neurodegeneration in PD has not been adequately investigated. In the present studies, we examined the long-term effects of MPTP treatment on regional concentrations of dopamine (DA), serotonin (5-HT) and norepinephrine (NE) in the striatum and prefrontal cortex (PFC). We also determined if there are differences in the age-dependent vulnerability of the monoaminergic system to MPTP. In young (3-month-old) mice, MPTP produced significant decreases in striatal DA but no changes in striatal 5-HT and NE three weeks after MPTP treatment. There was partial recovery of striatal DA concentrations 18 months later. This was accompanied by elevated striatal 5-HT. In the PFC, NE was decreased but there was complete recovery 18 months later. By contrast, we observed a long-term decrease in prefrontal 5-HT with no recovery of 5-HT concentrations 18 months after MPTP treatment. Striatal DA and NE but not 5-HT neurons exhibited age-dependent vulnerability to MPTP. Aging had no influence on the neurotoxic effects of MPTP in the PFC. Thus, there is divergence in the response of DA and 5-HT systems to MPTP neurotoxicity.
Keywords: Aging, dopamine, mice, MPTP, norepinephrine, prefrontal cortex, serotonin, striatum
Parkinson’s disease (PD) is characterized by the degeneration of nigrostriatal dopamine neurons and a decrease in striatal dopamine concentration [19, 24]. The decrease in striatal dopamine levels is thought to underlie the motor symptoms of PD [1]. However, over the past generation there has been an increasing awareness that PD is not only a disease of motor deficits, but also marked by extensive non-motor symptoms that range from autonomic dysfunction to depression and cognitive deficits [5, 30]. Most of the non-motor symptoms of PD have been suggested to be associated with changes in non-dopaminergic systems and changes in the function of extra-striatal sites [3, 4, 29]. In particular, the increased incidence of depression in PD has been speculated to be associated with loss of the forebrain serotonergic innervation [15, 16, 35].
Idiopathic PD is an age-related disorder: 1 – 2% of persons over the age of 65 years Based on data reporting that striatal dopamine concentrations decline as a function of age [20], it has been hypothesized that PD arises from the effects of some acute event, such as exposure to a neurotoxicant, superimposed on the age-related decline in striatal dopamine, ultimately reaching a threshold for manifestation of symptoms. Evidence in support of this hypothesis comes from studies showing that 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) produces more severe nigrostriatal dopamine neuron degeneration in older than young animals [7, 32].
Although it is has been known for some time that the forebrain serotonergic innervation is impacted in PD [15, 35], how aging influences non-dopaminergic systems in parkinsonism has not been extensively studied. We previously reported that there is a loss of serotonin and norepinephrine concentrations in the prefrontal and somatosensory cortices, but not the striatum, of the MPTP-treated mouse [28]; this corresponds well with the reported decreases in the cortical serotonergic and noradrenergic innervations in idiopathic PD [2, 28, 35]. Changes in the prefrontal cortex (PFC) are of particular interest because the PFC is critically involved in executive functions, including working memory and inhibitory control [6, 14], which are impaired in PD [10, 27], and because PFC volume declines with age in a manner that is positively correlated with the decline in executive function [31]. We therefore examined age-related changes in serotonin and norepinephrine as well as dopamine concentrations in the PFC and striatum of the MPTP-treated mouse. We first determined if any of MPTP-induced changes in the regional monoamine concentrations seen in animals sacrificed three weeks after toxin administration persist unabated at 18 months post-MPTP, or if there is some degree of recovery. We then examined striatal and PFC monoamine concentrations in mice 3 or 18 months of age at the time of MPTP treatment.
Materials and Methods
Animals
Male C57BL/6J mice, three months of age at the start of experiments, were obtained from Jackson Labs (Bar Harbor, ME). Animals were group-housed in a temperature- and humidity-controlled room maintained on a 12L:12D light-dark cycle (lights off at 1900h). Food and water were available ad libitum. All studies were performed in accordance with NIH guidelines on the ethical use of animals.
