Abstract
RATIONALE AND OBJECTIVES
To evaluate high spectral and spatial resolution (HiSS) MRI for diagnosis of breast cancer without injection of contrast media: to compare the performance of pre-contrast HiSS images to conventional contrast-enhanced fat-suppressed T1-weighted images, based on image quality and in the task of classifying benign and malignant breast lesions.
MATERIALS AND METHODS
Ten benign and 44 malignant lesions were imaged at 1.5T with HiSS (pre-contrast administration) and conventional fat-suppressed imaging (3–10 min post-contrast). This set of 108 images, after randomization, was evaluated by three experienced radiologists blinded to the imaging technique. BIRADS morphologic criteria (lesion shape; lesion margin; internal signal intensity pattern) and final assessment were used to measure reader performance. Image quality was evaluated based on boundary delineation and quality of fat suppression. An overall probability of malignancy was assigned to each lesion for HiSS and conventional images separately.
RESULTS
On boundary delineation and quality of fat-suppression, pre-contrast HiSS scored similarly to conventional post-contrast MRI. On benign vs. malignant lesion separation, there was no statistically significant difference in ROC performance between HiSS and conventional MRI, and HiSS met a reasonable non-inferiority condition.
CONCLUSION
Pre-contrast HiSS imaging is a promising approach for showing lesion morphology without blooming and other artifacts caused by contrast agents. HiSS images could be used to guide subsequent dynamic contrast-enhanced MRI scans, to maximize spatial and temporal resolution in suspicious regions. HiSS MRI without contrast agent injection may be particularly important for patients at risk for contrast-induced nephrogenic systemic fibrosis, or allergic reactions.
Keywords: breast cancer, breast lesion, high spectral and spatial resolution (HiSS) imaging, contrast-agent induced nephrotoxicity, echo-planar spectroscopic imaging
INTRODUCTION
Dynamic contrast-enhanced MRI (DCEMRI), combined with high-resolution post-contrast anatomical imaging, is an important tool for routine clinical detection and diagnosis of breast cancer. [1–3] However, although its sensitivity is reported as consistently high (75–100%), its specificity has been variable (29–90%). [4–8] There are a number of factors that can reduce the utility of DCEMRI in assessing the lesion morphology: magnetic susceptibility of contrast agents, as well as contrast agent diffusion and/or convection, causes increased blurring at tissue boundaries. [9] Rapid changes in contrast media concentration during image acquisition can change lesion contrast and thus contribute additional blurring in the phase-encoding direction. These effects can obscure the lesion margin and make morphology assessment more difficult (see Figure 1). In addition, contrast agent administration is contra-indicated in a small but significant percentage of the population due to the risk of contrast-agent induced nephrogenic systemic fibrosis or allergic reactions. [10] These issues could be addressed by effective imaging without contrast agents.
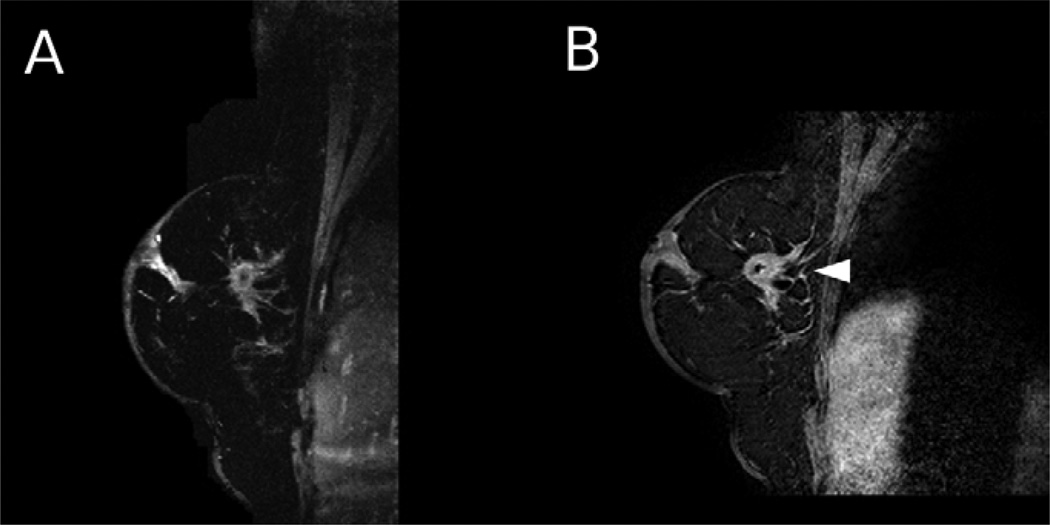
Figure 1.
A 69 year old woman with an invasive ductal carcinoma lesion was imaged using HiSS and conventional imaging. a) HiSS water peak height (TR/TE = 500/90 ms, in-plane resolution 1 mm) and b) conventional T1-weighted (TR/TE = 12.6/3.8 ms, in-plane resolution 1 mm) sagittal images are shown. The lesion is indicated with an arrow. Spiculations surrounding the lesion are much better visualized in the HiSS image, probably due to contrast agent diffusion from the lesion in the DCEMRI image.
