Abstract
Chloroplast movement was induced by partial cell illumination using a high-fluence blue microbeam in light-grown and dark-adapted prothallial cells of the fern Adiantum capillus-veneris. Chloroplasts inside the illuminated area moved out (high-fluence response [HFR]), whereas those outside moved toward the irradiated area (low-fluence response [LFR]), although they stopped moving when they reached the border. These results indicate that both HFR and LFR signals are generated by high-fluence blue light of the same area, and that an LFR signal can be transferred long-distance from the beam spot, although an HFR signal cannot. The lifetime of the HFR signal was calculated from the traces of chloroplast movement induced by a brief pulse from a high-fluence blue microbeam to be about 6 min. This is very short compared with that of the LFR (30–40 min; T. Kagawa, M. Wada [1994] J Plant Res 107: 389–398). These data indicate that the signal transduction pathways of the HFR and the LFR must be distinct.
Light plays an important role in the life of a plant, not only as an energy source but also as an environmental signal. Chloroplasts migrate to sites of better illumination to optimize photosynthesis. Under weak light chloroplasts spread only over surfaces perpendicular to the direction of the light, i.e. over periclinal walls, to maximize light absorption and generate maximum photosynthesis rates (LFR). Under strong light chloroplasts move to the anticlinal wall to avoid the light and minimize photodamage (HFR). In most plants these types of chloroplast rearrangements are controlled by blue light mediated by blue-light receptors. Although the identity of the photoreceptors is unknown, a single photoreceptor is thought to mediate both the LFR and the HFR, because the action spectra for both responses are very similar (Zurzycki, 1980). Light-induced chloroplast relocation can also be induced by partial cell irradiation by both LFR and HFR light. If a cell is partially irradiated with a beam of low-fluence blue light, the chloroplasts in the cell move toward the irradiated area; however, with a high-fluence beam they move away from the irradiated area (Yatsuhashi et al., 1985; Yatsuhashi and Wada, 1990).
In some plants, such as the fern Adiantum capillus-veneris and the green algae Mougeotia scalaris and Mesotaenium caldriorum, light-induced chloroplast movement is also induced by red light and is mediated by phytochrome (Wada et al., 1993). In A. capillus-veneris the effects of red and blue light in the LFR are very similar and both wavelengths work additively, suggesting that the signal transduction pathways from red and blue light converge (Kagawa and Wada, 1996). In M. scalaris simultaneous irradiation with red and blue light changes the direction of chloroplast photo-orientation defined by red light alone (Haupt and Häder, 1994). On the other hand, in M. caldriorum red light induces slow chloroplast photo-orientation and blue light potentiates the red-light effect without changing the direction of the response (Kraml et al., 1988). The effect of blue light on the red-light-induced chloroplast photo-orientation is thus quite different in these three examples.
Chloroplast relocation induced by low-fluence blue light has been analyzed in detail in a number of plants, but the response to high-fluence blue light has not. In the present study using dark- and light-adapted A. capillus-veneris prothallial cells and partial cell irradiation, we have examined how chloroplasts behave in and out of a beam of high-fluence blue light, especially at the border of the beam, as a first step in understanding the characteristics of the LFR and HFR signals. We have also analyzed the difference between HFR and LFR blue-light signals and whether red and blue light work additively in the HFR, as is the case in the LFR.
MATERIALS AND METHODS
Plant Materials and Culture Conditions
Prothalli of the fern Adiantum capillus-veneris L. were cultured as described by Kagawa and Wada (1993). Spores collected in the greenhouses at Tokyo Metropolitan University were precultured aseptically between two layers of thin agar-gelatin film in one-tenth-strength modified Murashige and Skoog mineral salt solution under red light (0.5 W m−2). After 1 week the culture medium was changed to White's mineral salt solution (W-0876, Sigma) and the resulting protonemata were cultured under continuous white light (5.5 W m−2) for at least another 3 weeks to induce growth of prothalli, which spread perpendicularly to the incident light. Before experiments using dark-adapted prothalli, the prothalli were kept in the dark for 2 d, which resulted in almost all chloroplasts being at the cell-dividing (anticlinal) walls (the dark position; Kagawa and Wada, 1993).