MPTP treatment
In the first experiment, we examined the effects of MPTP on regional monoamine concentrations in young adult (3 months old) mice, which were sacrificed three weeks or 18 months later. Animals were injected with saline or 20 mg/kg MPTP (ip; Sigma-Aldrich, St. Louis, MO) every two hours for a total of four injections, resulting in a cumulative dose of 80 mg/kg. Three weeks (saline: n = 15; MPTP: n = 18) or 18 months (saline: n = 7; MPTP: n = 8) after the last MPTP injection, mice were decapitated and the precommissural dorsolateral striatum and medial PFC (prelimbic and infralimbic cortices) dissected for subsequent determinations of monoamine concentrations [9].
In the second experiment, we compared the effects of MPTP administration to young (3 month old) or old” (18 month old) mice on regional monoamine concentrations. The mice received daily saline or MPTP (20 mg/kg, ip) injections over five consecutive days, for a cumulative dose of 100 mg/kg. Three weeks after the last MPTP injection, mice (young mice: saline, n = 6; MPTP, n = 7; old mice: saline, n = 6; MPTP, n = 7) were sacrificed and tissue dissected and saved for neurochemical analyses. The different MPTP protocol used in the two experiments was necessitated by the fact that the MPTP treatment protocol used in the first study was fatal in the older mice. We therefore opted to use a daily MPTP treatment protocol [28], in which we observed a mortality rate of 14% in aged mice as compared with no fatalities in young adult mice.
Tissue harvesting and determination of monoamine concentrations
Concentrations of dopamine, serotonin, and norepinephrine were determined using our previously described methods [8], with protein levels determined by the method of Lowry et al. [25].
Statistical Analysis
The effects of MPTP treatment in mice were analyzed by one-way ANOVA. When appropriate, post hoc comparisons were carried out with Tukey’s tests. Direct comparisons of regional monoamine concentrations in 3 month and 18 month old animals receiving vehicle (saline) injections were determined by a two-way ANOVA.
Results
Comparability of basal monoamine concentrations in young adult and 18 month old mice
A two-way ANOVA failed to uncover any differences in striatal or PFC monoamine concentrations between young adult and 18 month old mice receiving vehicle injections [dopamine: age F(1,64) = 0.230 NS; region × age F(1,64) = 0.251 NS; serotonin: age F(1,63) = 3.263 p = 0.076; region × age F(1,63) = 0.503 NS; norepinephrine: age F(1,64) = 0.546 NS; region × age F(1,64) = 0.199 NS]. We therefore performed subsequent statistical analyses using pooled monoamine concentrations from the young adult and 18 month old mice.
Temporally specific changes in regional monoamine concentrations in MPTP-treated mice
At three weeks after the MPTP treatment of young adult mice there was a significant overall MPTP effect on striatal monoamines [F(5,153) = 7.532; p < 0.0001] (Fig. 1A). Post hoc analyses revealed that striatal dopamine concentrations at three weeks post-MPTP were decreased by 80% in MPTP-treated relative to saline-injected mice (p < 0.001). A significant treatment effect was also seen in animals sacrificed 18 months after MPTP treatment [F(5,123) = 6.749, p < 0.0001; Fig. 1A], with post hoc analyses revealing that a significant but attenuated degree of striatal dopamine loss (−54%; p < 0.001).
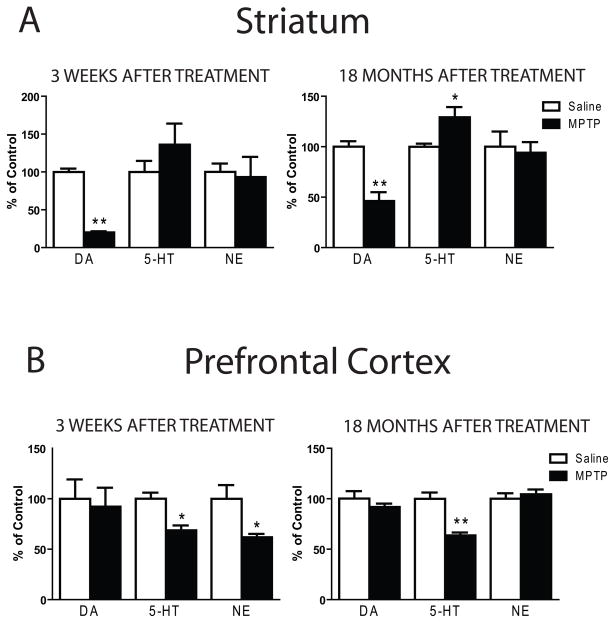
Figure 1.
