Abstract
The biodiversity of the marine environment and the associated chemical diversity constitute a practically unlimited resource of new antitumor agents in the field of the development of marine bioactive substances. In this review, the progress on studies of antitumor peptides from marine sources is provided. The biological properties and mechanisms of action of different marine peptides are described; information about their molecular diversity is also presented. Novel peptides that induce apoptosis signal pathway, affect the tubulin-microtubule equilibrium and inhibit angiogenesis are presented in association with their pharmacological properties. It is intended to provide useful information for further research in the fields of marine antitumor peptides.
Keywords: antitumor, peptides, marine organisms
1. Introduction
The sea, covering 70% of the Earth’s surface, offers a considerably broader spectrum of biological diversity than terra firma. Containing approximately 75% of all living organisms, the marine environment offers a rich source of natural products with potential therapeutic application. The discovery of the bio-regulatory role of different endogenous peptides in the organism as well as the understanding of the molecular mechanisms of action about some new bioactive peptides obtained from natural sources on specific cellular targets, contributed to developing peptides as promising lead drug candidates. Recently, marine peptides have opened a new perspective for pharmaceutical developments [1].
Peptides discovered from marine organism induce cell death with different mechanisms, including apoptosis, affecting the tubulin-microtubule equilibrium, or inhibiting angiogenesis (Figure 1). This finding has increased our knowledge about new potent cytotoxic, and many other properties with novel chemical structures associated to original mechanisms of pharmacological activity. These facts introduce marine peptides as a new choice for obtaining lead compounds on biomedical research. This review will account for the progress of the recent years and provide novel information in this field.
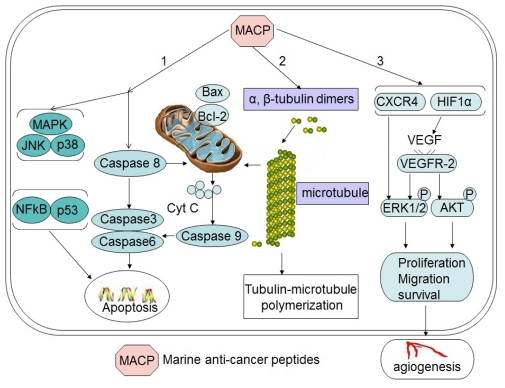
Figure 1.
Schematic depiction of major mechanisms for major antitumor peptides. Marine peptides induce cell death via the following pathways, apoptosis (1), affecting the tubulin-microtubule equilibrium (2) and angiogenesis pathway (3).
2. Peptides that Induce Apoptosis
Apoptosis as a form of programmed cell death is one of the major mechanisms of cell death in response to cancer therapies [2]. Also, apoptosis is a naturally occurring and evolutionarily conserved process by which cells that are no longer useful are directed to their deaths [3]. Apoptosis plays an indispensable role and is a fundamental process in development, physiology and homeostasis [4–7]. Its deregulation, i.e., either loss of pro-apoptotic signals or gain of anti-apoptotic signals, can lead to a variety of pathological conditions such as cancer initiation, promotion and progression or result in treatment failures [8,9]. As apoptosis does not usually trigger inflammatory or immune response, it becomes a preferable way of cancer cell death during cancer treatments. As such, modulation of apoptotic pathways and selective induction of apoptosis by chemical agents are likely to be a promising approach for cancer therapy [6,10–15]. In mammals, there are two major signaling systems that result in the activation of caspases, the extrinsic death receptor pathway [16–19] and the intrinsic mitochondrial pathway [20,21]. These two pathways include many cross talks between them. There is a long list of pro- or anti-apoptotic molecules that can trigger or regulate apoptosis. Therefore, developing anticancer peptides that target these molecules has become an important strategy for anticancer therapies.
Growing evidence shows that most marine anticancer peptides with cytotoxicity may trigger apoptosis by targeting many cellular proteins, and the induced apoptotic process involves both intracellular and extracellular pathways [14]. The balance between the pro-survival gene Bcl-2 and the pro-apoptotic gene Bax plays a key role in maintaining cell viability. Therefore, inhibition of Bcl-2 or induction of Bax becomes a good strategy for triggering an apoptotic process [22]. Identification of caspases activators becomes another approach for the discovery of novel anticancer agents because caspases are involved in intrinsic and extrinsic apoptosis pathways [23,24]. Some marine anticancer peptides can activate the Jun N-terminal kinase (JNK) and p38 mitogen-activated protein kinases (MAPK) pathways that lead to the release of cytochrome c (Cyt C) from mitochondria [25]. In brief, apoptosis is a complicated process and involves a huge number of signaling molecules, and failure of apoptosis activation is one of the major impediments to the treatment of cancer. Therefore, a good strategy for the development of new anticancer agents is to identify or develop such agents that can target multiple apoptosis regulating genes.
2.1. Peptides that Activate the Intrinsic Mitochondrial Pathway
The mitochondrial cell death pathway commences when apoptogenic molecules presented between the outer and inner mitochondrial membranes are released into the cytosol by mitochondrial outer membrane permeabilization. The release of pro-apoptotic factors such as cytochrome c from mitochondria leads to formation of a multimeric complex known as the apoptosome and initiates caspases activation cascades. These pathways are important for normal cellular homeostasis and play key roles in the pathogenesis of many diseases [21,26]. In the intrinsic mitochondrial pathway, at least 18 pro- and anti-apoptotic proteins of the Bcl-2 family are pivotal regulators of apoptosis, Bax as a proapoptotic member of the Bcl-2 family is very influential in the pro- and anti-apoptotic balance by regulating mitochondrial functions [22,27].
Jaspamide (Jasplakinolide), isolated from marine sponge Jaspis johnstoni, is a cyclic depsipeptide with a 15-carbon macrocyclic ring containing three amino acid residues (Figure 2A) [28]. Jasplakinolide induces apoptosis in Jurkat T cells as demonstrated by nucleosomal DNA ladder formation. Enhanced caspase-3 activity is also observed in Jaspamide-treated Jurkat T cells by using the fluorescent substrate DEVD-MAC. Transformed cell lines were more susceptible to Jasplakinolide-induced apoptosis than normal nontransformed cells [29]. Jaspamide-induced apoptosis is associated with caspase-3 activation and a decrease in Bcl-2 protein expression, but also with increased Bax levels. It seems that jaspamide induces a caspases independent pathway of cell death, which is responsible for the observed cytoplasmic and membrane changes in apoptosing cells, and also a caspases-dependent cell death, which is responsible for PARP proteolysis [30].
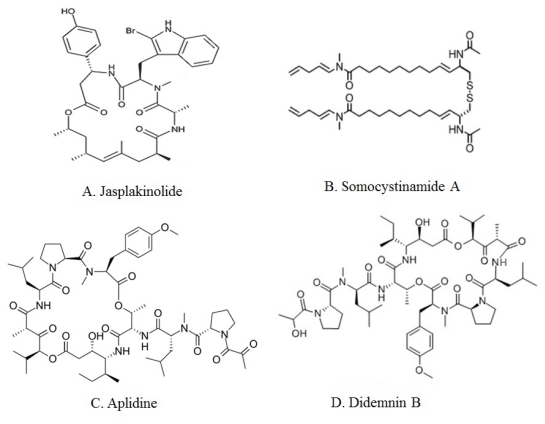
Figure 2.
Chemical structures of major marine peptides with apoptotic activity: Jasplakinolide (A), Somocystinamide A (B), Aplidine (C) and Didemnin B (D).
Some other peptides from marine sources, such as Somocystinamide A [31,32] and C-phycocyanin (C-PC) [33,34] have been observed to display potent caspases-dependent anti-apoptotic activity in different cancer cells. Somocystinamide A (ScA), a lipopeptide, was isolated from Lyngbya majuscula/Schizothrix sp. assemblage of marine cyanobacteria (Figure 2B) [31]. ScA stimulates apoptosis in a number of tumor cell lines and in angiogenic endothelial cells via both the intrinsic and extrinsic pathways, but the more effective mechanism is the activation of caspase-8 and its downstream pathways [32]. C-phycocyanin, a tetrapyrrole-protein complex isolated from the cyanobacteria Agmenellum quadruplicatum, Mastigocladus laminosus [33] and Spirulina platensis, could induce the activation of pro-apoptotic gene and down-regulation of anti-apoptotic gene expression, then facilitate the transduction of apoptosis signals that result in the apoptosis of HeLa cells in vitro. Caspases 2, 3, 4, 6, 8, 9, and 10 were activated in C-PC-treated HeLa cells, suggesting that C-PC-induced apoptosis was caspases-dependent. C-PC treatment of HeLa cells also results in release of cytochrome c from the mitochondria into the cytosol that was related to apoptosis of C-PC-treated HeLa cells [34].