In addition to effective pre-contrast imaging, the high spectral and spatial resolution (HiSS) spectroscopic imaging sequence [11–13] used in this work brings further advantages. First, in HiSS datasets, the spatial and spectral information are separated, allowing effective fat suppression in post-processing, rather than active suppression during image acquisition [14]. Early work by Harms and colleagues suggested that MRI with effective fat suppression could detect and characterize suspicious breast lesions without the need to inject contrast agents. [15, 16] However, even modern methods do not uniformly suppress fat across the breast, and they decrease signal intensity due to magnetization transfer effects. This laboratory introduced the use of HiSS imaging as an effective alternative to conventional fat-suppressed imaging. [12, 17, 18] Second, with HiSS, it is possible to construct images of a single Fourier component of the water resonance, e.g. water peak height images, which provide pure water images with excellent edge delineation, no fat contamination, and strong T2* contrast without the loss of image quality that is associated with conventional T2*-weighted images, as in HiSS, images from the entire echo train are used. Finally, analysis of the details of water resonance lineshape in each voxel can provide clinically useful information, and HiSS-derived quantitites have already been successfully correlated with tumor vascularization [14] and biopsy results. [19, 20]
If pre-contrast HiSS could effectively evaluate breast lesions, starting with their morphology, then pre-contrast HiSS MRI could in some cases substitute for the delayed contrast-enhanced images currently used to evaluate morphology. This would provide a safer alternative for patients who may not tolerate contrast agents. In addition, DCEMRI protocols with much higher temporal resolution could be applied, at the expense of very high (e.g. submillimeter) spatial resolution – an intriguing proposition that would be acceptable if a pre-contrast sequence could evaluate lesion morphology effectively and accurately. Higher temporal resolution could be useful for quantitative characterization of the contrast agent uptake and washout dynamics, or sometimes for simply detecting a small lesion against rapidly enhancing parenchyma. Alternatively, it would be possible to allocate higher spatial and temporal resolution during a DCEMRI acquisition to the regions of the breast found suspicious in pre-contrast imaging - essentially using pre-contrast HiSS images to guide subsequent DCEMRI. In both cases, sensitivity and specificity could be significantly improved. Earlier studies [21, 22] indicate that temporal resolution as high as 7 s may have potential for improving the differential diagnosis and characterization of DCIS, as well as evaluation of normal parenchyma. In addition, non-contrast enhanced protocols, perhaps combining a HiSS sequence for morphology evaluation with a diffusion-weighted sequence for effective lesion detection, could open possibilities for wider MRI breast cancer screening.
HiSS MRI requires sampling of the proton free induction decay (FID) and this increases the time required for data acquisition. However, recent technical development of HiSS has included demonstration of unilateral full-breast multi-slice imaging, [23] and implementation of parallel imaging (SENSE) [24] will further reduce run-times. Current SENSE-enabled technology would allow bilateral HiSS coverage in ~10 min. Thus HiSS MRI in the clinical setting can be practical.
Previous technical evaluation of HiSS images – as compared to conventional contrast-enhanced fat-suppressed T1-weighted gradient echo images (referred to in the following as ‘conventional’ images) – found that HiSS improved lesion conspicuity, margin and internal definition; contrast of parenchyma against the background, improved skin line visualization; and improved fat suppression. [11–13, 17, 23] However, clinical evaluation of HiSS water peak height images by radiologists has not been previously reported. In earlier studies, the morphology of lesions was not evaluated, and the lesions were not rated for probability of malignancy, nor correlated with biopsy. The purpose of the present study was to evaluate the performance of pre-contrast HiSS water peak height images, relative to that of conventional contrast-enhanced MRI, in image quality and the clinical task of diagnosing benign and malignant breast lesions based on morphological features.
MATERIALS AND METHODS
Patients
In our clinic, patients with suspicious breast lesions detected on X-ray mammography often receive clinical MRI scans. Fifty four patients from this population with mass-like lesions (average age 53 years, median age 53) were approached prospectively for consent to an additional research imaging acquisition, over the period of 2002–2008. Pathology results were first reviewed by an experienced pathologist and then reviewed at a multidisciplinary conference. The final results showed 10 benign (2 fibroadenomas, 1 sclerosing adenosis, 1 atypical ductal hyperplasia, 2 categorized as benign based on biopsy results, and 4 categorized as benign based on MRI reading) and 44 malignant lesions (19 invasive ductal carcinoma (IDC), 4 ductal carcinoma in situ (DCIS), 12 IDC with DCIS, 2 invasive lobular carcinoma (ILC), and 1 ILC with lobular carcinoma in situ). The average size of lesions was of 24 mm, and median size was 22 mm. The research protocol was approved by the Institutional Review Board; HIPAA guidelines were adhered to, and written informed consent was obtained from all subjects.
MRI protocol
MR images were acquired on a 1.5T Achieva scanner (Philips Healthcare, Cleveland, Ohio), and on a 1.5T GE SIGNA scanner equipped with Echo Speed™ gradients (GE Medical Systems, Waukesha, Wisconsin). The body coil was used for excitation, and a 7-element (Philips) or 4-element (GE) phased array coil designed for breast imaging was used to detect signal.
The imaging protocol used on the Philips scanners was as follows:
2D bilateral T2-weighted scan (axial)
Single-slice HiSS scan (sagittal)
3D Bilateral isotropic pre-contrast fat-suppressed gradient echo scan (axial, 3 min run time, spatial resolution of 1 × 1 mm in 1 mm thick slices)
3D Bilateral pre-contrast fat-suppressed gradient echo scan, (axial, 1 min temporal resolution, spatial resolution of 1 × 1 mm in 2 mm thick slices)
3D Bilateral dynamic fat-suppressed gradient echo scan during minutes 1 and 2 post contrast (axial, 1 min temporal resolution, spatial resolution of 1 × 1 mm in 2 mm thick slices)
3D Bilateral isotropic fat-suppressed post-contrast gradient echo scan during minutes 3–5 (axial, 3 min temporal resolution, spatial resolution of 1 × 1 mm in 1 mm thick slices)
3D Bilateral dynamic fat-suppressed gradient echo scan during minute 6 post contrast (axial, 1 min temporal resolution, spatial resolution of 1 × 1 mm in 2 mm thick slices)
The imaging protocol used on the GE scanners was as follows:
2D bilateral T2-weighted scan (axial)
Single or two-slice HiSS scan (sagittal)
3D Bilateral dynamic gradient echo scan (coronal, 1 min temporal resolution, pre and post contrast)
2D unilateral or bilateral fat-suppressed T1-weighted spoiled gradient echo (SPGR) scan (sagittal)
Based on the T2-weighted MR images and a previous X-ray mammogram, a sagittal slice through the suspicious lesion was selected by an experienced radiologist for HiSS acquisition. Only one or two HiSS slices were imaged (acquisition parameters listed in Table 1) using echo-planar spectroscopic imaging (EPSI), [25, 26] due to time constraints on the imaging protocol. Both spectral and spatial resolutions of HiSS images were sufficient to avoid truncation artifacts. HiSS images were acquired with the readout gradient applied in the antero-posterior direction to minimize artifacts due to respiratory and cardiac motion.