Temporal changes in regional monoamine concentrations in MPTP-treated mice. Mice were injected with saline or 20 mg/kg MPTP (ip) every two hours for a total of four injections, resulting in a cumulative dose of 80 mg/kg. Three weeks or 18 months after the last treatment, mice were sacrificed for neurochemical analysis. (A) The decreases in striatal dopamine (DA) produced by the acute MPTP protocol was apparent three weeks (left panel) and 18 months (right panel) after MPTP treatment. (B) In the PFC the acute MPTP treatment protocol produced decreases (p < 0.05) in serotonin (5-HT) and norepinephrine (NE) three weeks after MPTP treatment (left panel). Deficits in prefrontal 5-HT persisted 18 months (right panel) after MPTP treatment (p < 0.001). Control values (ng/mg protein) in the striatum are DA, 160.9±4.7; 5-HT, 8.26±0.56, NE, 2.33±0.15. Control values (ng/mg protein) in the PFC are DA, 1.64±0.10; 5-HT, 8.70±0.58, NE, 6.31±0.49. * p < 0.05 when compared to respective saline. ** p < 0.001 when compared to respective saline.
MPTP treatment did not elicit significant changes in striatal serotonin concentrations in animals examined three weeks after the last MPTP injection (Fig. 1A). However, in mice surviving for 18 months after MPTP treatment, there was a small but significant (29%) increase in MPTP- compared to saline-injected mice (p < 0.05).
In the PFC a different survival-dependent pattern of effects was observed. Significant changes in PFC monoamine levels [F(5,126) = 5.718; p < 0.0001] were uncovered three weeks after MPTP (Fig. 1B). Post hoc comparisons demonstrated significant decreases (p < 0.05) in both cortical serotonin (−33%) and norepinephrine (−38%) concentrations. These effects persisted: there was still a significant treatment effect in PFC monoamines when examined 18 months after MPTP treatment [F(5,105) = 4.965, p < 0.001; Fig. 1B]. However, the magnitude of the decrease in PFC serotonin concentration was the same in mice examined at 3 weeks (−38%) or 18 months (−36%) after MPTP treatment. In contrast, by the 18 month time point the decrease seen in PFC norepinephrine concentration at 3 weeks after MPTP treatment had completely resolved when examined at 18 months post-MPTP (Fig. 1B).
Age-dependent vulnerability of forebrain monoamine response to MPTP
We compared the effects of MPTP treatment on young adult (3 months old) and moderately old (18 months of age) mice, both sacrificed three weeks after drug administration. MPTP treatment protocol resulted in a 25% non-significant trend toward a decrease in striatal dopamine concentration in the young adult mice [F(5,119) = 1.159; NS] (Fig. 2A). In contrast, in the older mice the same MPTP treatment significantly decreased striatal monoamine concentrations [F(5,120) = 9.343, p < 0.0001; Fig. 2A], with post hoc analyses uncovering a significant decrease in striatal dopamine (−67%; p < 0.001) and norepinephrine (−32%; p < 0.05) but not serotonin levels.