Microbeam Irradiation
Prothallial cells were microbeam irradiated using an epimicrobeam irradiator (Yatsuhashi and Wada, 1990). The prothalli between agar-gelatin thin films were transferred into a cuvette composed of two round coverslips supported by a silicon-rubber, ring-shaped spacer (o.d. = 23 mm, i.d. = 17.5 mm; thickness approximately 0.8 mm). The cuvette was placed on the stage of the microbeam irradiator and individual cells were irradiated with a microbeam of blue or red light with a spot size 27 μm in diameter. Interference filters (Vacuum Optics, Tokyo, Japan) with transmission peaks at 453 and 660 nm (half-band-widths, 31 and 34 nm, respectively) provided monochromatic blue and red light. We used neutral-density filters (ND-3, ND-10, ND-30, and ND-50, Hoya, Tokyo, Japan) when necessary. We used a silicon photodiode (S1227-66BR, Hamamatsu Photonics, Hamamatsu, Japan) to measure the fluence rate of the microbeam. Red-light background illumination (30 W m−2) was used simultaneously in some experiments as an observation light; it was obtained by passing light from a halogen lamp through an interference filter (Vacuum Optics) with a transmission peak at 663.2 nm (half-band-width, 32 nm).
Analysis of Chloroplast Movement
Chloroplast movement induced by microbeam irradiation was observed under IR light obtained through a filter (IR-85, Hoya) or under background red light, obtained as described above, using an IR-sensitive video camera (C2400–07ER, Hamamatsu Photonics). (A quicktime movie sequence of the light-induced chloroplast relocation shown in Figs. 1, 3, and 6 is available at http://www.nibb.ac.jp/∼kagawa/ForPlantPhysiol/index.html.) Analysis of chloroplast movement was performed on a computer (Power Macintosh 7600, Apple Computer) using the public domain NIH image program (developed at the U.S. National Institutes of Health and available on the Internet at http://rsb.info.nih.gov/nih-image/). We obtained images of the observed cells at 1- or 2-min intervals, if not otherwise specified. Time courses of chloroplast movement were followed as the distance changes between a chloroplast and the center of the irradiation spot (see Figs. 2, 4, 5, 7, and 8). To estimate the duration of an avoidance response induced by brief blue-light irradiation (Figs. 7 and 8), each cell was recorded at 15-s intervals, chloroplast movement was plotted as tracks, and the times when the avoidance response began and ended were defined from this analysis. All experiments were repeated at least three times with different gametophytes.
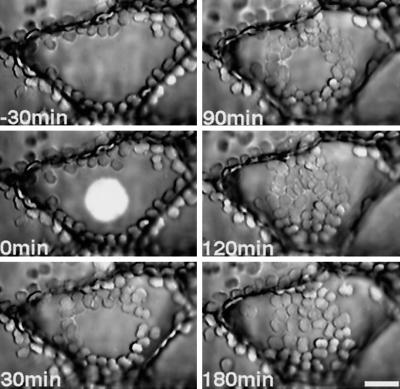
Figure 1.
Serial photographs of chloroplast relocations induced by microbeam irradiation with continuous blue light (the illumination spot is shown only at time 0) of a dark-adapted prothallial cell. Prothalli kept for 2 d in the dark were irradiated with a blue-light microbeam (10 W m−2, 27 μm in diameter). Photographs were taken at −30, 0, 30, 90, 120, and 180 min after the onset of blue-light irradiation. The prothallial cells were observed under IR light. Bar = 20 μm.
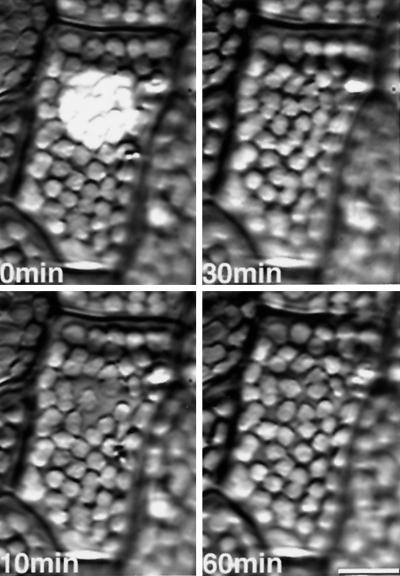
Figure 3.