Table 1.
HiSS imaging sequence parameters
| Philips Achieva | GE SIGNA | |
|---|---|---|
| TR [ms] | 500 | 250–500 |
| effective TE [ms] | 90 | 96–192 |
| flip angle | 90° | 60° |
| echo train length | 128 | 64–128 |
| echo spacing [ms] | 1.4 | 3 |
| spectral bandwidth [Hz] | 715 | 333 |
| spectral resolution [Hz] | 5.6 | 2.6–5.2 |
| field-of-view, readout direction [mm] | 256 | 240–360 |
| field-of-view, phase encode direction [mm] | 256 | 120–180 |
| acquisition matrix | 256 × 256 | 384 × 192 |
| in-plane resolution [mm] | 1 | 0.65–0.95 |
| slice thickness [mm] | 3 | 3–4 |
| number of slices imaged | 1 | 1–2 |
| acquisition time | 2’ 8” | 1’ 36” |
On the Philips Achieva scanner, the bilateral isotropic dynamic gradient echo scan (TR/TE = 12.6/3.8 ms, in-plane resolution 1 mm, slice thickness 1 mm) was reformatted in the sagittal plane for comparison with HiSS images. The data was acquired with an isotropic sequence, and therefore the reformatting was possible without loss of image quality. Fat suppression was achieved using SPAIR (spectral attenuated inversion recovery). On the GE SIGNA scanner, unilateral or bilateral sagittal fat saturated T1-weighted images were acquired 6–10 minutes post contrast injection (TR/TE = 175/4.2 ms, in-plane resolution 1 mm, slice thickness 3 mm). Fat suppression was achieved using SPECIAL™ (GE Medical Systems, Waukesha, Wisconsin). For both Philips and GE, the above images were used to evaluate lesion morphology in the comprehensive exam reading at the time of clinical care, and thus were selected for comparison to HiSS.
HiSS data processing
Data analysis was performed off-line, using software written in IDL (Interactive Data Language; ITT, Boulder, CO). A previously published algorithm [12, 18] was used to generate a proton spectrum in each voxel, identify fat and water peaks, and isolate the water component by subtracting a Lorentzian fit to the fat resonance and a baseline. [18] Images proportional to the water spectral peak height were generated. Thus HiSS images are effectively fat-suppressed in post-processing, rather than through active suppression during image acquisition, and this process is not affected by B0 field inhomogeneities.
Radiologist evaluation
One image from conventional fat-suppressed T1-weighted imaging (GE) or from the fat-suppressed T1-weighted isotropic sequence reformatted in the sagittal plane (Philips) that most closely corresponded to the HiSS image obtained for the same patient was selected for evaluation. One conventional and one HiSS image for each patient (a total of 108 images) were evaluated in random order (not paired) by three radiologists (years of experience reading breast MRI exams: 5, 6, and 4, for readers A, B, and C, respectively), separately and independently. The readers were blinded to the patients’ histology and prior imaging results, and the imaging method. Lesion morphology was evaluated according to the BIRADS lexicon, using the categories listed in Table 2. Boundary delineation and quality of fat suppression were evaluated on a 4-point scale (poor (0), fair (1), good (2), excellent (3)). An overall probability of malignancy was also assigned to each lesion image on a scale of 0–100% (in 5% increments). When no lesion was detected by a radiologist, the study was discarded. (The readers were aware that each image contained a lesion, and the sensitivity of either HiSS or conventional imaging was not assessed in this single-slice reading.)
Table 2.
BIRADS assessment categories used
| BIRADS category | Values |
|---|---|
| lesion shape | round, oval, lobular, irregular |
| lesion margin | smooth, irregular, spiculated |
| internal signal intensity pattern | homogeneous, heterogeneous, dark internal septations, bright internal septations, rim (peripheral hyper-intensity), central hyper-intensity |
| final BIRADS assessment | 0–5 |
Statistical analysis
The proportions of lesions detected by either conventional or HiSS images were compared using the McNemar test. The un-weighted kappa test was used to evaluate concordance between readers for the BIRADS categories evaluated here (average kappa is reported). The Wilcoxon signed rank test was used to compare the boundary delineation and quality of fat suppression for HiSS and conventional images, for the three readers separately. The Wilcoxon rank sum test was used to compare probability of malignancy scores (averaged over the three readers) of benign vs. malignant lesions, in HiSS and conventional images separately. The Wilcoxon signed rank test was used to compare probability of malignancy scores (averaged over the three readers) in HiSS vs. conventional images, for benign and malignant lesions separately.
ROC curves were generated using the probability of malignancy as a classifier for the task of separating benign and malignant lesions, for all three readers separately. The DBM-MRMC package for ROC analysis was used, [27–33] using the proper binormal model to fit ROC curves and a fixed readers ANOVA model. [34] Non-inferiority was tested using one-sided 95% CI, assuming normality of AUC and using the AUC CI as estimated by DBM-MRMC. The lower bound for non-inferiority was set at −0.1, which we believe to be a reasonable value for a preliminary clinical trial.
RESULTS
In Figure 1, HiSS water peak height and conventional sagittal images are shown for a 69 year old woman with an invasive ductal carcinoma lesion (arrow). In this case, spiculations surrounding the lesion are better visualized in the HiSS image. In Figure 2, similar images of a 53 year old woman with an atypical ductal hyperplasia lesion (arrow) are shown. Fat suppression is superior in the HiSS image. In Figure 3, similar images are shown for a 44 year old woman with a cancerous lesion (arrow) and lymph node invasion (not shown), and again, fat suppression is superior in the HiSS image.