2.2. Peptides that Target the JNK or p38 MAPK Pathway
Jun N-terminal kinases (JNKs) and p38 mitogen-activated protein kinases (MAPKs) play critical roles in the signaling mechanisms that orchestrate cellular responses to various types of cellular stress [35,36]. Unscheduled proliferation is a hallmark of cancer, and the JNK and p38 MAPK pathways regulate cell cycle progression at different points by both transcription-dependent and transcription-independent mechanisms, with profound effects on the development of various cancers. The pro- and anti-apoptotic effects of JNKs seem to be dependent not only on the stimuli, but also on the strength of the signals. Activation of the JNK and p38 MAPK pathways can trigger cytochrome c release and subsequently activate caspases cascades [35].
Aplidine (dehydrodidemnin B, DDB, Aplidin), a cyclic depsipeptide (Figure 2C), was isolated from the Mediterranean tunicate Aplidium albicans. Breast, melanoma and non-small-cell lung cancer appear to be sensitive to low concentrations of Aplidine [37,38]. Aplidine’s mechanism of action involves several pathways, including cell cycle arrest, inhibition of protein synthesis. Aplidine induces early oxidative stress and results in a rapid and persistent activation of JNK and p38 MAPK phosphorylation with activation of both kinases occurring very rapid, long before the execution of apoptosis, and full activation within 5–10 min of drug treatment in human HeLa tumor cells. JNK and p38 MAPK activation results in downstream cytochrome c release and activation of caspases-9 and -3 and PARP cleavage, demonstrating the mediation of the mitochondrial apoptotic pathway in this process. Protein kinase C delta (PKC-d) mediates the cytotoxic effect of Aplidin and that it is concomitantly processed and activated late in the apoptotic process by a caspases mediated mechanism [39]. Aplidin induces apoptosis in MDA-MB-231 breast cancer cells, resulting in sustained activation of the epidermal growth factor receptor (EGFR), the non-receptor protein-tyrosine kinase Src, and the serine/threonine kinases JNK and p38 MAPK. Two mechanisms by which Aplidin activates JNK: rapid activation of Rac1 small GTPase and down regulation of MKP-1 phosphatase. Aplidine, also called plitidepsin in clinical trials, is well-tolerated with minor toxicity in finished Phase I clinical trials [40–42]. Phase II studies are underway [43].
2.3. Peptides with an Unknown Mechanism of Apoptosis-Inducing Activity
Some marine peptides are known to induce cell death with apoptotic characteristics, including DNA fragmentation, nucleic shrinking and cell membrane swelling. However, the exact mode of action of cytotoxicity is unclear. Didemnins, originally reported in 1981 [44,45], are a family of depsipeptides with antitumor, antiviral and immunosuppressive activities primarily isolated from the Caribbean tunicate Trididemnum solidum, but later obtained from other species of the same genus [46,47]. Didemnin B, a branched N-methylated cyclic peptolide, originally was isolated from the Trididemnum genus of marine tunicates (Figure 2D). Didemnin B induces death of a variety of transformed cells with apoptotic morphology and DNA fragmentation within the cytosol and the generation of DNA ladders [48], but the exact mechanism for these effects is still obscure [49]. Until today, a lot of Didemnin analogues have been prepared semisynthetically and their biological activities evaluated, including cytotoxicity and antiviral and immunosuppressive properties [46,50]. However, Didemnin B continues to be a main focus in clinical investigation, being the first marine natural product currently in clinical trials as an anti-cancer agent [51,52].
Some other peptides from marine sources, such as Sansalvamide A [53,54], Cycloxazoline [55,56] and virenamides A–C [57] have been observed to display potent anti-apoptotic activity in different cancer cells but the exact targets by these chemicals have not yet been identified. Sansalvamide A, a cyclic depsipeptide produced by a marine fungus, has demonstrated significant anticancer activity [54]. One of Sansalvamide A analogs caused G (1) phase cell cycle arrest in two human pancreatic cancer cell lines (AsPC-1 and CD18); Sansalvamide A, an inhibitor of topoisomerase I, induces cell death with only some apoptotic characteristics in some cancer cells [53]. Cycloxazoline, a new cyclic hexapeptide is reported from a marine ascidian Lissoclinum bistratum [55]. Accumulation of HL-60 leukemia cells in G2/M and inhibition of cytokinesis was caused by cycloxazoline [56]. Three new linear cytotoxic tripeptides, virenamides A–C have been isolated from didemnid ascidian Diplosoma virens [57]. The virenamides showed modest cytotoxicity towards a panel of cultured cells: Virenamide A gave IC50 of 2.5 μg/mL against P388, and 10 μg/mL against A549, HT29 and CV1 cells, and exhibited topoisomerase II inhibitory activity. Virenamides B and C both gave IC50 of 2.5 μg/mL against P388, A549, HT29, and CV1 cells [57].
3. Peptides that Affect the Tubulin-Microtubule Equilibrium
Microtubules are intracellular organelles formed from the protein tubulin. These organelles have a number of essential cellular functions including chromosome segregation, the maintenance of cell shape, transport, motility, and organelle distribution. Drugs that affect the tubulin-microtubule equilibrium are effective anticancer drugs [58]. Tubulin binding molecules have generated considerable interest after the successful introduction of the taxanes into clinical oncology and the widespread use of the Vinca alkaloids Vincristine and Vinblastine. These compounds inhibit cell mitosis by binding to the protein tubulin in the mitotic spindle and preventing polymerization into the microtubules (MTs). This mode of action is also shared with other natural agents. Therefore, there is a strong need to design and develop new natural analogs as antimitotic agents to interact with tubulin at sites different from those of Vinca alkaloids and taxanes [59].
Dolastatin 10, a linear pentapeptide containing several unique amino acid subunits (Figure 3A), was derived from the marine mollusk Dolabella auricularia; it is the most potent member of a large class of related peptides [60,61]. Bai et al. reported that Dolastatin 10 inhibited the growth of L1210 murine leukemia cells in culture [62]. Preliminary studies indicated that Dolastatin 10 causes formation of a cold-stable tubulin aggregate at higher drug concentrations. Dolastatin 10 strongly inhibits microtubule assembly, tubulin-dependent GTP hydrolysis [62], and the binding of Vinca alkaloids to tubulin. Dolastatin 10 prevents loss of the stabilizing effects on the colchicine binding activity of tubulin. A tripeptide segment of Dolastatin 10 also effectively inhibits tubulin polymerization and GTP hydrolysis. The tripeptide did not significantly inhibit either vincristine binding or nucleotide exchange [63].
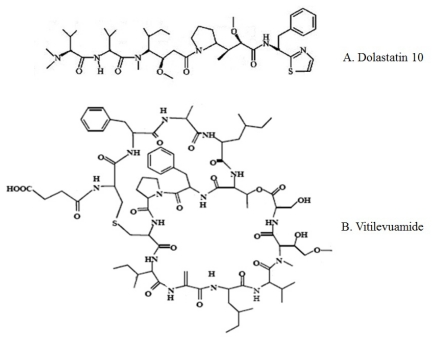
Figure 3.
Chemical structures of major marine peptides which affect the tubulin-microtubule equilibrium: Dolastatin 10 (A) and Vitilevuamide (B).
Vitilevuamide, a bicyclic 13 amino acid peptide (Figure 3B), was isolated from two marine ascidians, Didemnum cuculiferum and Polysyncranton lithostrotum. Vitilevuamide was strongly positive in a cell-based screen for inhibitors of tubulin polymerization, displaying activity in vivo against P388 lymphocytic leukemia. Vitilevuamide exhibits non-competitive inhibition of Vinblastine binding to tubulin. Colchicine binding to tubulin was stabilized in the presence of Vitilevuamide. GTP binding was also found to be weakly affected by the presence of Vitilevuamide, suggesting the possibility that Vitilevuamide inhibits tubulin polymerization via an interaction at a unique site [64].
Some other peptides from marine sources, such as Diazonamide A [65,66], Scleritodermin A [67,68], Hemiasterlin [69–72], Desmethoxymajusculamide C (DMMC) [73] and Milnamide D [74] have been observed to display potent inhibition of tubulin polymerization in different cancer cells. Diazonamide A, a complex cytotoxic peptide, was isolated from the marine ascidian Diazona angulata [65]. Diazonamide A and the analog have a unique binding site on tubulin differing from the Vinca alkaloid and Dolastatin 10 binding sites. Diazonamide A and the analog bind weakly to unpolymerized tubulin but strongly to microtubule ends [65,66]. Scleritodermin A is a new cyclic peptide isolated from the lithistid sponge Scleritoderma nodosum. Scleritodermin A showed significant in vitro cytotoxicity against human tumor cell lines and inhibited tubulin polymerization [67,68]. Hemiasterlin, a natural tripeptide derived from marine sponges Auletta and Siphonochalina sp., binds to the Vinca-peptide site in tubulin, disrupts normal microtubule dynamics; depolymerize microtubules [69,70]. One analogue of Hemiasterlin, HTI-286, inhibits the polymerization of purified tubulin, disrupts microtubule organization in cells. HTI-286 is considered as a potent inhibitor of proliferation and has substantially less interaction with multidrug resistance protein (P-glycoprotein) than currently used antimicrotubule agents [71,72]. Desmethoxymajusculamide C (DMMC), a new cyclic depsipeptide was extracted from the Fijian Cyanobacterium Lyngbya majuscula. DMMC exhibited potent and selective anti-solid tumor activity against the HCT-116 human colon carcinoma cell line via disruption of cellular microfilament networks. Linearized DMMC was also evaluated in the biological assays and found to maintain potent actin depolymerization characteristics while displaying solid tumor selectivity equivalent to DMMC in the disk diffusion assay [73].