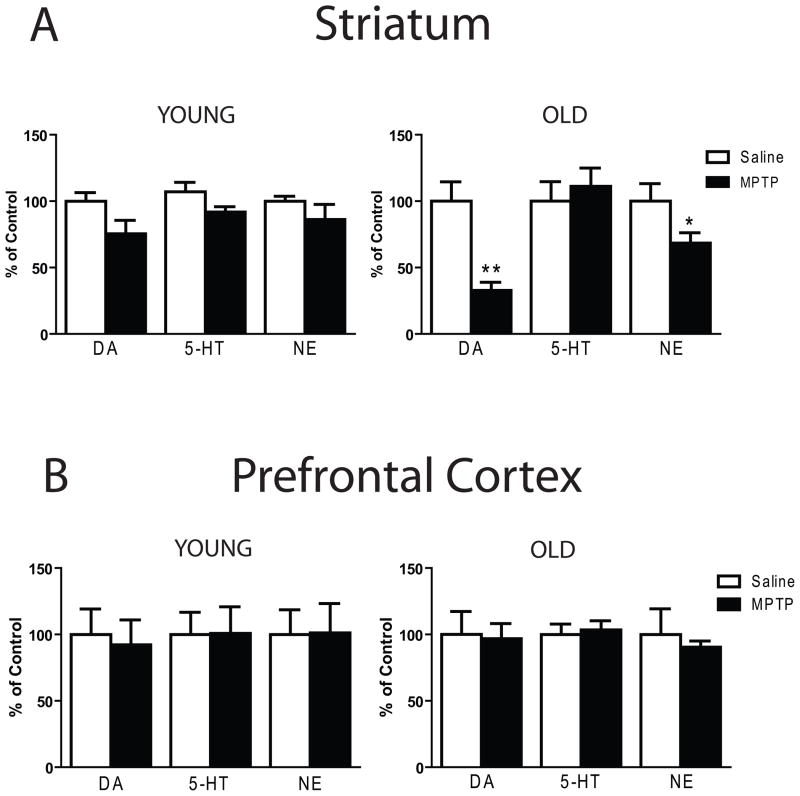
Figure 2.
MPTP treatment produces age-related neurochemical changes in the striatum but not the PFC. Young (3 months old) or old (18 months old) mice received daily saline or MPTP (20 mg/kg, ip) injections for five consecutive days, for a cumulative dose of 100 mg/kg. Three weeks after the last treatment mice were sacrificed for neurochemical analysis. (A) Despite lack of effects in young mice (left panel), the subchronic MPTP treatment protocol produced significant decreases in DA (p < 0.001) and NE (p < 0.05) in the striatum of old mice (right panel). (B) The subchronic MPTP treatment protocol failed to produce changes in monoamine concentrations in the PFC of young or old mice. * p < 0.05 when compared to respective saline. ** p < 0.001 when compared to respective saline.
In contrast, the MPTP treatment did not cause any significant change in cortical dopamine, serotonin, or norepinephrine concentrations in either the young or older mice (Fig. 2B).
Discussion
Striatal dopamine concentrations in mice that survived for 18 months after MPTP treatment recovered significantly from the markedly decreased concentrations observed in animals examined three weeks after MPTP treatment. In contrast, at 18 months survival there was a marginally significant increase in striatal serotonin that was not seen at the shorter survival interval. Serotonin concentrations were decreased in the PFC of young adult mice sacrificed at three weeks after MPTP treatment, but there was no recovery in cortical concentrations of the indoleamine over the next 18 months. In contrast, the relatively marked decrease in cortical norepinephrine seen at three weeks survival had completely recovered to control levels by 18 months after MPTP administration.
These data on time-dependent changes in regional monoamine concentrations after MPTP treatment confirm and extend previous results. Most previous studies have reported a very rapid decrease in striatal dopamine concentrations that recovers by the second post-treatment week and is then stable over the ensuing four-five weeks [17, 36]. A few studies have examined striatal dopamine levels at intermediate (up to 5 months) survival periods [7, 33], but none has assessed recovery in striatal dopamine over long (>12 month) survival periods. What is consistent across all studies is that after a short period there is a gradual partial recovery of striatal dopamine concentrations [7, 17, 33]. Our data extend these reports, with substantial recovery seen at 18 months survival, although there is still a significant (54%) decrease relatively to age-matched controls at this time point.