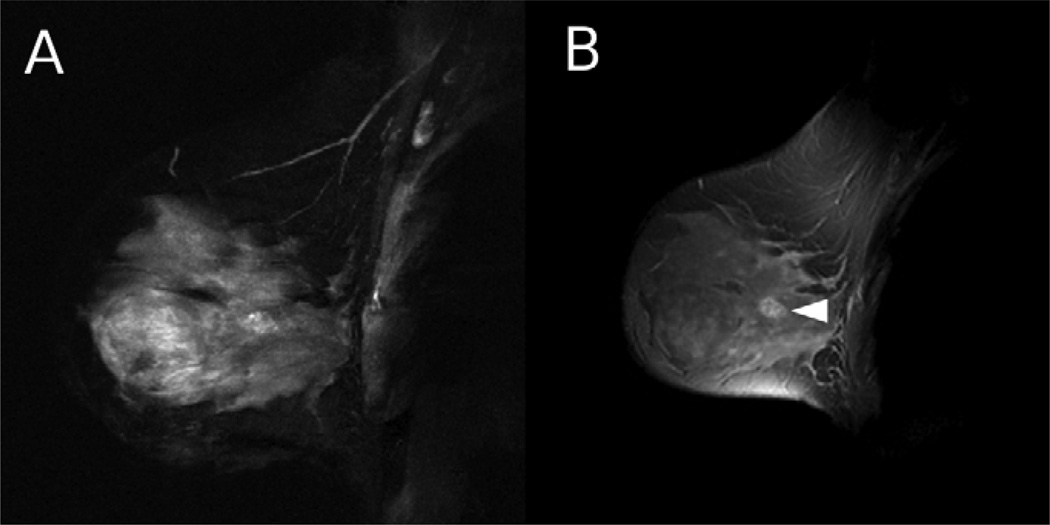
Figure 2.
A 53 year old woman with an atypical ductal hyperplasia lesion was imaged using HiSS and conventional imaging. a) HiSS water peak height (TR/TE = 500/192 ms, in-plane resolution 1 mm) and b) conventional T1-weighted (TR/TE = 175/4.2 ms, in-plane resolution 1 mm) sagittal images are shown. The lesion is indicated with an arrow. Fat suppression is superior in the HiSS image.
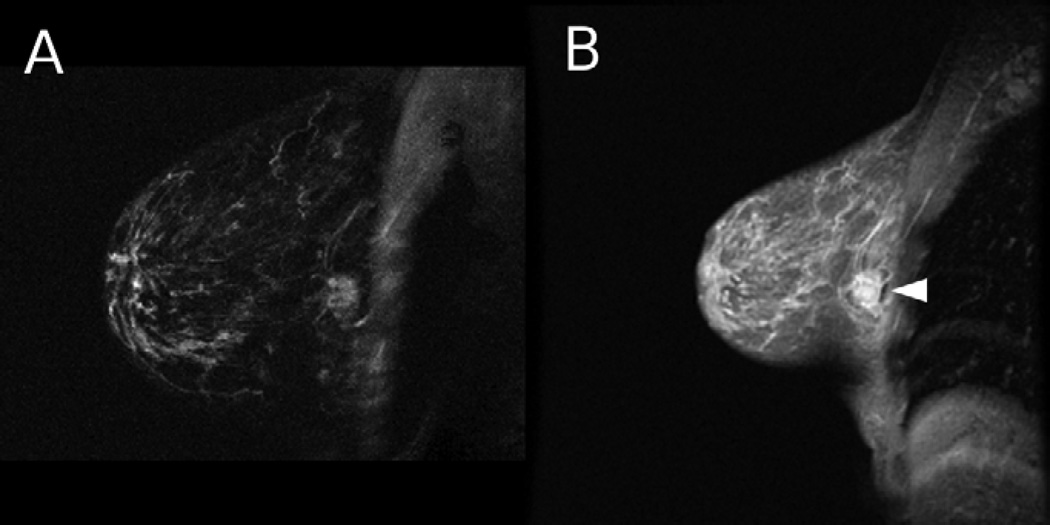
Figure 3.
A 44 year old woman with a cancerous lesion and lymph node invasion (not shown) was imaged using HiSS and conventional imaging. a) HiSS water peak height (TR/TE = 250/96 ms, in-plane resolution 0.63 mm) and b) conventional T1-weighted (TR/TE = 175/4.2 ms, in-plane resolution 1 mm) sagittal images are shown. The lesion is indicated with an arrow. Fat suppression is superior in the HiSS image.
Overall
All lesions were identified by at least one reader. Fifty three of 54 lesions on conventional, and all lesions on HiSS images were identified by at least two readers. Fifty of 54 on conventional, and 51 of 54 lesions on HiSS images were identified by all three readers. Although the detection rates were marginally higher for HiSS images compared to conventional images, these differences were not statistically significant.
BIRADS measures
The three BIRADS descriptive measures and the final BIRADS assessment are category measures and were used to evaluate concordance between readers. The concordance was found to be in the fair to moderate range for lesion shape, lesion margin and internal signal intensity pattern, which is not surprising given the large number of descriptor choices. Final BIRADS category assessment prevalence (approximately 80% of the images were assessed as BIRADS category 4) precluded meaningful application of the ‘kappa’ test. Thus the continuous ‘probability of malignancy’ was a more appropriate measure for comparison of performance. There were no substantial differences in ‘kappa’ test results between HiSS and conventional images. We looked at the pattern of differences between HiSS and conventional images in rating for each BIRADS category. For this purpose, the majority reading was used (when all three readings differed, the data point was discarded). The radiologists’ evaluation of lesion shape in HiSS and conventional images differed in 13 of 46 (23%, when ‘Round’ and ‘Oval’ were pooled together) of the patients, with no particular pattern. The radiologists’ evaluation of lesion margin differed in 25 of 48 (52%) of the patients, with no particular pattern. Internal signal intensity pattern was rated differently in 13 of 52 (25%) of patients. In 11 cases the internal pattern was classified as ‘homogeneous’ in conventional images and ‘heterogeneous’ or ‘dark internal septations’ in HiSS images. In an additional 8 (15%) patients, the internal signal intensity pattern was ‘Rim’ – related to contrast agent uptake – in conventional images, and a different pattern in HiSS images (pre-contrast). The final BIRADS assessment differed in 2 of 49 (4%) of patients.