We purified an antitumor protein from the coelomic fluid of Meretrix meretrix Linnaeus, MML, which exhibited significant cytotoxicity to several cancer cell types, including human hepatoma BEL-7402, human breast cancer MCF-7 and human colon cancer HCT-116 cells. Further studies demonstrated that MML increased cell membrane permenbility and inhibition of tubulin polymerization [75].
4. Peptides that Inhibit Angiogenesis
Angiogenesis, the formation of new blood vessels, is a complex multistep process, including the destabilization of established vessel, endothelial cell proliferation, migration and new tube formation. Angiogenesis plays an important role in the growth, invasion and metastasis of most solid tumors. Both tumor growth and metastasis depend on the expansion of host vasculatures into tumors through angiogenesis [76–79]. Vascular endothelial growth factor (VEGF) and its receptor, VEGFR-2 (Flk-1/KDR), play a key role in tumor angiogenesis [80,81]. Tumor growth may be inhibited via blocking the VEGF-VEGFR-2 pathway and downstream intracellular signaling. These pathways include VEGF-induced phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2), serine/threonine protein kinase family protein kinase B (Akt), two tumor promoters CXC chemokine Receptor (CXCR4) and Hypoxia inducible factor 1alpha (HIF1α) [82,83]. HIF1α, a subunit of HIF1 transcription factor, regulates not only adaptive responses to hypoxia, but also many cellular functions under normoxia. HIF1α induces VEGF aggregation, actions known to be important for cellular survival and endovascular differentiation [84,85].
Neovastat (AE-941) is a derivative of shark cartilage extract. Rather than being a specific monomolecular compound, AE-941 is a defined standardized liquid extract comprising the <500 kDa fraction from the cartilage of shark, Squalus acanthias, directly inhibits tumor cell growth and angiogenesis [86,87]. Lee et al. founded that Neovastat was mediated via inhibition of VEGF and HIF2 alpha pathway [88]. Mice treated with Neovastat had significantly reduced inflammatory cell count in BAL fluid. Furthermore, Mice treated with Neovastat showed significantly reduced VEGF and HIF2 alpha expression on lung tissue [88].
We purified a novel linear polypeptide with MW 15500, PG155, with potent anti-angiogenic activity from the cartilage of shark, Prionace glauca. The anti-angiogenic effects of PG155 were evaluated using zebrafish embryos model in vivo. Our study confirmed that PG155 inhibited the growth of SIV of zebrafish embryos. In vitro transwell experiment revealed that the polypeptide inhibited VEGF induced migration and tubulogenesis of human umbilical vein endothelial cells (HUVECs) [89].
Mycothiazole, a mixed polyketide/peptide-derived compound with a central thiazole moiety, inhibited hypoxic HIF1 signaling in tumor cells that correlated with the suppression of HIF1 target gene VEGF expression. Mechanistic studies revealed that mycothiazole selectively suppresses mitochondrial respiration at complex I (NADH-ubiquinone oxidoreductase), may serve as a valuable molecular probe for mitochondrial biology and HIF-mediated hypoxic signaling [90].
5. Peptides with Unknown Mechanism for Their Anti-Tumor Activity
Although a huge effort has been put on the development of anticancer agents from marine sources, this area is still a virgin field and many fewer peptides have been identified so far compared with the research on the peptides isolated from other natural sources. There are still many other peptides with unknown mechanisms for their induction of cytotoxicity, likely because they involve complicated mechanisms. A lot of peptides were isolated from ascidians, including patellamides [91–94], Styelin D [95], Eusynstyelamide [96], botryllamides A–D (1–4) [97], Lissoclinamides [46,98,99] and Mollamides [100,101], which display potent cytotoxicity, but their exact mechanism have not been well documented. Styelin D, a 32-residue C-terminally amidated antimicrobial peptide, isolated from blood cells of the ascidian Styela clava is discovered a cytotoxic peptide, was quite cytotoxic and hemolytic to eukaryotic cells [95]. Lissoclinamides (Lissoclinamides 4 and 5), a cyclic peptide isolated from the aplousobranch ascidian Lissoclinum patella [98], showed relevant antineoplastic as well as other pharmacological properties against human fibroblast and bladder carcinoma cell lines and normal lymphocytes [46]. The most potent is Lissoclinamide 7, containing two thiazoline rings, which rivals Didemnin B in cytotoxicity in vitro [99].
Sponge is another rich resource of peptides, such as Geodiamolides A–G [102,103], Orbiculamide A [104], Koshikamide B [105], Phakellistatins [106–109], Microcionamides A and B [110], Halicylindramides [111], Haligramides [112], Hemiasterlin [113], Milnamide A [114], Corticiamide A [115], Theopapuamide [116], Taumycins A [117], Koshikamide A1 [118], Koshikamide A2 [119] and Efrapeptin G [120] have been observed to display potent cytotoxicity in different cancer cells but the exact targets by these chemicals have not yet been identified. Geodiamolides A–G initially isolated from the Caribbean sponge Geodia sp. are a group of cytotoxic peptides in which there is three amino acids forming a cyclic peptide with a common polyketide unit [102,103]. Orbiculamide A, acyclic peptide from a marine sponge Theonella sp., is cytotoxic against P388 murine leukemia cells (ED50 = 0.34 μg/mL), but also against different melanoma cell lines [104]. Koshikamide B, a cytotoxic peptide lactone from a marine sponge Theonella sp., is a 17-residue peptide lactone composed of six proteinogenic amino acids, two D-isomers of proteinogenic amino acids, seven N-methylated amino acids, and two unusual amino acid residues. Koshikamide B exhibits cytotoxicity against P388 murine leukemia cells and the human colon tumor (HCT-116) cell line [105]. Phakellistatins, isolated from two Indo-Pacific sponges, Phakellia costata and Stylotella aurantium, are a group of proline rich cyclic heptapeptides; Phakellistatin 1, showed potent activity against P388 murine leukemia cells and other different melanoma cell lines [106]. Microcionamides A and B, new linear peptides cyclized via a cysteine moiety and isolated from the Philippine Sponge Clathria (Thalysias) abietina, exhibited significant cytotoxicity against the human breast tumor cells lines MCF-7 and SKBR-3 and displayed inhibitory activity against Mycobacterium tuberculosis H37Ra [110]. Similarly, several other marine anticancer peptides, including Keenamide A [121], Kulokekahilide-1 [122], Kulokekahilide-2 [123] and Scopularide A and B [124], also elicit antitumor activity via unknown mechanisms. Keenamide A, a new cytotoxic cyclic hexapeptide, was isolated from the notaspidean mollusk Pleurobranchus forskalii. Keenamide A exhibited significant activity against the P388, A549, MEL-20, and HT-29 tumor cell lines [121].
Some other antitumor peptides including Symplocamide A [125,126], Apratoxin D [127,128] and Mitsoamide [129] have been isolated recently from Cyanobacteria. Symplocamide A is a newly discovered 3-amino-6-hydroxy-2-piperidone (Ahp) cyclodepsipeptide, isolated from a marine cyanobacteria Symploca sp. in Papua New Guinea [126]. Symplocamide A is an extremely potent cytotoxin, with IC50 in nanomole level for H460 lung cancer and neuro-2A neuroblastoma cell lines [125]. Apratoxin D was extracted from marine cyanobacteria Lyngbya majuscula and Lyngbya sordida. Apratoxin D showed potent in vitro cytotoxicity against H-460 human lung cancer cells with an IC50 value of 2.6 nM [127]. Recently, a new cytotoxic and linear peptide was isolated from the marine cyanobacteria Geitlerinema sp. [129]. Additionally, two novel cyclodepsipeptides, Scopularides A and B were found in the fungus Scopulariopsis brevicaulis and activity of Scopularides against several tumor cell lines was significant at 10 μg/mL [124]. However, the exact mechanisms by these peptides have not yet been identified.
6. Conclusions
The discussed marine peptides and their mode actions are summarized in Table 1. However, a lot of marine peptides display antitumor activity via multiple targets. Dolastatin 10 not only inhibits microtubule assembly, but also induces apoptosis associating with a decrease in Bcl-2 level and an increase in p53 expression in the lymphoma cell line [130]. Aplidine’s mechanism of action involves several pathways, including cell cycle arrest, inhibition of protein synthesis and anti-angiogenic activity [131].
Table 1.