Temporal changes in striatal serotonin in response to MPTP treatment have not been extensively studied. Various studies have reported an increase, no change, or a decrease in striatal serotonin concentrations over the first several weeks after MPTP treatment [17, 34, 38]. We observed a non-significant trend toward an increase in striatal serotonin at 3 weeks survival, which evolved to a relatively small but significant (29%) increase at 18 months survival. Zhou et al. [39] reported that when serotonin levels are increased in the striatum some serotonin is accumulated into dopaminergic terminals by the dopamine transporter, and subsequently co-released with dopamine. The increase in tissue serotonin concentrations that we observed at the long-survival interval may in part contribute to the capacity of the animal to continue overtly normal motor activity. Interestingly, in neonatal animals with striatal dopamine denervation there is a large serotonergic hyperinnervation of the striatum, but the magnitude of this response is reduced if animals are dopamine denervated as adults [21, 34]. Thus, the same increase in the striatal serotonin innervation may be seen in adult animals, but only after long survival intervals.
The temporally specific changes in monoamine concentrations that we observed in the PFC differ from those seen in the striatum. Consistent with our earlier data, we did not observe any significant change in PFC dopamine levels at three weeks post-MPTP. Hallman et al. [17] did observe a small but significant decrease in PFC dopamine concentrations at one but not five weeks after MPTP treatment. Thus, these data are consistent with a rapid decrease in cortical dopamine stores over the first week after MPTP treatment, with recovery to normal levels seen by about three weeks. In contrast to these rodent data, studies in non-human primates, which have a considerably expanded cortical dopamine innervation relative to that seen in rodents [13], have reported that MPTP administration significantly decreases cortical dopamine concentrations [11], but studies at different times after MPTP administration have not been performed.
We were surprised to note that striatal and PFC monoamine concentrations did not differ significantly across vehicle-treated mice of different ages: monoamine concentrations in mice injected with vehicle and sacrificed 18 months later (and thus were approximately 21 months old) were not significantly different from monoamine concentrations seen in vehicle-injected animals sacrificed at ~4 months of age (three weeks after MPTP). While it is clear that there are age-dependent changes in dopamine-mediated behaviors [22, 26], some previous studies have found that rodent forebrain monoamine levels do not decrease as function of age [18, 37], although others have reported age-related decreases in dopamine concentrations [7, 12, 20]. Despite a broadly-held view that the number of substantia nigra dopamine neurons declines with age, Kubis et al. [23] reported that the number of tyrosine hydroxylase-positive (dopamine) neurons in the substantia nigra or other midbrain dopamine regions did not change significantly in humans ranging from 44 to 110 years of age. While some of these data are consistent with a lack of age-related declines in forebrain monoamine concentrations once young adulthood is reached, major changes in the dynamic release of the monoamines may occur in aged animals [18], and more work is needed to define the precise development trajectory of monoamine changes in the mature and aged mouse.
Although we observed that monoamine concentrations do not differ significantly between 4 month- and 21 month-old vehicle-injected mice, it was possible that the long-term persistence of the PFC serotonin decrease reflects an interaction between MPTP treatment and altered release characteristics of monoamines in the older animals [18]. We decided to examine the effect of MPTP on striatal and PFC monoamine levels in mice treated with MPTP at ~3 months or 18 months of age and then sacrificed three weeks later. Unfortunately, we were unable to use the same MPTP treatment protocol in the older animals that was used in our initial study on young adult mice because our standard MPTP protocol was uniformly lethal to the older animals. We therefore treated 3 and 18 month old mice with a relatively low (20 mg/kg) dose of MPTP, injected over five successive days; this treatment regimen was marked by a low incidence of animal loss (14%). We found that the striatal dopamine innervation of the older mouse was much more susceptible to MPTP treatment, showing a >60% decrease in striatal dopamine concentrations relative to only a ~25% (non-significant) decrease in striatal dopamine stores in the young adult mouse. However, the cortical dopamine innervation was not affected by MPTP treatment in either the young or older mice.
The low dose MPTP treatment protocol we followed in our study of the susceptibility of young vs older mice to MPTP did not elicit any significant decrease in PFC serotonin levels in either age group of mice. This suggests that some cumulative threshold dose for MPTP must be reached if any cortical serotonin deafferentation is to be seen. However, if higher MPTP doses are employed to partially disrupt cortical serotonin, the serotonin system does not recover, suggesting that serotonergic neurons do not enjoy the plasticity of catecholaminergic neurons in the mature rodent.