Serial photographs of chloroplast relocation induced by microbeam irradiation with continuous blue light (30 W m−2) from 0 to 50 min in a light-adapted prothallial cell. The blue light was switched off at 50 min. Chloroplasts in the beam moved out of the irradiated area during the light treatment but relocated in the beam after the light was switched off. Other details are the same as in Figure 1.
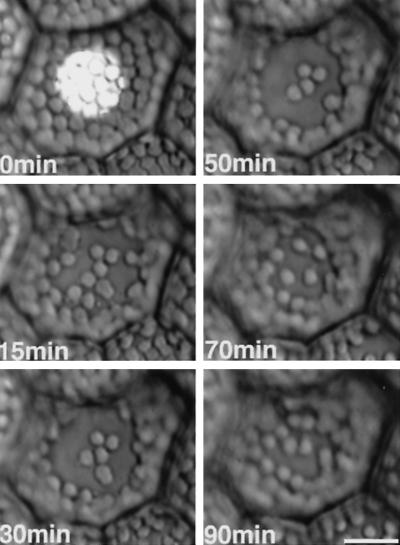
Figure 6.
Serial photographs of chloroplast relocation induced by a microbeam irradiation with a brief blue-light irradiation (30 W m−2 for 1 min) in a light-adapted prothallial cell. Other details are the same as for Figure 1.
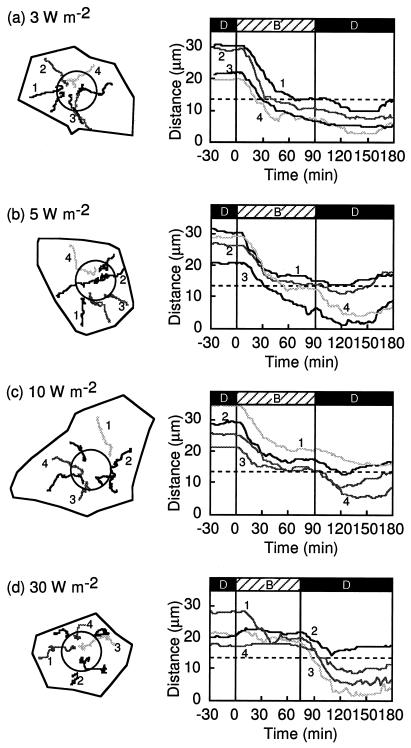
Figure 2.
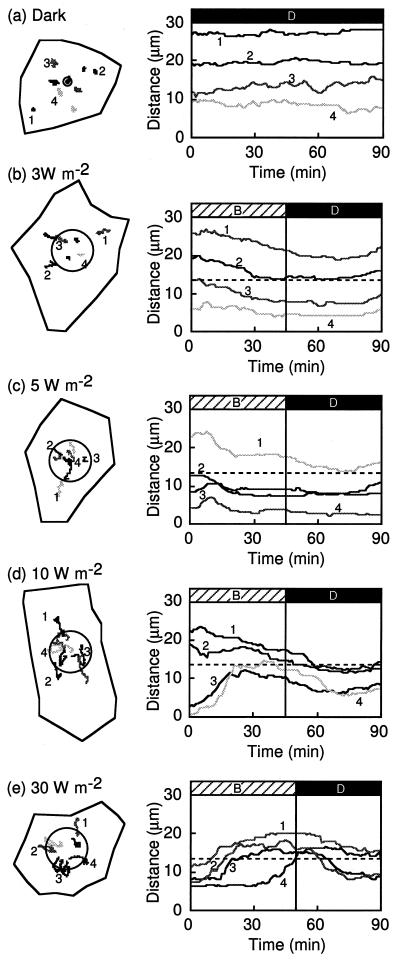
Blue-light-induced chloroplast relocation in dark-adapted prothallial cells. Schedules of light treatment are shown at the top of the figure (D, dark; B, blue light). a through d, Each dark-adapted prothallial cell was irradiated continuously with a blue-light microbeam of 3, 5, 10, or 30 W m−2 and chloroplast movement was recorded under IR light from −30 min to 180 min after the onset of blue-light irradiation. On the left are tracks of chloroplast movements in the cells (shown as closed lines) during the experimental treatment. Circles show the irradiated areas of the cells. On the right are graphs of the changes in distance between the center of the irradiated area and the chloroplasts with time. The line numbers in the right panels correspond to the chloroplast numbers shown in the left panels. The cell in c is the same one shown in Figure 1. These experiments were repeated at least three times in different cells with high reproducibility.