A summary of marine peptides and their mode of actions.
| Name of peptides | Sources | Class/types | Mode of action and references |
|---|---|---|---|
| Jaspamide (Jasplakinolide) | Marine sponge, Jaspis johnstoni | Cyclic depsipeptide | Caspase-3 activation, decreasing in Bcl-2 protein expression [28–30] |
| Somocystinamide A (ScA) | Lyngbya majuscula/Schizothrix sp. assemblage of marine cyanobacteria | Lipopeptide | Caspase-8 activation [31,32] |
| C-phycocyanin (C-PC) | Cyanobacteria Agmenellum quadruplicatum, Mastigocladus laminosus, Spirulina platensis | Tetrapyrrole-protein complex | Caspases-dependent apoptosis [34] |
| Aplidine (dehydrodidemnin B, DDB, Aplidin) | Tunicate, Aplidium albicans | Cyclic depsipeptide | JNK and p38 MAPK phosphorylation activation [37–39] |
| Didemnin B | Tunicate, Trididemnum solidum | Cyclic depsipeptide | Apoptosis, but unclear [44–49] |
| Sansalvamide A | Marine fungus | Cyclic depsipeptide | Apoptosis, but unclear [53,54] |
| Cycloxazoline | Marine ascidian, Lissoclinum bistratum | Cyclic depsipeptide | Apoptosis, but unclear [55,56] |
| Virenamides A–C | Didemnid ascidian, Diplosoma virens | Linear tripeptides | Apoptosis, but unclear [57] |
| Dolastatin 10 | Marine mollusk, Dolabella auricularia | Linear peptide | Microtubule assembly Inhibition [60,62] |
| Vitilevuamide | Marine ascidians, Didemnum cuculiferum and Polysyncranton lithostrotum | Bicyclic peptide | Tubulin polymerization inhibition [64] |
| Diazonamide | Marine ascidian, Diazona angulata | Macrocyclic peptide | Tubulin polymerization inhibition [65,66] |
| Scleritodermin A | Lithistid sponge, Scleritoderma nodosum | Cyclic peptide | Tubulin polymerization inhibition [67,68] |
| Hemiasterlin | Marine sponges, Auletta sp. and Siphonochalina sp. | Tripeptide | Tubulin polymerization inhibition [70–72] |
| DMMC | Cyanobacterium Lyngbya majuscula | Cyclic depsipeptide | Tubulin polymerization inhibition [73] |
| MML | Coelomic fluid, Meretrix meretrix | Protein | Tubulin polymerization inhibition [75] |
| Neovastat (AE-941) | Shark cartilage, Squalus acanthias | Extract < 500 kDa | VEGF and HIF2 alpha pathway inhibition [86–88] |
| PG155 | Shark cartilage, Prionace glauca | Polypeptide | VEGF induced angiogenesis inhibition [89] |
| Styelin D | Ascidian, Styela clava | C-terminally amidated peptide | Unknown [95] |
| Lissoclinamides | Aplousobranch ascidian, Lissoclinum patella | Cyclic peptide | Unknown [46,98,99] |
| Geodiamolides A–G | Caribbean sponge, Geodia sp. | Cyclic peptide | Unknown [102,103] |
| Orbiculamide A | Marine sponge, Theonella sp. | Cyclic peptide | Unknown [104] |
| Koshikamide B | Marine sponge, Theonella sp. | Peptide lactone | Unknown [105] |
| Phakellistatins | Marine sponges | Cyclic heptapeptides | Unknown [106–109] |
| Microcionamides A and B | Philippine Sponge, Clathria (Thalysias) abietina | Linear peptides | Unknown [110] |
| Keenamide A | Notaspidean mollusk, Pleurobranchus forskalii | Cyclic hexapeptide | Unknown [121] |
| Scopularides A and B | Fungus Scopulariopsis brevicaulis | Cyclodepsipeptide | Unknown [124] |
| Symplocamide A | Marine cyanobacteria Symploca sp. | Cyclodepsipeptide | Unknown [125,126] |
| Apratoxin D | Marine cyanobacteria Lyngbya majuscula and Lyngbya sordida | Macrocycle peptide | Unknown [127,128] |
| Mitsoamide | Marine cyanobacteria Geitlerinema sp. | Linear peptide | Unknown [129] |
Peptide compounds reviewed here are obtained from very different marine organisms with different mechanism on their antitumor activity. Because of the peculiarities of the life in the sea, many of these molecules can be found only in a single source For example, Jaspamide (Jasplakinolide) is only found in marine sponge Jaspis species [28–30], and Trididemnum genus of marine tunicate is the only source for Didemnin B [44–49]. Although the underling mechanism of source specificity of the marine peptides is unclear, it is conceivable that the special environment of marine offers the diversity of marine natural products. It is also possible that the organisms, which may contain the peptides, have not been found since the study of marine peptides is still in its infancy. Compared with the peptides found from other sources, there is more diversity on the style/classes of marine peptides; more cyclic peptides or depsipeptide were found in marine organism. These marine peptides seem to be very useful and promising for biomedical research. There is no doubt that the diversity of marine peptides in its structure and mode of action provide a rich source for the design of very specific and potent new pharmaceuticals for a wide variety of diseases.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 30801453 and 81072065) and National Innovative Drug Development Programs of China (No. 2009ZX09102-240 and 2009ZX09103-661).
Footnotes
Samples Availability: Available from the authors.
References
- 1.de Vries DJ, Beart PM. Fishing for drugs from the sea: Status and strategies. Trends Pharmacol. Sci. 1995;16:275–279. doi: 10.1016/s0165-6147(00)89045-8. [DOI] [PubMed] [Google Scholar]
- 2.Reed JC. Mechanisms of apoptosis. Am. J. Pathol. 2000;157:1415–1430. doi: 10.1016/S0002-9440(10)64779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Danial NN, Korsmeyer SJ. Cell death: Critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 4.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 5.Rowinsky EK. Targeted induction of apoptosis in cancer management: The emerging role of tumor necrosis factor-related apoptosis-inducing ligand receptor activating agents. J. Clin. Oncol. 2005;23:9394–9407. doi: 10.1200/JCO.2005.02.2889. [DOI] [PubMed] [Google Scholar]
- 6.Call JA, Eckhardt SG, Camidge DR. Targeted manipulation of apoptosis in cancer treatment. Lancet Oncol. 2008;9:1002–1011. doi: 10.1016/S1470-2045(08)70209-2. [DOI] [PubMed] [Google Scholar]
- 7.Iannolo G, Conticello C, Memeo L, de Maria R. Apoptosis in normal and cancer stem cells. Crit. Rev. Oncol. Hematol. 2008;66:42–51. doi: 10.1016/j.critrevonc.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Burz C, Berindan-Neagoe I, Balacescu O, Irimie A. Apoptosis in cancer: Key molecular signaling pathways and therapy targets. Acta Oncol. 2009;48:811–821. doi: 10.1080/02841860902974175. [DOI] [PubMed] [Google Scholar]
- 9.Fulda S, Pervaiz S. Apoptosis signaling in cancer stem cells. Int. J. Biochem. Cell Biol. 2009;42:31–38. doi: 10.1016/j.biocel.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Ghobrial IM, Witzig TE, Adjei AA. Targeting apoptosis pathways in cancer therapy. CA Cancer J. Clin. 2005;55:178–194. doi: 10.3322/canjclin.55.3.178. [DOI] [PubMed] [Google Scholar]
- 11.Eberle J, Fecker LF, Forschner T, Ulrich C, Rowert-Huber J, Stockfleth E. Apoptosis pathways as promising targets for skin cancer therapy. Br. J. Dermatol. 2007;156S:18–24. doi: 10.1111/j.1365-2133.2007.07855.x. [DOI] [PubMed] [Google Scholar]
- 12.Ziegler DS, Kung AL. Therapeutic targeting of apoptosis pathways in cancer. Curr. Opin. Oncol. 2008;20:97–103. doi: 10.1097/CCO.0b013e3282f310f6. [DOI] [PubMed] [Google Scholar]
- 13.Cheng L, Wang X, Zhang J, Zhang SB, Zheng SQ, Zheng J. Targeting apoptosis signaling pathways in cancer therapy. Zhonghua Bing Li Xue Za Zhi. 2009;38:639–642. [PubMed] [Google Scholar]