Postmortem studies of the PFC in PD have found that the concentrations or cortical innervation densities of dopamine, serotonin, and norepinephrine are all decreased relative to values observed in normal control subjects [2, 28, 35]. PD, which typically strikes older (>50 years of age) persons, invariably involves patients who survived for relatively long periods after receiving a diagnosis of PD. A recent staging system for PD based upon the presence of synuclein-immunoreactive inclusions in neurons has reported that serotonergic neurons of the dorsal and median raphe are not impacted until stage IV [3], i.e., until after dopamine neuron loss is present. It is interesting to note that once PFC serotonin levels are decreased in the MPTP-treated mouse, they remain decreased, regardless of age of the animal. It is therefore probably fortunate in idiopathic PD that serotonin involvement is not seen until relatively late in the course of the illness.
Highlights.
MPTP produced decreases in striatal DA which partially recovered 18 months later.
By contrast, striatal 5-HT was increased 18 months after MPTP treatment.
In the cortex, decreases in NE were transient but those in 5-HT were long-lasting.
Only striatal DA and NE exhibited age-related vulnerability to MPTP.
5-HT neurons lack the plasticity exhibited by DA neurons to MPTP toxicity.
Acknowledgments
This work was supported by U01 NS041071 (TAA) from the National Institute of Neurological Disorders and Stroke and by the National Parkinson Foundation Center of Excellence at Vanderbilt (AYD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Agid Y. Parkinson’s disease: pathophysiology. Lancet. 1991;337:1321–1324. doi: 10.1016/0140-6736(91)92989-f. [DOI] [PubMed] [Google Scholar]
- 2.Azmitia EC, Nixon R. Dystrophic serotonergic axons in neurodegenerative diseases. Brain Res. 2008;1217:185–194. doi: 10.1016/j.brainres.2008.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 4.Broussolle E, Dentresangle C, Landais P, Garcia-Larrea L, Pollak P, Croisile B, Hibert O, Bonnefoi F, Galy G, Froment JC, Comar D. The relation of putamen and caudate nucleus 18F-Dopa uptake to motor and cognitive performances in Parkinson’s disease. J Neurol Sci. 1999;166:141–151. doi: 10.1016/s0022-510x(99)00127-6. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhuri KR, Healy DG, Schapira AH. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5:235–245. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 6.Dalley JW, Cardinal RN, Robbins TW. Prefrontal executive and cognitive functions in rodents: neural and neurochemical substrates. Neurosci Biobehav Rev. 2004;28:771–784. doi: 10.1016/j.neubiorev.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 7.Date I, Felten DL, Felten SY. Long-term effect of MPTP in the mouse brain in relation to aging: neurochemical and immunocytochemical analysis. Brain Res. 1990;519:266–276. doi: 10.1016/0006-8993(90)90088-s. [DOI] [PubMed] [Google Scholar]
- 8.Deutch AY, Cameron DS. Pharmacological characterization of dopamine systems in the nucleus accumbens core and shell. Neuroscience. 1992;46:49–56. doi: 10.1016/0306-4522(92)90007-o. [DOI] [PubMed] [Google Scholar]
- 9.Deutch AY, Tam SY, Roth RH. Footshock and conditioned stress increase 3,4-dihydroxyphenylacetic acid (DOPAC) in the ventral tegmental area but not substantia nigra. Brain Res. 1985;333:143–146. doi: 10.1016/0006-8993(85)90134-9. [DOI] [PubMed] [Google Scholar]
