Abstract
Strong seed dormancy has been an obstacle for field production of Echinacea species. Previous research on overcoming Echinacea seed dormancy has been extensive and focused on treatment methods, which involve time and expense, and are incompatible with organic production if synthetic chemicals are used. We have attempted to genetically reduce seed dormancy through selection and breeding in Echinacea, by using E. pallida as a model species. Three accessions were used in this study. Nine parent plants of each accession selected from early, in-dark germinated seeds (in-dark plants) or from late, in-light seeds (in-light plants) were planted and grouped by accession and germination treatment method for seed production through a polycross method. Germination tests indicated that these in-dark plants produced seed (in-dark seed) with significantly reduced seed dormancy when tested under light or dark conditions in comparison to the seed of the in-light plants (in-light seed). Among the three accessions, the in-dark seed germinated at much higher rates than did the in-light seed, more than 2× at 25°C under light and up to an 83× increase in darkness, and up to an 8× increase over the corresponding parental seed lots under comparable germination conditions. In addition to these increases in germination, the in-dark seed showed early and synchronized germination as compared to the in-light seed. Since these results were achieved through only one cycle of selection and breeding, they strongly suggest that we have developed a very effective method for modifying seed dormancy in Echinacea.
Keywords: seed germination, pale purple coneflower, medicinal crop, breeding method
1. Introduction
Seed dormancy, in simple terms, is a phenomenon blocking intact viable seeds from germinating under favorable conditions and has been an extensively studied area in plant biology (Baskin and Baskin, 2004; Finch-Savage and Leubner-Metzger, 2006; Simpson, 1990). Strong seed dormancy exists in many wild and weedy plant species and in semi-domesticated crops (Finkelstein et al., 2008). Over the years, researchers have developed a wide range of methods to break dormancy for promoting seed germination (AOSA, 2010). For crops that are typically propagated vegetatively, such as tree fruits, breeders and curators often can rely upon complex protocols to germinate their seeds. But when seed-propagation systems are used directly in commercial crop production, the application of dormancy breaking methods may be inconvenient, expensive, or lead to inconsistent results.
Preparations from Echinacea species have been among the top botanical dietary supplements in Europe and North America since late 1980s, purportedly for general stimulation of the immune system and for treatment or prevention of the common cold (Barrett et al., 2010; Woelkart et al., 2008). Contradictory laboratory and clinical test results have been published regarding the effectiveness of Echinacea products (Barrett et al., 2010; Ross, 2010; Shah et al., 2007; Woelkart et al., 2008), but they are received well in the marketplace, and this trend seems likely to continue (Barrett et al., 2010).
According to the classification system by McGregor (McGregor, 1968), the genus Echinacea contains nine species and four varieties, all native to North America. Among them, E. angustifolia DC., E. purpurea (L.) Moench, and E. pallida have been the most widely used as dietary supplements (Kindscher et al., 2008; Li, 1998). In recent years, E. purpurea has become the primary species for field cultivation and volume of product, and is also the most researched Echinacea species, as reflected in a recent search of the PubMed (National Center for Biotechnology Information, 2011) database. This may be in part because less effort is required for its cultivation, resulting from the fact that it displays little or no seed dormancy in commercial seed lots (Qu et al., 2005), relatively rapid growth, and broad adaptation to various soil types (Li, 1998). However, E. angustifolia was the primary species initially used by Native Americans for its medicinal properties (Volker et al., 2001), and, through the 1920s, this species was the most prescribed medicine made from an American plant (Foster, 1991). While the proportion of E. angustifolia in Echinacea preparations has declined in recent years, during the same time period, the market price of E. angustifolia root has been more than triple that of E. purpurea. For example, dry roots were priced at $4–6 and $14–18 per pound for E. purpurea and E. angustifolia, respectively, in 2006 and 2007 (LeRoy Millard, Nature’s Cathedral, personal communication). Difficulties in the cultivation of E. angustifolia, due to its slower growth and strong requirement for good soil drainage (Li, 1998), may contribute significantly to this cost differential and limitations to field production. Another notable factor that has discouraged the cultivation of E. angustifolia is its strong seed dormancy, requiring complex protocols (Feghahati and Reese, 1994) for promoting germination.