- 14.Lin X, Liu M, Hu C, Liao DJ. Targeting cellular proapoptotic molecules for developing anticancer agents from marine sources. Curr. Drug Targets. 2010;11:708–715. doi: 10.2174/138945010791170824. [DOI] [PubMed] [Google Scholar]
- 15.von Schwarzenberg K, Vollmar AM. Targeting apoptosis pathways by natural compounds in cancer: Marine compounds as lead structures and chemical tools for cancer therapy. Cancer Lett. 2010 doi: 10.1016/j.canlet.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Abe K, Kurakin A, Mohseni-Maybodi M, Kay B, Khosravi-Far R. The complexity of TNF-related apoptosis-inducing ligand. Ann. N. Y. Acad. Sci. 2000;926:52–63. doi: 10.1111/j.1749-6632.2000.tb05598.x. [DOI] [PubMed] [Google Scholar]
- 17.Ozoren N, El-Deiry WS. Cell surface Death Receptor signaling in normal and cancer cells. Semin. Cancer Biol. 2003;13:135–147. doi: 10.1016/s1044-579x(02)00131-1. [DOI] [PubMed] [Google Scholar]
- 18.Peter ME, Krammer PH. The CD95(APO-1/Fas) DISC and beyond. Cell Death Differ. 2003;10:26–35. doi: 10.1038/sj.cdd.4401186. [DOI] [PubMed] [Google Scholar]
- 19.Thorburn A. Death receptor-induced cell killing. Cell Signal. 2004;16:139–144. doi: 10.1016/j.cellsig.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Kroemer G. Mitochondrial control of apoptosis: An introduction. Biochem. Biophys. Res. Commun. 2003;304:433–435. doi: 10.1016/s0006-291x(03)00614-4. [DOI] [PubMed] [Google Scholar]
- 21.Gupta S, Kass GE, Szegezdi E, Joseph B. The mitochondrial death pathway: A promising therapeutic target in diseases. J. Cell. Mol. Med. 2009;13:1004–1033. doi: 10.1111/j.1582-4934.2009.00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398–6406. doi: 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]
- 23.Okun I, Balakin KV, Tkachenko SE, Ivachtchenko AV. Caspase activity modulators as anticancer agents. Anticancer Agents Med. Chem. 2008;8:322–341. doi: 10.2174/187152008783961914. [DOI] [PubMed] [Google Scholar]
- 24.Ghavami S, Hashemi M, Ande SR, Yeganeh B, Xiao W, Eshraghi M, Bus CJ, Kadkhoda K, Wiechec E, Halayko AJ, Los M. Apoptosis and cancer: Mutations within caspase genes. J. Med. Genet. 2009;46:497–510. doi: 10.1136/jmg.2009.066944. [DOI] [PubMed] [Google Scholar]
- 25.Park HJ, Kim BC, Kim SJ, Choi KS. Role of MAP kinases and their cross-talk in TGF-beta1-induced apoptosis in FaO rat hepatoma cell line. Hepatology. 2002;35:1360–1371. doi: 10.1053/jhep.2002.33205. [DOI] [PubMed] [Google Scholar]
- 26.Kang MH, Reynolds CP. Bcl-2 inhibitors: Targeting mitochondrial apoptotic pathways in cancer therapy. Clin. Cancer Res. 2009;15:1126–1132. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong WW, Puthalakath H. Bcl-2 family proteins: The sentinels of the mitochondrial apoptosis pathway. IUBMB Life. 2008;60:390–397. doi: 10.1002/iub.51. [DOI] [PubMed] [Google Scholar]
- 28.Braekman JC, Daloze D, Moussiaux B, Riccio R. Jaspamide from the marine sponge Jaspis johnstoni. J. Nat. Prod. 1987;50:994–995. [Google Scholar]
- 29.Odaka C, Sanders ML, Crews P. Jasplakinolide induces apoptosis in various transformed cell lines by a caspase-3-like protease-dependent pathway. Clin. Diagn. Lab. Immunol. 2000;7:947–952. doi: 10.1128/cdli.7.6.947-952.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cioca DP, Kitano K. Induction of apoptosis and CD10/neutral endopeptidase expression by jaspamide in HL-60 line cells. Cell. Mol. Life Sci. 2002;59:1377–1387. doi: 10.1007/s00018-002-8515-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nogle LM, Gerwick WH. Somocystinamide A, a novel cytotoxic disulfide dimer from a Fijian marine cyanobacterial mixed assemblage. Org. Lett. 2002;4:1095–1098. doi: 10.1021/ol017275j. [DOI] [PubMed] [Google Scholar]
- 32.Wrasidlo W, Mielgo A, Torres VA, Barbero S, Stoletov K, Suyama TL, Klemke RL, Gerwick WH, Carson DA, Stupack DG. The marine lipopeptide somocystinamide A triggers apoptosis via caspase 8. Proc. Natl. Acad. Sci. USA. 2008;105:2313–2318. doi: 10.1073/pnas.0712198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schirmer T, Huber R, Schneider M, Bode W, Miller M, Hackert ML. Crystal structure analysis and refinement at 2.5 Å of hexameric C-phycocyanin from the cyanobacterium Agmenellum quadruplicatum: The molecular model and its implications for light-harvesting. J. Mol. Biol. 1986;188:651–676. doi: 10.1016/s0022-2836(86)80013-4. [DOI] [PubMed] [Google Scholar]
- 34.Li B, Gao MH, Zhang XC, Chu XM. Molecular immune mechanism of C-phycocyanin from Spirulina platensis induces apoptosis in HeLa cells in vitro. Biotechnol. Appl. Biochem. 2006;43:155–164. doi: 10.1042/BA20050142. [DOI] [PubMed] [Google Scholar]
- 35.Dhanasekaran DN, Reddy EP. JNK signaling in apoptosis. Oncogene. 2008;27:6245–6251. doi: 10.1038/onc.2008.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wagner EF, Nebreda AR. Signal integration by JNK and p38 MAPK pathways in cancer development. Nat. Rev. Cancer. 2009;9:537–549. doi: 10.1038/nrc2694. [DOI] [PubMed] [Google Scholar]
- 37.Depenbrock H, Peter R, Faircloth GT, Manzanares I, Jimeno J, Hanauske AR. In vitro activity of aplidine, a new marine-derived anti-cancer compound, on freshly explanted clonogenic human tumour cells and haematopoietic precursor cells. Br. J. Cancer. 1998;78:739–744. doi: 10.1038/bjc.1998.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Urdiales JL, Morata P, de Castro IN, Sánchez-Jiménez F. Antiproliferative effect of dehydrodidemnin B (DDB), a depsipeptide isolated from Mediterranean tunicates. Cancer Lett. 1996;102:31–37. doi: 10.1016/0304-3835(96)04151-1. [DOI] [PubMed] [Google Scholar]
- 39.Garcia-Fernandez LF, Losada A, Alcaide V, Alvarez AM, Cuadrado A, Gonzalez L, Nakayama K, Nakayama KI, Fernandez-Sousa JM, Munoz A, et al. Aplidin induces the mitochondrial apoptotic pathway via oxidative stress-mediated JNK and p38 activation and protein kinase C delta. Oncogene. 2002;21:7533–7544. doi: 10.1038/sj.onc.1205972. [DOI] [PubMed] [Google Scholar]
- 40.Rinehart KL. Antitumor compounds from tunicates. Med. Res. Rev. 2000;20:1–27. doi: 10.1002/(sici)1098-1128(200001)20:1<1::aid-med1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 41.Faivre S, Chieze S, Delbaldo C, Ady-Vago N, Guzman C, Lopez-Lazaro L, Lozahic S, Jimeno J, Pico F, Armand JP, et al. Phase I and pharmacokinetic study of aplidine, a new marine cyclodepsipeptide in patients with advanced malignancies. J. Clin. Oncol. 2005;23:7871–7880. doi: 10.1200/JCO.2005.09.357. [DOI] [PubMed] [Google Scholar]
- 42.Maroun JA, Belanger K, Seymour L, Matthews S, Roach J, Dionne J, Soulieres D, Stewart D, Goel R, Charpentier D, et al. Phase I study of Aplidine in a dailyx5 one-hour infusion every 3 weeks in patients with solid tumors refractory to standard therapy. A National Cancer Institute of Canada Clinical Trials Group study: NCIC CTG IND 115. Ann. Oncol. 2006;17:1371–1378. doi: 10.1093/annonc/mdl165. [DOI] [PubMed] [Google Scholar]
- 43.Nalda-Molina R, Valenzuela B, Ramon-Lopez A, Miguel-Lillo B, Soto-Matos A, Perez-Ruixo JJ. Population pharmacokinetics meta-analysis of plitidepsin (Aplidin) in cancer subjects. Cancer Chemother. Pharmacol. 2009;64:97–108. doi: 10.1007/s00280-008-0841-4. [DOI] [PubMed] [Google Scholar]