- 10.Dubois B, Pillon B. Cognitive deficits in Parkinson’s disease. J Neurol. 1997;244:2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- 11.Elsworth JD, Deutch AY, Redmond DE, Jr, Sladek JR, Jr, Roth RH. MPTP reduces dopamine and norepinephrine concentrations in the supplementary motor area and cingulate cortex of the primate. Neurosci Lett. 1990;114:316–322. doi: 10.1016/0304-3940(90)90583-u. [DOI] [PubMed] [Google Scholar]
- 12.Emborg ME, Ma SY, Mufson EJ, Levey AI, Taylor MD, Brown WD, Holden JE, Kordower JH. Age-related declines in nigral neuronal function correlate with motor impairments in rhesus monkeys. J Comp Neurol. 1998;401:253–265. [PubMed] [Google Scholar]
- 13.Gaspar P, Berger B, Febvret A, Vigny A, Henry JP. Catecholamine innervation of the human cerebral cortex as revealed by comparative immunohistochemistry of tyrosine hydroxylase and dopamine-beta-hydroxylase. J Comp Neurol. 1989;279:249–271. doi: 10.1002/cne.902790208. [DOI] [PubMed] [Google Scholar]
- 14.Goldman-Rakic PS. The physiological approach: functional architecture of working memory and disordered cognition in schizophrenia. Biol Psychiatry. 1999;46:650–661. doi: 10.1016/s0006-3223(99)00130-4. [DOI] [PubMed] [Google Scholar]
- 15.Guttman M, Boileau I, Warsh J, Saint-Cyr JA, Ginovart N, McCluskey T, Houle S, Wilson A, Mundo E, Rusjan P, Meyer J, Kish SJ. Brain serotonin transporter binding in non-depressed patients with Parkinson’s disease. Eur J Neurol. 2007;14:523–528. doi: 10.1111/j.1468-1331.2007.01727.x. [DOI] [PubMed] [Google Scholar]
- 16.Haapaniemi TH, Ahonen A, Torniainen P, Sotaniemi KA, Myllyla VV. [123I]beta-CIT SPECT demonstrates decreased brain dopamine and serotonin transporter levels in untreated parkinsonian patients. Mov Disord. 2001;16:124–130. doi: 10.1002/1531-8257(200101)16:1<124::aid-mds1007>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 17.Hallman H, Olson L, Jonsson G. Neurotoxicity of the meperidine analogue N-methyl-4-phenyl-1,2,3,6-tetrahydropyridine on brain catecholamine neurons in the mouse. Eur J Pharmacol. 1984;97:133–136. doi: 10.1016/0014-2999(84)90521-1. [DOI] [PubMed] [Google Scholar]
- 18.Hebert MA, Gerhardt GA. Normal and drug-induced locomotor behavior in aging: comparison to evoked DA release and tissue content in fischer 344 rats. Brain Res. 1998;797:42–54. doi: 10.1016/s0006-8993(98)00370-9. [DOI] [PubMed] [Google Scholar]
- 19.Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N Engl J Med. 1988;318:876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- 20.Kish SJ, Shannak K, Rajput A, Deck JH, Hornykiewicz O. Aging produces a specific pattern of striatal dopamine loss: implications for the etiology of idiopathic Parkinson’s disease. J Neurochem. 1992;58:642–648. doi: 10.1111/j.1471-4159.1992.tb09766.x. [DOI] [PubMed] [Google Scholar]
- 21.Kostrzewa RM, Reader TA, Descarries L. Serotonin neural adaptations to ontogenetic loss of dopamine neurons in rat brain. J Neurochem. 1998;70:889–898. doi: 10.1046/j.1471-4159.1998.70030889.x. [DOI] [PubMed] [Google Scholar]
- 22.Kubanis P, Zornetzer SF. Age-related behavioral and neurobiological changes: a review with an emphasis on memory. Behav Neural Biol. 1981;31:115–172. doi: 10.1016/s0163-1047(81)91195-x. [DOI] [PubMed] [Google Scholar]
- 23.Kubis N, Faucheux BA, Ransmayr G, Damier P, Duyckaerts C, Henin D, Forette B, Le Charpentier Y, Hauw JJ, Agid Y, Hirsch EC. Preservation of midbrain catecholaminergic neurons in very old human subjects. Brain. 2000;123(Pt 2):366–373. doi: 10.1093/brain/123.2.366. [DOI] [PubMed] [Google Scholar]
- 24.Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373:2055–2066. doi: 10.1016/S0140-6736(09)60492-X. [DOI] [PubMed] [Google Scholar]