Figure 4.
Blue-light-induced chloroplast relocation in light-adapted prothallial cells. On the left are tracks of chloroplast movements in the cells (shown as closed lines) during the experimental treatment. Circles show the irradiated areas of the cells. On the right are graphs of the changes in distance between the center of the irradiated area and the chloroplasts with time. The line numbers in the right panels correspond to the chloroplast numbers shown in the left panels. a, Dark control immediately after transferring from light to the dark. A light-grown prothallial cell was observed in the dark under IR light. A symbol (⊙) shows the designated center of irradiation. b through e, Light-grown prothallial cells irradiated with blue light of 3, 5, 10, or 30 W m−2 for 45 or 50 min, respectively, and then kept in the dark. Each line is separated by a 1-min interval. The cell in e is the same one shown in Figure 3. Other details are the same as for Figure 2.
Figure 5.
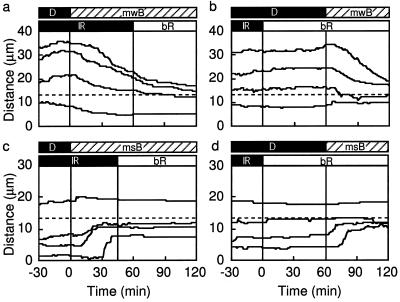
Effects of background red-light illumination on blue-light-microbeam-induced chloroplast relocation. The schedules of light treatment are shown as bars at the top of the panels. The top bars are microbeam irradiation; the bottom bars are background illumination. The other details are the same as for Figure 2. a and c, Light-adapted prothallial cells kept in the dark for 30 min before light treatment continuously irradiated with a blue-light microbeam of 3 W m−2 (mwB) or 10 W m−2 (msB), and with background red-light illumination (bR; 30 W m−2) started 45 or 60 min after the onset of the blue-light microbeam. Tracks of chloroplast relocation are shown as the distance from the center of the blue-light microbeam. b and d, The sequence of blue-light microbeam and red-light background illumination was changed as shown in the bars at the top of the panels. Four tracks are shown for each treatment.
Figure 7.
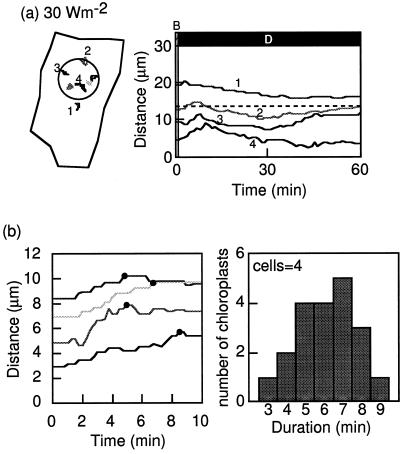
Chloroplast-avoidance response induced by a brief blue-light irradiation in light-adapted prothallial cells. a, Light-adapted prothallial cell irradiated with blue light of 30 W m−2 for 1 min. On the left are tracks of chloroplast movements in the cell (shown as closed lines) during the experimental treatment. The circle shows the irradiated area of the cell. On the right is a graph of the changes in distance between the center of the irradiated area and the chloroplasts with time. The line numbers in the right panel correspond to the chloroplast numbers shown in the left panel. The cell is the same one shown in Figure 6. b, Examples of the time courses measured as the distance changes between the chloroplast and the center of the beam spot (left), and a histogram of duration of the avoidance response (right). The duration was estimated from the time course of chloroplast movement obtained at 15-s intervals as a period between the onset of blue-light irradiation. Black circles indicate when chloroplasts began to move back. Average of this histogram is 6.2 ± 0.4 min (mean ± se). Other details are the same as for Figure 2.
Figure 8.