- 44.Rinehart KL, Gloer JB, Cook JC, Mizsak SA, Scahill TA. Structures of the didemnins, antiviral and cytotoxic depsipeptides from a Caribbean tunicate. J. Am. Chem. Soc. 1981;103:1857–1859. [Google Scholar]
- 45.Rinehart K, Jr, Gloer J, Hughes R, Jr, Renis H, McGovren J, Swynenberg E, Stringfellow D, Kuentzel S, Li L. Didemnins: Antiviral and antitumor depsipeptides from a caribbean tunicate. Science. 1981;212:933–935. doi: 10.1126/science.7233187. [DOI] [PubMed] [Google Scholar]
- 46.Schmitz F, Bowden B, Toth S. Antitumor and cytotoxic compounds from marine organisms. Mar. Biotechnol. 1993;1:197–308. [Google Scholar]
- 47.Blunden G. Biologically active compounds from marine organisms. Phytother. Res. 2001;15:89–94. doi: 10.1002/ptr.982. [DOI] [PubMed] [Google Scholar]
- 48.Grubb DR, Wolvetang EJ, Lawen A. Didemnin B induces cell death by apoptosis: The fastest induction of apoptosis ever described. Biochem. Biophys. Res. Commun. 1995;215:1130–1136. doi: 10.1006/bbrc.1995.2580. [DOI] [PubMed] [Google Scholar]
- 49.Johnson KL, Lawen A. Rapamycin inhibits didemnin B-induced apoptosis in human HL-60 cells: Evidence for the possible involvement of FK506-binding protein 25. Immunol. Cell Biol. 1999;77:242–248. doi: 10.1046/j.1440-1711.1999.00821.x. [DOI] [PubMed] [Google Scholar]
- 50.Vervoort H, Fenical W, Epifanio RA. Tamandarins A and B: New cytotoxic depsipeptides from a Brazilian ascidian of the family Didemnidae. J. Org. Chem. 2000;65:782–792. doi: 10.1021/jo991425a. [DOI] [PubMed] [Google Scholar]
- 51.Chun H, Davies B, Hoth D, Suffness M, Plowman J, Flora K, Grieshaber C, Leyland-Jones B. Didemnin B. Investig. New Drugs. 1986;4:279–284. doi: 10.1007/BF00179597. [DOI] [PubMed] [Google Scholar]
- 52.Rinehart KL, Holt TG, Fregeau NL, Keifer PA, Wilson GR, Perun TJ, Sakai R, Thompson AG, Stroh JG, Shield LS, Seigler DS, Li LH, Martin DG, Grimmelikhuijzen CJP, Gäde G. Bioactive Compounds from Aquatic and Terrestrial Sources. J. Nat. Prod. 1990;53:771–792. doi: 10.1021/np50070a001. [DOI] [PubMed] [Google Scholar]
- 53.Hwang Y, Rowley D, Rhodes D, Gertsch J, Fenical W, Bushman F. Mechanism of inhibition of a poxvirus topoisomerase by the marine natural product sansalvamide A. Mol. Pharmacol. 1999;55:1049–1053. doi: 10.1124/mol.55.6.1049. [DOI] [PubMed] [Google Scholar]
- 54.Pan PS, Vasko RC, Lapera SA, Johnson VA, Sellers RP, Lin CC, Pan CM, Davis MR, Ardi VC, McAlpine SR. A comprehensive study of Sansalvamide A derivatives: The structure-activity relationships of 78 derivatives in two pancreatic cancer cell lines. Bioorg. Med. Chem. 2009;17:5806–5825. doi: 10.1016/j.bmc.2009.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hambley TW, Hawkins CJ, Lavin MF, van den Brenk A, Watters DJ. Cycloxazoline: A cytotoxic cyclic hexapeptide from the ascidian lissoclinum bistratum. Tetrahedron. 1992;48:341–348. [Google Scholar]
- 56.Watters DJ, Beamish HJ, Marshall KA, Gardiner RA, Seymour GJ, Lavin MF. Accumulation of HL-60 leukemia cells in G2/M and inhibition of cytokinesis caused by two marine compounds, bistratene A and cycloxazoline. Cancer Chemother. Pharmacol. 1994;33:399–409. doi: 10.1007/BF00686269. [DOI] [PubMed] [Google Scholar]
- 57.Carroll AR, Feng Y, Bowden BF, Coll JC. Studies of Australian Ascidians. 5. Virenamides A–C, New Cytotoxic Linear Peptides from the Colonial Didemnid Ascidian Diplosoma virens. J. Org. Chem. 1996;61:4059–4061. doi: 10.1021/jo951379o. [DOI] [PubMed] [Google Scholar]
- 58.Hadfield JA, Ducki S, Hirst N, McGown AT. Tubulin and microtubules as targets for anticancer drugs. Prog. Cell Cycle Res. 2003;5:309–325. [PubMed] [Google Scholar]
- 59.Islam MN, Iskander MN. Microtubulin binding sites as target for developing anticancer agents. Mini Rev. Med. Chem. 2004;4:1077–1104. doi: 10.2174/1389557043402946. [DOI] [PubMed] [Google Scholar]
- 60.Pettit GR, Kamano Y, Fujii Y, Herald CL, Inoue M, Brown P, Gust D, Kitahara K, Schmidt JM, Doubek DL, Michel C. Marine animal biosynthetic constituents for cancer chemotherapy. J. Nat. Prod. 1981;44:482–485. doi: 10.1021/np50016a016. [DOI] [PubMed] [Google Scholar]
- 61.Luesch H, Moore RE, Paul VJ, Mooberry SL, Corbett TH. Isolation of Dolastatin 10 from the Marine Cyanobacterium Symploca Species VP642 and Total Stereochemistry and Biological Evaluation of Its Analogue Symplostatin 1. J. Nat. Prod. 2001;64:907–910. doi: 10.1021/np010049y. [DOI] [PubMed] [Google Scholar]
- 62.Bai R, Pettit GR, Hamel E. Dolastatin 10, a powerful cytostatic peptide derived from a marine animal. Inhibition of tubulin polymerization mediated through the Vinca alkaloid binding domain. Biochem. Pharmacol. 1990;39:1941–1949. doi: 10.1016/0006-2952(90)90613-p. [DOI] [PubMed] [Google Scholar]
- 63.Bai RL, Pettit GR, Hamel E. Binding of dolastatin 10 to tubulin at a distinct site for peptide antimitotic agents near the exchangeable nucleotide and Vinca alkaloid sites. J. Biol. Chem. 1990;265:17141–17149. [PubMed] [Google Scholar]
- 64.Edler MC, Fernandez AM, Lassota P, Ireland CM, Barrows LR. Inhibition of tubulin polymerization by vitilevuamide, a bicyclic marine peptide, at a site distinct from colchicine, the Vinca alkaloids, and dolastatin 10. Biochem. Pharmacol. 2002;63:707–715. doi: 10.1016/s0006-2952(01)00898-x. [DOI] [PubMed] [Google Scholar]
- 65.Cruz-Monserrate Z, Vervoort HC, Bai R, Newman DJ, Howell SB, Los G, Mullaney JT, Williams MD, Pettit GR, Fenical W, Hamel E. Diazonamide A and a synthetic structural analog: Disruptive effects on mitosis and cellular microtubules and analysis of their interactions with tubulin. Mol. Pharmacol. 2003;63:1273–1280. doi: 10.1124/mol.63.6.1273. [DOI] [PubMed] [Google Scholar]
- 66.Lachia M, Moody CJ. The synthetic challenge of diazonamide A, a macrocyclic indole bis-oxazole marine natural product. Nat. Prod. Rep. 2008;25:227–253. doi: 10.1039/b705663j. [DOI] [PubMed] [Google Scholar]
- 67.Schmidt EW, Raventos-Suarez C, Bifano M, Menendez AT, Fairchild CR, Faulkner DJ. Scleritodermin A, a cytotoxic cyclic peptide from the lithistid sponge Scleritoderma nodosum. J. Nat. Prod. 2004;67:475–478. doi: 10.1021/np034035z. [DOI] [PubMed] [Google Scholar]
- 68.Liu S, Cui YM, Nan FJ. Total synthesis of the originally proposed and revised structures of scleritodermin A. Org. Lett. 2008;10:3765–3768. doi: 10.1021/ol801419m. [DOI] [PubMed] [Google Scholar]
- 69.Anderson HJ, Coleman JE, Andersen RJ, Roberge M. Cytotoxic peptides hemiasterlin, hemiasterlin A and hemiasterlin B induce mitotic arrest and abnormal spindle formation. Cancer Chemother. Pharmacol. 1997;39:223–226. doi: 10.1007/s002800050564. [DOI] [PubMed] [Google Scholar]
- 70.Gamble WR, Durso NA, Fuller RW, Westergaard CK, Johnson TR, Sackett DL, Hamel E, Cardellina Ii JH, Boyd MR. Cytotoxic and tubulin-interactive hemiasterlins from Auletta sp. and Siphonochalina spp. sponges. Bioorg. Med. Chem. 1999;7:1611–1165. doi: 10.1016/s0968-0896(99)00089-9. [DOI] [PubMed] [Google Scholar]
- 71.Loganzo F, Discafani CM, Annable T, Beyer C, Musto S, Hari M, Tan X, Hardy C, Hernandez R, Baxter M, et al. HTI-286, a synthetic analogue of the tripeptide hemiasterlin, is a potent antimicrotubule agent that circumvents P-glycoprotein-mediated resistance in vitro and in vivo. Cancer Res. 2003;63:1838–1845. [PubMed] [Google Scholar]