Image quality measures
For all readers, there was no statistically significant difference in average scores of HiSS and conventional T1-weighted images on either boundary delineation or quality of fat suppression (p > 0.25 in all cases).
Probability of malignancy measure
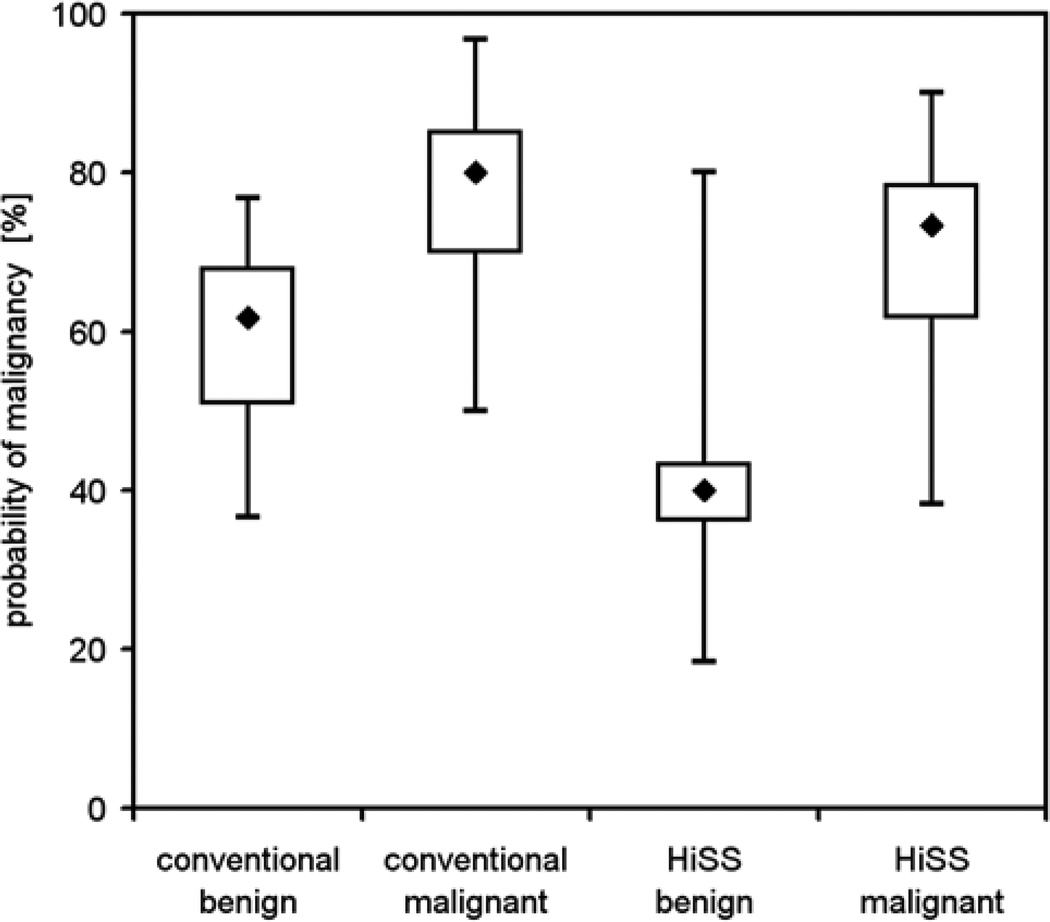
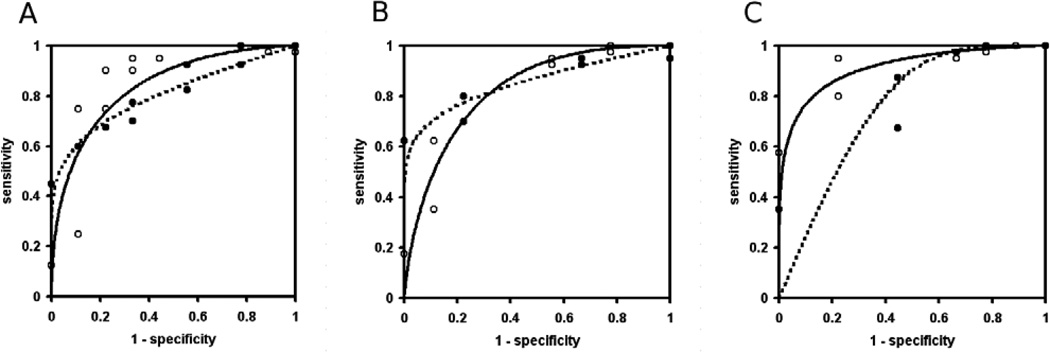
Both conventional T1-weighted and HiSS images discriminated well between benign and malignant lesions (p < 0.001), though in Figure 4 the separation of benign and malignant lesions appears to be more obvious for HiSS images. The differences in the mean of the probability of malignancy distribution between conventional and HiSS images were statistically significant for both benign (p < 0.05, difference −16.8, 95% CI: (−31.7; −2.0)) and malignant lesions (p = 0.001, difference −7.4, 95% CI: (−12.4; −2.5)), indicating that conventional imaging was less likely to label a lesion as malignant in both cases. The impact of these differences on individual patient management is best evaluated via ROC analysis. ROC curves that used probability of malignancy as the decision variable for the task of separating benign and malignant lesions are shown in Figure 5, for HISS (open circles, solid line) and conventional images (solid circles, dotted lines), for readers A, B and C. The lines represent proper binormal model fits to the actual data. AUC values for conventional T1-weighted and HiSS images are 0.81 and 0.84 respectively (difference 0.036, 95% CI: (−0.25;0.32)) for reader A, 0.86 and 0.83 (difference −0.028, 95% CI: (−0.26;0.20) for reader B, and 0.76 and 0.91 (difference 0.16, 95% CI: (0.02;0.30) for reader C. No statistical significance was found in the difference in the overall performance of the two imaging methods (p = 0.6). The overall AUCs for conventional and HiSS imaging were 0.807 and 0.862 respectively. The difference in AUCs was +0.056 ± 0.091, with a 95% one-sided CI lower bound of −0.095 which is larger than −0.1; thus pre-contrast HiSS satisfies our non-inferiority condition with respect to post-contrast conventional imaging. In fact, the AUC values for HiSS may be somewhat better than for conventional imaging - but this difference is not statistically significant. The post hoc power of the test is 0.52, as can be expected when the lower bounds for non-inferiority test and the confidence interval are very close.