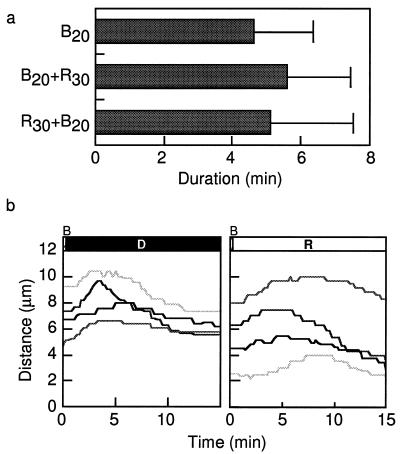
Effects of a brief irradiation with a red-light microbeam on the chloroplast-avoidance response induced by a brief blue-light microbeam in the same area. a, Duration of chloroplast-avoidance response induced by a brief blue-light microbeam (30 W m−2 for 20 s) with or without a red-light microbeam (30 W m−2 for 30 s) . B20, Blue-light irradiation for 20 s; B20+R30, blue-light irradiation for 20 s followed by red-light irradiation for 30 s; R30+B20, red-light irradiation for 30 s followed by blue-light irradiation for 20 s. Bars = se. b, Time course of chloroplast-avoidance response induced by a brief blue-light irradiation followed or not by a red-light (R) microbeam (30 W m−2) . D, Darkness.
RESULTS
Blue-Light-Induced Chloroplast Relocation in Dark-Adapted Prothallial Cells
Chloroplast relocation was induced by a continuous blue-light microbeam with different fluence rates (3, 5, 10, and 30 W m−2) at the center of the cell surface of dark-adapted prothallial cells in which chloroplasts were localized at the anticlinal walls but not at the upper surface. Chloroplasts moved toward the blue-light-irradiated area (Figs. 1 and 2) from the cell periphery. When the chloroplasts reached the edge of the beam spot, their behavior was different depending upon the fluence rate. At 3 to 5 W m−2 blue light, the chloroplasts that had located there earlier entered the beam spot and their directionality disappeared without a break in movement, whereas those that arrived later could not enter the irradiated area because of space constraints. In a continuous blue-light beam of 10 or 30 W m−2, almost all of the chloroplasts that moved toward the irradiated area stopped at the beam edge and did not enter the illuminated area. After the light was switched off, however, the chloroplasts entered this area (Figs. 1 and 2, c and d).
Blue-Light-Induced Chloroplast Relocation in Light-Adapted Prothallial Cells
Light-adapted prothallial cells were irradiated with continuous blue-light microbeams of different fluence rates (Figs. 3 and 4). As a control the light-adapted cells were transferred to darkness and the chloroplast behavior was observed for 1.5 h, but no chloroplast relocation could be detected (Fig. 4a). When the cells were irradiated with 3 W m−2 blue light, chloroplasts that had located outside of the beam moved toward the irradiated area (Fig. 4b, lines 1 and 2) and chloroplasts located at the border moved inside (Fig. 4b, line 3). However, chloroplasts inside the beam remained in their original position (Fig. 4b, line 4). With 5 W m−2 blue-light treatment, inside-located chloroplasts moved toward the beam edge for the first 10 min as if they were avoiding the high-fluence beam, but then changed their direction of movement back toward the center (Fig. 4c, lines 3 and 4). Chloroplasts that were located outside moved toward the irradiated area, but stopped if they touched other chloroplasts farther inside (Fig. 4c, line 1). When cells were irradiated with 10 or 30 W m−2 blue light, a typical HFR was observed. Inside-located chloroplasts began to move toward the beam border (Figs. 3 and 4, d and e), and stopped moving when they came out of the beam. In contrast, chloroplasts that had been located far from the beam moved toward the irradiated area as far as they could (Fig. 4d, line 1). After the light was switched off, the chloroplasts moved inside or toward the inside of the beam.
Effects of Background Red-Light Illumination
We next examined whether the blue-light-induced chloroplast-avoidance response was affected by simultaneous illumination with background red light. Light-adapted cells were incubated in the dark (observed under IR conditions) for 30 min and then irradiated with a blue-light microbeam of 3 or 10 W m−2 (Fig. 5, a and c, respectively) simultaneously with IR light for 60 or 45 min, respectively, followed by 30 W m−2 red light over the whole cell area. Under these conditions chloroplast movement before and after the transition from IR to red light was not affected. Similarly, after 30 min of observation under IR conditions, the cells were irradiated with background red light over the whole cell area. Sixty minutes after the onset of the red-light irradiation, parts of the cells were irradiated with a blue-light microbeam of 3 or 10 W m−2 (Fig. 5, b and d, respectively). Again, we observed the expected pattern of blue-light-induced chloroplast relocation. Our data indicate that the blue-light-induced chloroplast relocation was not affected by red light under LFR or HFR conditions (Fig. 5).