As compared to E. angustifolia or E. purpurea, E. pallida is not as widely used in dietary supplements, but research on E. pallida has demonstrated several potential medicinal properties, including anti-cancer (Chicca et al., 2007), anti-viral (Schneider et al., 2010), and anti-inflammatory activities (Zhang et al., in preparation), and the promotion of wound healing (Zhai et al., 2009). Strong seed dormancy is also present in E. pallida, and treatments that are effective for breaking dormancy for E. angustifolia have similar benefits for E. pallida (Qu et al., 2004; Sari et al., 2001).
Development of protocols to improve Echinacea seed germination has been extensive, involving the use of inorganic salts (Gao et al., 1998), growth regulators (Bishnoi et al., 2010; Pill and Haynes, 1996; Qu et al., 2004), cold, moist stratification (Bishnoi et al., 2010; Bratcher et al., 1993; Parmenter, 1996; Qu et al., 2004, Romero et al., 2005; Wartidiningsih et al., 1994) and mechanical scarification (Bishnoi et al., 2010; Feghahati and Reese, 1994; Sorenson and Holden, 1974). Without such treatments, Echinacea seeds (except for some commercial seed lots of E. purpurea) tend to germinate poorly and/or erratically. However, such treatments require additional time and cost, and their results may be species or seed-lot specific.
In addition, if the treatment methods involve synthetic chemicals, they cannot be used by growers who produce these crops organically. Organic field production is favored for botanical dietary supplements, and trends towards organic production have been increasing. Of methods that do not involve synthetic chemicals, cold, moist stratification is time consuming, requiring several weeks under controlled conditions, and results have been uneven (Bratcher et al., 1993; Parmenter, 1996; Qu et al., 2004, Romero et al., 2005; Wartidiningsih et al., 1994). Light is another factor that can be effective in promoting Echinacea seed germination (Feghahati and Reese, 1994; Smith-Jochum and Albrecht, 1987), and has been used both during and following cold, moist stratification (Romero et al., 2005). It is important to note that any light requirements for seed germination present risks of seed desiccation and seed loss due to wind or bird predation when seeds are sown under field conditions with little or no covering (Qu et al., 2004).
Although Echinacea plants can be propagated vegetatively (Feghahati and Reese, 1994), field production of Echinacea for dietary-supplement feedstocks has been solely from seed propagation. Commercial E. purpurea seed lots present little or no dormancy, displaying 82–97% germination without dormancy-breaking treatments (Qu et al., 2005); this has been achieved through unintended selection over repeated cycles of regeneration and gradual domestication, but strong dormancy is still found in wild populations (Qu et al., 2005). In contrast, seed dormancy is still problematic and ubiquitous for E. angustifolia and E. pallida, particularly for organic producers of these crops. Therefore, the development of cultivars of these species with seeds possessing little or no dormancy would be quite desirable. To this end, we initiated an experiment to reduce seed dormancy in Echinacea through artificial selection and breeding. We selected E. pallida as a model species, because of its strong seed dormancy and relative ease of cultivation for producing seeds, especially at our site in Ames, Iowa, which has clay loam soils that do not drain quickly. Herein, we report our success following this line of this research and discuss its applications in Echinacea and other crops that may benefit from genetically reduced seed dormancy.
2. Materials and Methods
2.1. Initial seed germination
Seeds used in this experiment were obtained from the USDA-ARS North Central Regional Plant Introduction Station (NCRPIS), in Ames, IA. In April 2009, three seed lots of E. pallida (PI 631276, PI 631322 and PI 649036) with strong dormancy were identified by examining historical germination test results (Table 1). We germinated these seeds in a growth chamber (Conviron-CMP500, Winnipeg, Manitoba) under light or in darkness at a constant 25 °C. Our experiment followed a completely randomized design, with each treatment containing 200 seeds divided into four even replicates. After soaking in tap water at room temperature for 15 min, seeds were placed into clear, plastic germination boxes on water-saturated blotter paper, 50 seeds per box. For the light treatment, cool white fluorescent lamps provided photosynthetically active radiation at 40 μmol·m−2·s−1. Seeds subjected to darkness were placed in the same growth chamber, but the boxes were wrapped with aluminum foil to prevent light penetration. Germination (emergence of radicles >2 mm long) was recorded daily for the light treatment and on three occasions (days 7, 14 and 20) under dim light for the dark treatment. Germinated seeds were removed when counted. This procedure was completed in < 1 min for each germination box. It has been reported (Qu et al., 2004) that short exposure to light during germination evaluation has little effect on final germination. All tests lasted for 20 d after initiation. After removal from the germination box, the germinating seeds were transplanted into flats and grown in the NCRPIS greenhouse for about 10 weeks before field planting.