Figure 4.
Boxplots for probability of malignancy distribution are shown for benign and malignant lesions, for conventional T1-weighted and HiSS images. Both conventional and HiSS imaging methods show a statistically significant separation of benign and malignant lesions (p < 0.001), but this appears to be more obvious in HiSS data. The differences in the mean of the probability distribution between conventional and HiSS images were statistically significant for both benign and malignant lesions (p < 0.05).
Figure 5.
ROC plots with probability of malignancy as a classifier, and proper binormal model fits to the data, are shown for conventional T1-weighted (solid circles, dotted line) and HiSS (open circles, solid line) images. The AUC values for conventional and HiSS images are 0.81 and 0.84 respectively (difference 0.036, 95% CI: (−0.25;0.32)) for reader A, 0.86 and 0.83 (difference −0.028, 95% CI: (−0.26;0.20) for reader B, and 0.76 and 0.91 (difference 0.16, 95% CI: (0.02;0.30) for reader C. There is no statistically significant difference in the overall performance of the two imaging methods.
DISCUSSION
We have compared pre-contrast HiSS and conventional post-contrast fat-suppressed T1-weighted images based on several morphologic and quantitative criteria. There is substantial variation in BIRADS descriptive morphological measures between HiSS and conventional images. This could be a consequence of different weighting in the images (T1 in conventional, vs. a mixture of T1 and T2* weighing in HiSS images), the presence of contrast agent in conventional images, or simply a test-retest variation in reading the images. In a large number of patients, internal signal intensity pattern was found to be ‘homogeneous’ in conventional, but ‘heterogeneous’ or ‘dark internal septations’ on HiSS. This is consistent with previous reports [12] demonstrating that HiSS images appear to provide better internal contrast than conventional images, potentially a consequence of the high dynamic range of HiSS water peak height images and the sensitivity of HiSS images to subtle local magnetic susceptibility gradients, including gradients due to deoxyhemoglobin and micro-calcifications. Alternatively, with the elimination of some of the blurring inherent to conventional imaging, or due to contrast agent administration, it may have been possible to visualize internal contrast within lesions with clarity. Previous quantitative analyses showing superior fat-suppression [11, 23] in HiSS images were not reproduced here with statistical significance. This could be due to the fact that in the present study a subjective overall impression by the radiologists was measured, which carries a larger statistical uncertainty compared to objective quantitative evaluation used in earlier studies.
The performance of the overall ‘probability for malignancy’ measure in differentiating benign from malignant lesions is the most important result of the study. Within the uncertainties of this trial, pre-contrast HiSS imaging showed promising non-inferior performance compared to conventional contrast-enhanced imaging in the task of separating benign and malignant breast lesions. In fact, the overall AUC value was slightly higher for HiSS than for conventional imaging, although this difference was not statistically significant due to the uncertainty of our estimate. The non-inferiority test is meaningful because the new method provides considerable advantages not directly related to the diagnosis, at least for the subset of the population that cannot tolerate contrast agents. It is interesting to note that the averages of the ‘probability of malignancy’ measure in conventional images are not clustered preferentially below 50% for benign lesions. This is almost certainly due to the fact that only a single-slice image was used for evaluation, rather than the whole exam, which would include the information on surrounding tissue, and on contrast uptake and washout. In HiSS images, however, the ‘probability of malignancy’ scores for benign lesions are below 50% on average, even with the limited singleslice information. Notably, pre-contrast HiSS images separate benign and malignant lesions as well as conventional post-contrast images. This is not surprising, as non-contrast imaging has advantages for evaluation of morphology, as discussed in the 'Introduction'. A more comprehensive study evaluating multi-slice 3D lesion morphology derived from HiSS images, as an addition to the full set of clinical images including a pre-contrast T2 weighted and the complete dynamic sequence, would provide an even better and more realistic evaluation of HiSS performance.
The data presented here suggest that HiSS is a promising technique for detection and evaluation of lesions before injection of contrast media. This is consistent with the results of previous work [12, 16, 35, 36] although, as all lesions in this study were visible on pre-contrast HiSS images, the sensitivity of HiSS imaging was not evaluated here. Reliable visualization of small lesions before contrast injection would have advantages. First, perceived lesion morphology often changes after contrast injection because contrast media may cause ‘blooming’ and blurring (as illustrated in Figure 1). [9] HiSS imaging would allow more accurate evaluation of lesion morphology and this might be diagnostically useful, as is illustrated in Figure 3. HiSS might also prove useful during MRI-guided biopsy where it might eliminate the need for repeated contrast injections. Second, reliable detection of lesions with contrast-enhanced MRI requires subtraction of a pre-contrast image from a post contrast image. This reduces signal-to-noise ratio and introduces blurring due to motion.
Finally, accurate morphological evaluation of suspicious lesions before contrast media injection could allow optimization – perhaps via integration with computer-aided detection and diagnostics – of the subsequent DCEMRI protocol. One way to achieve this is to provide high temporal and/or spatial resolution in suspicious areas, with more modest resolution elsewhere, especially during the critical early phase of contrast media uptake and washout. It is likely that this would increase both sensitivity and specificity. [22] Specifically, a very high temporal resolution (on the order of 10–15 s per bilateral acquisition) DCEMRI sequence would allow fully quantitative analysis of the DCEMRI data using the two-compartment (or more complex) model of contrast agent uptake and washout. Alternatively, screening could also be improved by using fast DCEMRI to visualize tumor contrast uptake in the first 15–30 s, when it is not accompanied by the parenchymal enhancement background signal. Such acceleration would come at the expense of very high spatial resolution, but intermediate resolution – on the order of 1 mm – would be acceptable if a pre-contrast sequence is available that evaluates morphology of any detected lesion effectively and accurately. Also, in non-contrast enhanced screening protocols (e.g.including diffusion-weighted MRI), HiSS could provide accurate morphological assessment of any detected lesions.