A Brief Chloroplast-Avoidance Response Induced by Blue Light
In traditional experiments HFR was usually induced by continuous blue-light irradiation. We studied whether chloroplast-avoidance responses could be induced by a brief blue-light irradiation in light-adapted prothallial cells (Figs. 6 and 7a). When the cells were irradiated with a blue-light microbeam of 30 W m−2 for 1 min and transferred to darkness, inside-located chloroplasts began to move toward the outside of the beam, as if they were avoiding the light (Fig. 7a, right panel, lines 3 and 4). In contrast, outside-located chloroplasts moved toward the beam (Fig. 7a, right panel, line 1). Most of the inside-located chloroplasts stopped outward movement within 10 min after the light was switched off. The average duration of the outward movement was 6.2 ± 0.4 min (Fig. 7b). The outward movement of inside-located chloroplasts could also be induced with a blue-light irradiation of 30 W m−2 for 20 s (Fig. 8), but 6 s was not enough (data not shown).
Next we examined the sequential irradiation with red and blue microbeams in the same area to determine whether red-light irradiation affected the brief blue-light-induced avoidance response. Red-light irradiation with a microbeam of 30 W m−2 for 30 s alone as a control did not induce a chloroplast-avoidance response (data not shown). The brief red-light irradiation (30 s) did not affect the outcome of the blue-light irradiation with 30 W m−2 for 6 or 20 s, whether given before or after. There was no effect on the avoidance response of inside-located chloroplasts, on the accumulation response of outside-located chloroplasts (data not shown), or on the duration of the outward movement of inside-located chloroplasts (Fig. 8a).
Finally, we examined the effect of a continuous red-light microbeam (30 W m−2) after the blue-light microbeam pulse (30 W m−2 for 6 or 20 s) on blue-light-induced chloroplast movement. Under blue light for 20 s, a chloroplast-avoidance response was observed, for the first several minutes in both cases with or without red-light irradiation (Fig. 8b). In contrast, under blue-light irradiation for 6 s with or without continuous red-light irradiation of 30 W m−2, we did not observe a chloroplast-avoidance response, but did find an accumulation response (data not shown). These experiments indicated that red light had no effect on the blue-light-induced HFR.
DISCUSSION
Light-induced chloroplast relocation was induced by a microbeam of high-fluence blue light given in the center of fern prothallial cells. When we observed the behavior of each chloroplast in a given cell continuously, both LFRs and HFRs, even in a single cell, could be seen outside and inside of the beam spot of blue light, respectively. This result indicates that both LFR and HFR signals were elicited in the beam-irradiated area, although it is not known whether they shared one or more common photoreceptors. Furthermore, these findings suggest that the LFR signal can be transferred long distances from the beam to the cell periphery, but that the HFR signal cannot. In very high-fluence light (30 W m−2), the chloroplasts moved approximately 5 μm out of the beam, but this may have been a consequence of stray light entering through the cell wall. The lifetimes of the LFR and HFR signals were also found to differ: the LFR lifetime was about 30 to 40 min (Kagawa and Wada, 1994), whereas the HFR lifetime was less than 10 min (Fig. 7). These findings suggest that the signal transduction pathways for the LFR and HFR responses must differ.
The photoreceptors may also be distinct, although the photoreceptors for the LFR and HFR responses of Lemna trisuka and Funaria hygrometrica were thought to be the same, because the shapes of the action spectra of these phenomena were those of a typical blue-light response (Zurzycki, 1980). We do not yet know the identity of the blue-light receptor for the LFR and HFR in A. capillus-veneris, although we have recently cloned five cryptochromes (Wada et al., 1997; Kanegae and Wada, 1998) and one NPH1-like phytochrome (Adi phy3) (Wada et al., 1997; Nozue et al., 1998) that may be candidates. All of these genes are expressed in gametophytes. Further investigation is needed to identify which blue-light receptor corresponds to which chloroplast movement.