- 72.Yamashita A, Norton EB, Kaplan JA, Niu C, Loganzo F, Hernandez R, Beyer CF, Annable T, Musto S, Discafani C, et al. Synthesis and activity of novel analogs of hemiasterlin as inhibitors of tubulin polymerization: Modification of the A segment. Bioorg. Med. Chem. Lett. 2004;14:5317–5322. doi: 10.1016/j.bmcl.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 73.Simmons TL, Nogle LM, Media J, Valeriote FA, Mooberry SL, Gerwick WH. Desmethoxymajusculamide C, a cyanobacterial depsipeptide with potent cytotoxicity in both cyclic and ring-opened forms. J. Nat. Prod. 2009;72:1011–1016. doi: 10.1021/np9001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chevallier C, Richardson AD, Edler MC, Hamel E, Harper MK, Ireland CM. A new cytotoxic and tubulin-interactive milnamide derivative from a marine sponge Cymbastela sp. Org. Lett. 2003;5:3737–3739. doi: 10.1021/ol035476c. [DOI] [PubMed] [Google Scholar]
- 75.Ning X, Zhao J, Zhang Y, Cao S, Liu M, Ling P, Lin X. A novel anti-tumor protein extracted from Meretrix meretrix Linnaeus induces cell death by increasing cell permeability and inhibiting tubulin polymerization. Int. J. Oncol. 2009;35:805–812. doi: 10.3892/ijo_00000393. [DOI] [PubMed] [Google Scholar]
- 76.Folkman J. The role of angiogenesis in tumor growth. Semin. Cancer Biol. 1992;3:65–71. [PubMed] [Google Scholar]
- 77.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat. Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 78.Bouck N, Stellmach V, Hsu SC. How tumors become angiogenic. Adv. Cancer Res. 1996;69:135–174. doi: 10.1016/s0065-230x(08)60862-3. [DOI] [PubMed] [Google Scholar]
- 79.Folkman J. Angiogenesis and angiogenesis inhibition: An overview. EXS. 1997;79:1–8. doi: 10.1007/978-3-0348-9006-9_1. [DOI] [PubMed] [Google Scholar]
- 80.Ferrara N. VEGF: An update on biological and therapeutic aspects. Curr. Opin. Biotechnol. 2000;11:617–624. doi: 10.1016/s0958-1669(00)00153-1. [DOI] [PubMed] [Google Scholar]
- 81.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 82.Nakamura S, Chikaraishi Y, Tsuruma K, Shimazawa M, Hara H. Ruboxistaurin, a PKCbeta inhibitor, inhibits retinal neovascularization via suppression of phosphorylation of ERK1/2 and Akt. Exp. Eye Res. 2009;90:137–145. doi: 10.1016/j.exer.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 83.Ushio-Fukai M. Redox signaling in angiogenesis: Role of NADPH oxidase. Cardiovasc. Res. 2006;71:226–235. doi: 10.1016/j.cardiores.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 84.Chiavarina B, Whitaker-Menezes D, Migneco G, Martinez-Outschoorn UE, Pavlides S, Howell A, Tanowitz HB, Casimiro MC, Wang C, Pestell RG, et al. HIF1-alpha functions as a tumor promoter in cancer associated fibroblasts, and as a tumor suppressor in breast cancer cells: Autophagy drives compartment-specific oncogenesis. Cell Cycle. 2010;9:3534–3551. doi: 10.4161/cc.9.17.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fukushima K, Murata M, Hachisuga M, Tsukimori K, Seki H, Takeda S, Asanoma K, Wake N. Hypoxia inducible factor 1 alpha regulates matrigel-induced endovascular differentiation under normoxia in a human extravillous trophoblast cell line. Placenta. 2008;29:324–331. doi: 10.1016/j.placenta.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 86.Lee A, Langer R. Shark cartilage contains inhibitors of tumor angiogenesis. Science. 1983;221:1185–1187. doi: 10.1126/science.6193581. [DOI] [PubMed] [Google Scholar]
- 87.Dupont E, Brazeau P, Juneau C. Extracts of shark cartilage having an anti-angiogenic activity and an effect on tumor regression: Process of making thereof. 5,985,839. US Patents. 1999 16 November;
- 88.Lee SY, Chung SM. Neovastat (AE-941) inhibits the airway inflammation via VEGF and HIF-2 alpha suppression. Vasc. Pharmacol. 2007;47:313–318. doi: 10.1016/j.vph.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 89.Zheng L, Ling P, Wang Z, Niu R, Hu C, Zhang T, Lin X. A novel polypeptide from shark cartilage with potent anti-angiogenic activity. Cancer Biol. Ther. 2007;6:775–780. doi: 10.4161/cbt.6.5.4002. [DOI] [PubMed] [Google Scholar]
- 90.Morgan JB, Mahdi F, Liu Y, Coothankandaswamy V, Jekabsons MB, Johnson TA, Sashidhara KV, Crews P, Nagle DG, Zhou YD. The marine sponge metabolite mycothiazole: A novel prototype mitochondrial complex I inhibitor. Bioorg. Med. Chem. 2010;18:5988–5994. doi: 10.1016/j.bmc.2010.06.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.McDonald LA, Ireland CM. Patellamide E: A new cyclic peptide from the ascidian Lissoclinum patella. J. Nat. Prod. 1992;55:376–379. doi: 10.1021/np50081a016. [DOI] [PubMed] [Google Scholar]
- 92.Rashid MA, Gustafson KR, Cardellina JH, II, Boyd MR, Patellamide F. A new cytotoxic cyclic peptide from the colonial ascidian Lissoclinum patella. J. Nat. Prod. 1995;58:594–597. doi: 10.1021/np50118a020. [DOI] [PubMed] [Google Scholar]
- 93.Fu X, Do T, Schmitz FJ, Andrusevich V, Engel MH. New cyclic peptides from the ascidian Lissoclinum patella. J. Nat. Prod. 1998;61:1547–1551. doi: 10.1021/np9802872. [DOI] [PubMed] [Google Scholar]
- 94.Fu X, Su J, Zeng L. Prepatellamide A, a new cyclic peptide from the ascidian Lissoclinum patella. Sci. China Ser. B Chem. 2000;43:643–648. [Google Scholar]
- 95.Taylor SW, Craig AG, Fischer WH, Park M, Lehrer RI. Styelin D, an extensively modified antimicrobial peptide from ascidian hemocytes. J. Biol. Chem. 2000;275:38417–38426. doi: 10.1074/jbc.M006762200. [DOI] [PubMed] [Google Scholar]
- 96.Swersey JC, Ireland CM, Cornell LM, Peterson RW. Eusynstyelamide, a highly modified dimer peptide from the ascidian Eusynstyela misakiensis. J. Nat. Prod. 1994;57:842–845. doi: 10.1021/np50108a027. [DOI] [PubMed] [Google Scholar]
- 97.McDonald LA, Christopher Swersey J, Ireland CM, Carroll AR, Coll JC, Bowden BF, Fairchild CR, Cornell L. Botryllamides A–D, new brominated tyrosine derivatives from styelid ascidians of the genus Botryllus. Tetrahedron. 1995;51:5237–5244. [Google Scholar]
- 98.Degnan BM, Hawkins CJ, Lavin MF, McCaffrey EJ, Parry DL, van den Brenk AL, Watters DJ. New cyclic peptides with cytotoxic activity from the ascidian Lissoclinum patella. J. Med. Chem. 1989;32:1349–1354. doi: 10.1021/jm00126a034. [DOI] [PubMed] [Google Scholar]
- 99.Hawkins CJ, Lavin MF, Marshall KA, van den Brenk AL, Watters DJ. Structure-activity relationships of the lissoclinamides: Cytotoxic cyclic peptides from the ascidian Lissoclinum patella. J. Med. Chem. 1990;33:1634–1638. doi: 10.1021/jm00168a016. [DOI] [PubMed] [Google Scholar]
- 100.Donia MS, Wang B, Dunbar DC, Desai PV, Patny A, Avery M, Hamann MT. Mollamides B and C, Cyclic hexapeptides from the indonesian tunicate Didemnum molle. J. Nat. Prod. 2008;71:941–945. doi: 10.1021/np700718p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Carroll AR, Bowden BF, Coll JC, Hockless DCR, Skelton BW, White AH. Studies of Australian Ascidians. IV. Mollamide, a Cytotoxic Cyclic Heptapeptide from the Compound Ascidian Diplosoma virens. Aust. J. Chem. 1994;47:61–69. [Google Scholar]
- 102.Sonnenschein RN, Farias JJ, Tenney K, Mooberry SL, Lobkovsky E, Clardy J, Crews P. A further study of the cytotoxic constituents of a milnamide-producing sponge. Org. Lett. 2004;6:779–782. doi: 10.1021/ol036446c. [DOI] [PubMed] [Google Scholar]
- 103.Coleman JE, van Soest R, Andersen RJ. New geodiamolides from the sponge Cymbastela sp. collected in Papua New Guinea. J. Nat. Prod. 1999;62:1137–1141. doi: 10.1021/np990155o. [DOI] [PubMed] [Google Scholar]