- 25.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 26.Mizoguchi K, Shoji H, Tanaka Y, Tabira T. Orbitofrontal dopaminergic dysfunction causes age-related impairment of reversal learning in rats. Neuroscience. 2010;170:1110–1119. doi: 10.1016/j.neuroscience.2010.08.037. [DOI] [PubMed] [Google Scholar]
- 27.Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65:1239–1245. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- 28.Nayyar T, Bubser M, Ferguson MC, Diana Neely M, Shawn Goodwin J, Montine TJ, Deutch AY, Ansah TA. Cortical serotonin and norepinephrine denervation in parkinsonism: preferential loss of the beaded serotonin innervation. Eur J Neurosci. 2009;30:207–216. doi: 10.1111/j.1460-9568.2009.06806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pavese N, Metta V, Bose SK, Chaudhuri KR, Brooks DJ. Fatigue in Parkinson’s disease is linked to striatal and limbic serotonergic dysfunction. Brain. 2010;133:3434–3443. doi: 10.1093/brain/awq268. [DOI] [PubMed] [Google Scholar]
- 30.Poewe W. Non-motor symptoms in Parkinson’s disease. Eur J Neurol. 2008;15(Suppl 1):14–20. doi: 10.1111/j.1468-1331.2008.02056.x. [DOI] [PubMed] [Google Scholar]
- 31.Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- 32.Ricaurte GA, Irwin I, Forno LS, DeLanney LE, Langston E, Langston JW. Aging and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced degeneration of dopaminergic neurons in the substantia nigra. Brain Res. 1987;403:43–51. doi: 10.1016/0006-8993(87)90120-x. [DOI] [PubMed] [Google Scholar]
- 33.Ricaurte GA, Langston JW, Delanney LE, Irwin I, Peroutka SJ, Forno LS. Fate of nigrostriatal neurons in young mature mice given 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine: a neurochemical and morphological reassessment. Brain Res. 1986;376:117–124. doi: 10.1016/0006-8993(86)90905-4. [DOI] [PubMed] [Google Scholar]
- 34.Rozas G, Liste I, Guerra MJ, Labandeira-Garcia JL. Sprouting of the serotonergic afferents into striatum after selective lesion of the dopaminergic system by MPTP in adult mice. Neurosci Lett. 1998;245:151–154. doi: 10.1016/s0304-3940(98)00198-0. [DOI] [PubMed] [Google Scholar]
- 35.Scatton B, Javoy-Agid F, Rouquier L, Dubois B, Agid Y. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson’s disease. Brain Res. 1983;275:321–328. doi: 10.1016/0006-8993(83)90993-9. [DOI] [PubMed] [Google Scholar]
- 36.Stephenson D, Ramirez A, Long J, Barrezueta N, Hajos-Korcsok E, Matherne C, Gallagher D, Ryan A, Ochoa R, Menniti F, Yan J. Quantification of MPTP-induced dopaminergic neurodegeneration in the mouse substantia nigra by laser capture microdissection. J Neurosci Methods. 2007;159:291–299. doi: 10.1016/j.jneumeth.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 37.Thiruchelvam M, McCormack A, Richfield EK, Baggs RB, Tank AW, Di Monte DA, Cory-Slechta DA. Age-related irreversible progressive nigrostriatal dopaminergic neurotoxicity in the paraquat and maneb model of the Parkinson’s disease phenotype. Eur J Neurosci. 2003;18:589–600. doi: 10.1046/j.1460-9568.2003.02781.x. [DOI] [PubMed] [Google Scholar]
- 38.Vuckovic MG, Wood RI, Holschneider DP, Abernathy A, Togasaki DM, Smith A, Petzinger GM, Jakowec MW. Memory, mood, dopamine, and serotonin in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-lesioned mouse model of basal ganglia injury. Neurobiol Dis. 2008;32:319–327. doi: 10.1016/j.nbd.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou FM, Liang Y, Salas R, Zhang L, De Biasi M, Dani JA. Corelease of dopamine and serotonin from striatal dopamine terminals. Neuron. 2005;46:65–74. doi: 10.1016/j.neuron.2005.02.010. [DOI] [PubMed] [Google Scholar]