There are several limitations to this study. Most importantly, the contrast kinetics of the DCEMRI scans was not considered in the comparison with HiSS imaging. Instead, this study focused on morphological, rather than functional, information in both HiSS and DCEMRI images. Functional imaging using HiSS and its potential for improving diagnostic performance has been pursued in other studies, [14] [19, 20] but also was not evaluated here. As DCEMRI images are currently used for morphological assessment of lesions, it is appropriate to ask whether a non-contrast sequence can provide the same information, allowing development of DCEMRI protocols that focus on functional (contrast kinetics), rather than anatomical imaging, as discussed above.
Another significant limitation is the single-slice implementation of HiSS imaging, used in this research due to the time constraints on the imaging protocol. For example, this guided our choice to study mass-like lesions, as they are easier to target. However, recent results demonstrate significant improvements in HiSS imaging speed using parallel imaging [24] and parallel sampling of multiple lines of k-space. Volumetric imaging has already been implemented in clinically feasible times. [23] Furthermore, long run times are less critical when performed pre-contrast, since there is no dynamic change in the images. The positive outcome of this trial suggests that these avenues of research should be pursued, and future studies will include volumetric (multi-slice or 3D) HiSS imaging, with the ultimate goal of full bilateral coverage.
In conclusion, this first clinical evaluation of pre-contrast HiSS water peak height imaging, in the task of separating known malignant and benign lesions, demonstrates that HiSS performs similarly to conventional contrast-enhanced fat-suppressed T1 weighted images. This finding is promising for patients for whom use of contrast agent is contra-indicated, e.g., patients with compromised renal function, or for design of non-contrast enhanced screening protocols. In addition, optimization of the DCEMRI protocol based on pre-contrast HiSS data may be possible.
ACKNOWLEDGMENTS
This work was supported by grants from the NIBIB (RO1 EB003108), the NCI (RO1CA78803), the NIH/NCI (P50 CA125183), the Army Breast Cancer Research Program (DAMD 17-02-1-0033), and the Segal Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Saslow D, Boetes C, Burke W, et al. American Cancer Society guidelines for breast screening with MRI as an adjunct to mammography. CA: a cancer journal for clinicians. 2007;57:75–89. doi: 10.3322/canjclin.57.2.75. [DOI] [PubMed] [Google Scholar]
- 2.Swayampakula AK, Dillis C, Abraham J. Role of MRI in screening, diagnosis and management of breast cancer. Expert review of anticancer therapy. 2008;8:811–817. doi: 10.1586/14737140.8.5.811. [DOI] [PubMed] [Google Scholar]
- 3.Warner E, Messersmith H, Causer P, et al. Systematic review: using magnetic resonance imaging to screen women at high risk for breast cancer. Annals of internal medicine. 2008;148:671–679. doi: 10.7326/0003-4819-148-9-200805060-00007. [DOI] [PubMed] [Google Scholar]
- 4.Kuhl CK, Mielcareck P, Klaschik S, et al. Dynamic breast MR imaging: are signal intensity time course data useful for differential diagnosis of enhancing lesions? Radiology. 1999;211:101–110. doi: 10.1148/radiology.211.1.r99ap38101. [DOI] [PubMed] [Google Scholar]
- 5.Huang W, Fisher PR, Dulaimy K, et al. Detection of breast malignancy: diagnostic MR protocol for improved specificity. Radiology. 2004;232:585–591. doi: 10.1148/radiol.2322030547. [DOI] [PubMed] [Google Scholar]
- 6.Bluemke DA, Gatsonis CA, Chen MH, et al. Magnetic resonance imaging of the breast prior to biopsy. JAMA : the journal of the American Medical Association. 2004;292:2735–2742. doi: 10.1001/jama.292.22.2735. [DOI] [PubMed] [Google Scholar]
- 7.Kuhl CK, Schild HH, Morakkabati N. Dynamic bilateral contrast-enhanced MR imaging of the breast: trade-off between spatial and temporal resolution. Radiology. 2005;236:789–800. doi: 10.1148/radiol.2363040811. [DOI] [PubMed] [Google Scholar]
- 8.Kneeshaw PJ, Lowry M, Manton DM, et al. Differentiation of benign from malignant breast disease associated with screening detected microcalcifications using dynamic contrast enhanced magnetic resonance imaging. Breast. 2006;15:29–38. doi: 10.1016/j.breast.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Penn A, Thompson S, Brem R, et al. Morphologic blooming in breast MRI as a characterization of margin for discriminating benign from malignant lesions. Academic radiology. 2006;13:1344–1354. doi: 10.1016/j.acra.2006.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perazella MA. Gadolinium-contrast toxicity in patients with kidney disease: nephrotoxicity and nephrogenic systemic fibrosis. Current drug safety. 2008;3:67–75. doi: 10.2174/157488608783333989. [DOI] [PubMed] [Google Scholar]
- 11.Fan X, Abe H, Medved M, et al. Fat suppression with spectrally selective inversion vs. high spectral and spatial resolution MRI of breast lesions: qualitative and quantitative comparisons. Journal of magnetic resonance imaging : JMRI. 2006;24:1311–1315. doi: 10.1002/jmri.20732. [DOI] [PubMed] [Google Scholar]
- 12.Medved M, Newstead GM, Abe H, et al. High spectral and spatial resolution MRI of breast lesions: preliminary clinical experience. AJR American journal of roentgenology. 2006;186:30–37. doi: 10.2214/AJR.04.1704. [DOI] [PubMed] [Google Scholar]
- 13.Karczmar GS, Du W, Medved M, et al. Spectrally inhomogeneous effects of contrast agents in breast lesion detected by high spectral and spatial resolution MRI. Academic radiology. 2002;9 Suppl 2:S352–S354. doi: 10.1016/s1076-6332(03)80227-1. [DOI] [PubMed] [Google Scholar]