- 104.Fusetani N, Sugawara T, Matsunaga S, Hirota H. Orbiculamide A: A novel cytotoxic cyclic peptide from a marine sponge Theonella sp. J. Am. Chem. Soc. 1991;113:7811–7812. [Google Scholar]
- 105.Araki T, Matsunaga S, Nakao Y, Furihata K, West L, Faulkner DJ, Fusetani N. Koshikamide B, a cytotoxic peptide lactone from a marine sponge Theonella sp. J. Org. Chem. 2008;73:7889–7894. doi: 10.1021/jo801032n. [DOI] [PubMed] [Google Scholar]
- 106.Pettit GR, Cichacz Z, Barkoczy J, Dorsaz AC, Herald DL, Williams MD, Doubek DL, Schmidt JM, Tackett LP, Brune DC, et al. Isolation and structure of the marine sponge cell growth inhibitory cyclic peptide phakellistatin 1. J. Nat. Prod. 1993;56:260–267. doi: 10.1021/np50092a011. [DOI] [PubMed] [Google Scholar]
- 107.Pettit GR, Tan R, Ichihara Y, Williams MD, Doubek DL, Tackett LP, Schmidt JM, Cerny RL, Boyd MR, Hooper JN. Antineoplastic agents, 325. Isolation and structure of the human cancer cell growth inhibitory cyclic octapeptides phakellistatin 10 and 11 from Phakellia sp. J. Nat. Prod. 1995;58:961–965. doi: 10.1021/np50120a025. [DOI] [PubMed] [Google Scholar]
- 108.Pettit GR, Tan R. Antineoplastic agents 390. Isolation and structure of phakellistatin 12 from a Chuuk archipelago marine sponge. Bioorg. Med. Chem. Lett. 2003;13:685–688. doi: 10.1016/s0960-894x(02)01054-5. [DOI] [PubMed] [Google Scholar]
- 109.Pettit GR, Tan R. Isolation and structure of phakellistatin 14 from the Western Pacific marine sponge Phakellia sp. J. Nat. Prod. 2005;68:60–63. doi: 10.1021/np040092w. [DOI] [PubMed] [Google Scholar]
- 110.Davis RA, Mangalindan GC, Bojo ZP, Antemano RR, Rodriguez NO, Concepcion GP, Samson SC, de Guzman D, Cruz LJ, Tasdemir D, Harper MK, Feng X, Carter GT, Ireland CM. Microcionamides A and B, Bioactive Peptides from the Philippine Sponge Clathria (Thalysias) abietina. J. Org. Chem. 2004;69:4170–4176. doi: 10.1021/jo040129h. [DOI] [PubMed] [Google Scholar]
- 111.Li H, Matsunaga S, Fusetani N. Halicylindramides D and E, antifungal peptides from the marine sponge Halichondria cylindrata. J. Nat. Prod. 1996;59:163–166. doi: 10.1021/np9600309. [DOI] [PubMed] [Google Scholar]
- 112.Rashid MA, Gustafson KR, Boswell JL, Boyd MR. Haligramides A and B, two new cytotoxic hexapeptides from the marine sponge Haliclona nigra. J. Nat. Prod. 2000;63:956–959. doi: 10.1021/np000051+. [DOI] [PubMed] [Google Scholar]
- 113.Bai R, Durso NA, Sackett DL, Hamel E. Interactions of the sponge-derived antimitotic tripeptide hemiasterlin with tubulin: Comparison with dolastatin 10 and cryptophycin 1. Biochemistry. 1999;38:14302–14310. doi: 10.1021/bi991323e. [DOI] [PubMed] [Google Scholar]
- 114.Crews P, Farias JJ, Emrich R, Keifer PA. Milnamide A, an unusual cytotoxic tripeptide from the marine sponge Auletta cf. constricta. J. Org. Chem. 1994;59:2932–2934. [Google Scholar]
- 115.Laird DW, LaBarbera DV, Feng X, Bugni TS, Harper MK, Ireland CM. Halogenated cyclic peptides isolated from the sponge Corticium sp. J. Nat. Prod. 2007;70:741–746. doi: 10.1021/np060489v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ratnayake AS, Bugni TS, Feng X, Harper MK, Skalicky JJ, Mohammed KA, Andjelic CD, Barrows LR, Ireland CM. Theopapuamide, a cyclic depsipeptide from a Papua New Guinea lithistid sponge Theonella swinhoei. J. Nat. Prod. 2006;69:1582–1586. doi: 10.1021/np060229d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bishara A, Rudi A, Aknin M, Neumann D, Ben-Califa N, Kashman Y. Taumycins A and B, two bioactive lipodepsipeptides from the Madagascar sponge Fascaplysinopsis sp. Org. Lett. 2008;10:4307–4309. doi: 10.1021/ol801750y. [DOI] [PubMed] [Google Scholar]
- 118.Fusetani N, Warabi K, Nogata Y, Nakao Y, Matsunaga S, van Soest RRM. Koshikamide A1, a new cytotoxic linear peptide isolated from a marine sponge, Theonella sp. Tetrahedron Lett. 1999;40:4687–4690. [Google Scholar]
- 119.Araki T, Matsunaga S, Fusetani N. Koshikamide A2, a cytotoxic linear undecapeptide isolated from a marine sponge of Theonella sp. Biosci. Biotechnol. Biochem. 2005;69:1318–1322. doi: 10.1271/bbb.69.1318. [DOI] [PubMed] [Google Scholar]
- 120.Boot CM, Tenney K, Valeriote FA, Crews P. Highly N-methylated linear peptides produced by an atypical sponge-derived Acremonium sp. J. Nat. Prod. 2006;69:83–92. doi: 10.1021/np0503653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wesson KJ, Hamann MT. Keenamide A, a bioactive cyclic peptide from the marine mollusk Pleurobranchus forskalii. J. Nat. Prod. 1996;59:629–631. doi: 10.1021/np960153t. [DOI] [PubMed] [Google Scholar]
- 122.Kimura J, Takada Y, Inayoshi T, Nakao Y, Goetz G, Yoshida WY, Scheuer PJ. Kulokekahilide-1, a cytotoxic depsipeptide from the cephalaspidean mollusk Philinopsis speciosa. J. Org. Chem. 2002;67:1760–1767. doi: 10.1021/jo010176z. [DOI] [PubMed] [Google Scholar]
- 123.Nakao Y, Yoshida WY, Takada Y, Kimura J, Yang L, Mooberry SL, Scheuer PJ. Kulokekahilide-2, a cytotoxic depsipeptide from a cephalaspidean mollusk Philinopsis speciosa. J. Nat. Prod. 2004;67:1332–1340. doi: 10.1021/np049949f. [DOI] [PubMed] [Google Scholar]
- 124.Yu Z, Lang G, Kajahn I, Schmaljohann R, Imhoff JF. Scopularides A and B, cyclodepsipeptides from a marine sponge-derived fungus, Scopulariopsis brevicaulis. J. Nat. Prod. 2008;71:1052–1054. doi: 10.1021/np070580e. [DOI] [PubMed] [Google Scholar]
- 125.Linington RG, Edwards DJ, Shuman CF, McPhail KL, Matainaho T, Gerwick WH. Symplocamide A, a potent cytotoxin and chymotrypsin inhibitor from the marine Cyanobacterium Symploca sp. J. Nat. Prod. 2008;71:22–27. doi: 10.1021/np070280x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stolze SC, Meltzer M, Ehrmann M, Kaiser M. Solid phase total synthesis of the 3-amino-6-hydroxy-2-piperidone (Ahp) cyclodepsipeptide and protease inhibitor Symplocamide A. Chem. Commun. 2010;46:8857–8859. doi: 10.1039/c0cc02889d. [DOI] [PubMed] [Google Scholar]
- 127.Gutierrez M, Suyama TL, Engene N, Wingerd JS, Matainaho T, Gerwick WH. Apratoxin D, a potent cytotoxic cyclodepsipeptide from papua new guinea collections of the marine cyanobacteria Lyngbya majuscula and Lyngbya sordida. J. Nat. Prod. 2008;71:1099– 1103. doi: 10.1021/np800121a. [DOI] [PubMed] [Google Scholar]
- 128.Liu L, Rein KS. New peptides isolated from Lyngbya species: A review. Mar. Drugs. 2010;8:1817–1837. doi: 10.3390/md8061817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Andrianasolo EH, Goeger D, Gerwick WH. Mitsoamide: A cytotoxic linear lipopeptide from the Madagascar marine cyanobacterium Geitlerinema sp. Pure Appl. Chem. 2007;79:593–602. [Google Scholar]
- 130.Maki A, Diwakaran H, Redman B, al-Asfar S, Pettit GR, Mohammad RM, al-Katib A. The bcl-2 and p53 oncoproteins can be modulated by bryostatin 1 and dolastatins in human diffuse large cell lymphoma. Anticancer Drugs. 1995;6:392–397. doi: 10.1097/00001813-199506000-00005. [DOI] [PubMed] [Google Scholar]
- 131.Taraboletti G, Poli M, Dossi R, Manenti L, Borsotti P, Faircloth GT, Broggini M, D’Incalci M, Ribatti D, Giavazzi R. Antiangiogenic activity of aplidine, a new agent of marine origin. Br. J. Cancer. 2004;90:2418–2424. doi: 10.1038/sj.bjc.6601864. [DOI] [PMC free article] [PubMed] [Google Scholar]