The LFR in A. capillus-veneris gametophytes can be induced by red or blue light (Yatsuhashi et al., 1985; Kagawa and Wada, 1994). The signal transduction pathways are known to be at least partly shared (Kagawa and Wada, 1996). Sequential irradiation with blue or red light of subthreshold fluences (60 and 10 J m−2, respectively, under which no chloroplast movement could be induced), did induce an LFR in dark-adapted A. capillus-veneris gametophytes. In the present study we have examined whether red-light signals could also play a role as a part of the signal for blue-light-induced HFR (Figs. 6 and 8). If the red-light signal is the same as the blue-light HFR signal, then irradiation with blue light slightly lower than the threshold plus red light should induce HFR. Two different experiments were performed to address this question: (a) blue-light microbeam plus simultaneous red-light background illumination, and (b) sequential irradiation with blue and red microbeams in the same area. However, we obtained no positive response, again indicating that the signal transduction pathways for the blue-light-induced LFR and HFR are different.
Further advancement of the study of light-induced chloroplast relocation will require mutants deficient in this phenomenon (Trojan and Gabrys, 1996), and we are now screening such mutants from A. capillus-veneris. If we could isolate LFR- and HFR-deficient mutants in different clones, then we could be sure that the LFR and HFR are indeed distinct phenomena controlled by different genes and by different signal transduction pathways.
ACKNOWLEDGMENT
We are grateful to Dr. Jane Silverthorne of the University of California (Santa Cruz) for her critical reading of the manuscript.
Abbreviations:
- HFR
high-fluence response
- LFR
low-fluence response
Footnotes
This work was partially supported by a Grant-in-Aid for Scientific Research (no. 09440270 to M.W.) from the Ministry of Education, Science, Sports and Culture of Japan.
LITERATURE CITED
- Haupt W, Häder D-P. Photomovement. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants, Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 707–732. [Google Scholar]
- Kagawa T, Wada M. Light-dependent nuclear positioning in prothallial cells of Adiantum capillus-veneris. Protoplasma. 1993;177:82–85. [Google Scholar]
- Kagawa T, Wada M. Brief irradiation with red or blue light induces orientational movement of chloroplasts in dark-adapted prothallial cells of the fern Adiantum. J Plant Res. 1994;107:389–398. [Google Scholar]
- Kagawa T, Wada M. Phytochrome- and blue-light-absorbing pigment-mediated directional movement of chloroplasts in dark-adapted prothallial cells of fern Adiantum as analyzed by microbeam irradiation. Planta. 1996;198:488–493. [Google Scholar]
- Kanegae T, Wada M. Isolation and characterization of the plant blue light photoreceptor (cryptochrome) homologousgenes of the fern Adiantum capillus-veneris. Mol Gen Genet. 1998;259:345–353. doi: 10.1007/s004380050821. [DOI] [PubMed] [Google Scholar]
- Kraml M, Büttner G, Haupt W, Herrmann H. Chloroplast orientation in Mesotaenium: the phytochrome effect is strongly potentiated by interaction with blue light. Protoplasma. 1988;S1:172–179. [Google Scholar]
- Nozue K, Kanegae T, Imaizmi T, Fukoda S, Okamoto H, Yeh K-C, Lagarias JC, Wada M. A phytochrome from the fern Adiantum with features of putative photoreceptor NPHI. Proc Natl Acad Sci USA. 1998;95:15826–15830. doi: 10.1073/pnas.95.26.15826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trojan A, Gabrys H. Chloroplast distribution in Arabidopsis thaliana (L.) depends on light conditions during growth. Plant Physiol. 1996;111:419–425. doi: 10.1104/pp.111.2.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M, Grolig F, Haupt W. Light-oriented chloroplast positioning: contribution to progress in photobiology. J Photochem Photobiol. 1993;31:415–418. [Google Scholar]
- Wada M, Kanegae T, Nozue K, Fukuda S. Cryptogam phytochrome. Plant Cell Environ. 1997;20:685–690. [Google Scholar]
- Yatsuhashi H, Kadota A, Wada M. Blue- and red-light action in photo-orientation of chloroplasts in Adiantum protonemata. Planta. 1985;165:43–50. doi: 10.1007/BF00392210. [DOI] [PubMed] [Google Scholar]
- Yatsuhashi H, Wada M. High-fluence rate responses in the light-oriented chloroplast movement in Adiantum protonemata. Plant Sci. 1990;68:87–94. [Google Scholar]
- Zurzycki J. Blue light-induced intracellular movement. In: Senger H, editor. Blue Light Syndrome. New York: Springer-Verlag; 1980. pp. 50–68. [Google Scholar]