- 14.Foxley S, Fan X, Mustafi D, et al. Sensitivity to tumor microvasculature without contrast agents in high spectral and spatial resolution MR images. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2009;61:291–298. doi: 10.1002/mrm.21801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harms SE, Flamig DP, Hesley KL, et al. Fat-suppressed three-dimensional MR imaging of the breast. Radiographics : a review publication of the Radiological Society of North America, Inc. 1993;13:247–267. doi: 10.1148/radiographics.13.2.8460218. [DOI] [PubMed] [Google Scholar]
- 16.Soderstrom CE, Harms SE, Copit DS, et al. Three-dimensional RODEO breast MR imaging of lesions containing ductal carcinoma in situ. Radiology. 1996;201:427–432. doi: 10.1148/radiology.201.2.8888235. [DOI] [PubMed] [Google Scholar]
- 17.Du W, Du YP, Bick U, et al. Breast MR imaging with high spectral and spatial resolutions: preliminary experience. Radiology. 2002;224:577–585. doi: 10.1148/radiol.2242011022. [DOI] [PubMed] [Google Scholar]
- 18.Medved M, Du W, Zamora MA, et al. The effect of varying spectral resolution on the quality of high spectral and spatial resolution magnetic resonance images of the breast. Journal of magnetic resonance imaging : JMRI. 2003;18:442–448. doi: 10.1002/jmri.10378. [DOI] [PubMed] [Google Scholar]
- 19.Medved M, Newstead GM, Fan X, et al. Fourier component imaging of water resonance in the human breast provides markers for malignancy. Physics in medicine and biology. 2009;54:5767–5779. doi: 10.1088/0031-9155/54/19/007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Medved M, Newstead GM, Fan X, et al. Fourier components of inhomogeneously broadened water resonances in breast: a new source of MRI contrast. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2004;52:193–196. doi: 10.1002/mrm.20115. [DOI] [PubMed] [Google Scholar]
- 21.Pinker K, Grabner G, Bogner W, et al. A combined high temporal and high spatial resolution 3 Tesla MR imaging protocol for the assessment of breast lesions: initial results. Investigative radiology. 2009;44:553–558. doi: 10.1097/RLI.0b013e3181b4c127. [DOI] [PubMed] [Google Scholar]
- 22.Jansen SA, Fan X, Medved M, et al. Characterizing early contrast uptake of ductal carcinoma in situ with high temporal resolution dynamic contrast-enhanced MRI of the breast: a pilot study. Physics in medicine and biology. 2010;55:N473–N485. doi: 10.1088/0031-9155/55/19/N02. [DOI] [PubMed] [Google Scholar]
- 23.Medved M, Newstead GM, Abe H, et al. Clinical implementation of a multislice high spectral and spatial resolution-based MRI sequence to achieve unilateral full-breast coverage. Magnetic resonance imaging. 2010;28:16–21. doi: 10.1016/j.mri.2009.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Medved M, Ivancevic MK, Olopade OI, et al. Echo-planar spectroscopic imaging (EPSI) of the water resonance structure in human breast using sensitivity encoding (SENSE) Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2010;63:1557–1563. doi: 10.1002/mrm.22332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doyle M, Mansfield P. Chemical-shift imaging: a hybrid approach. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1987;5:255–261. doi: 10.1002/mrm.1910050306. [DOI] [PubMed] [Google Scholar]
- 26.Mansfield P. Spatial mapping of the chemical shift in NMR. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 1984;1:370–386. doi: 10.1002/mrm.1910010308. [DOI] [PubMed] [Google Scholar]
- 27.Dorfman DD, Berbaum KS, Metz CE. Receiver operating characteristic rating analysis. Generalization to the population of readers and patients with the jackknife method. Investigative radiology. 1992;27:723–731. [PubMed] [Google Scholar]
- 28.Dorfman DD, Berbaum KS, Lenth RV, et al. Monte Carlo validation of a multireader method for receiver operating characteristic discrete rating data: factorial experimental design. Academic radiology. 1998;5:591–602. doi: 10.1016/s1076-6332(98)80294-8. [DOI] [PubMed] [Google Scholar]
- 29.Hillis SL, Berbaum KS. Power estimation for the Dorfman-Berbaum-Metz method. Academic radiology. 2004;11:1260–1273. doi: 10.1016/j.acra.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 30.Hillis SL, Berbaum KS. Monte Carlo validation of the Dorfman-Berbaum-Metz method using normalized pseudovalues and less data-based model simplification. Academic radiology. 2005;12:1534–1541. doi: 10.1016/j.acra.2005.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hillis SL, Obuchowski NA, Schartz KM, et al. A comparison of the Dorfman-Berbaum-Metz and Obuchowski-Rockette methods for receiver operating characteristic (ROC) data. Statistics in medicine. 2005;24:1579–1607. doi: 10.1002/sim.2024. [DOI] [PubMed] [Google Scholar]
- 32.Hillis SL. A comparison of denominator degrees of freedom methods for multiple observer ROC analysis. Statistics in medicine. 2007;26:596–619. doi: 10.1002/sim.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hillis SL, Berbaum KS, Metz CE. Recent developments in the Dorfman-Berbaum-Metz procedure for multireader ROC study analysis. Academic radiology. 2008;15:647–661. doi: 10.1016/j.acra.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pesce LL, Metz CE. Reliable and computationally efficient maximum-likelihood estimation of "proper" binormal ROC curves. Academic radiology. 2007;14:814–829. doi: 10.1016/j.acra.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harms SE, Flamig DP, Hesley KL, et al. MR imaging of the breast with rotating delivery of excitation off resonance: clinical experience with pathologic correlation. Radiology. 1993;187:493–501. doi: 10.1148/radiology.187.2.8475297. [DOI] [PubMed] [Google Scholar]
- 36.Soderstrom CE, Harms SE, Farrell RS, Jr., et al. Detection with MR imaging of residual tumor in the breast soon after surgery. AJR American journal of roentgenology. 1997;168:485–488. doi: 10.2214/ajr.168.2.9016232. [DOI] [PubMed] [Google Scholar]