Table 1.
Historical germination results (in %) of the parental seed lots of E. pallida used in this experiment.
| Treatment | Seed lots
|
||
|---|---|---|---|
| PI 631276-00ncai01 | PI 631322-00ncai01 | PI 649036-04ncai01 | |
| Ethephon, Light 25 °Cz | 56 (2001) | 64 (2001) | 39 (2005) |
| Light, 20/30 °Cy | 22 (2002) | 5 (2002) | No test |
Germination test duration was 20 and 12 days, respectively.
2.2. Selection of seeds for growing parent plants
Since it has been documented that light promotes Echinacea seed germination, we reasoned that the earlier an individual seed germinated in darkness, the less that seed displayed dormancy. On the other hand, seeds that germinated late in light are likely to have stronger dormancy. Therefore, seedlings growing from the first nine, in-dark germinated seeds from each seed lot were selected as the parent plants for producing the next generation of seeds for evaluating dormancy reduction. And, to serve as an effective control, seedlings from the last nine, in-light germinated seeds from each lot were also selected for seed production.
2.3. Seed production
Seed production was achieved through a polycross method. The selected E. pallida seedlings were transplanted into the field in June 2009 at the NCRPIS farm. The field soils are classified as a silty clay loam. The seedlings were grouped by accession and germination treatment method. Each group of nine plants was planted in separate beds with 60 cm in-row spacing and 45 cm between rows. The plants did not flower in 2009 and overwintered well in the field without losses. In early June 2010, the beds were individually caged (Widrlechner et al., 1997). When flowers were ready for pollination in about mid-June, honeybee nucleus hives were placed in the cages to effect pollen transfer among plants. Seed heads were harvested in late August 2010, when seeds were fully mature (seed color turned brown), and placed in paper bags at room temperature for about two months before seed processing. All seed heads from each group of nine plants were pooled together. After threshing and cleaning, seeds were stored for about four months in an office (at ~20 °C and 50% RH) in paper bags before germination testing.
2.4. Seed germination of samples produced in 2010
Germination testing of E. pallida seeds harvested in 2010 was conducted in March 2011. Basic germination conditions and treatment methods were the same as those described for the initial experiments. However, for these samples, we tested 300 seeds from each seed lot, placed into three germination boxes with 100 seeds each as replicates. In addition, for seed lots from PI 631322 and 631276, the 2010 samples were also germinated in a growth chamber under 12h alternating temperatures of 20/30 °C with continuous light, for a comparison to germination test conditions used historically for tests of the parental seed lots (Table 1). For this round of tests, seed preparation was the same as described earlier, except that 99 seeds were used and divided into three replications.
For data analysis, germination percentages were normalized by transformation (arcsin√%) before being subjected to analysis of variance, following the methods of Wartidiningsih and Geneve (1994).
3. Results and Discussion
We experienced severe, unfavorable weather conditions in Ames, IA during the 2010 growing season, with excessive precipitation that led to atypically saturated soils, high humidity, and reduced sunlight. However, our E. pallida selections were little affected by the unfavorable weather and grew relatively well in 2010, suggesting that this species can tolerate wet, poorly drained soils. All nine plants of each treatment group flowered and set seeds that matured well by the time of harvest.
3.1. In-light germination
Test results for 2010 intermated seed lots are presented in Table 2. Significant germination differences were observed among these lots. Seeds resulting from the intermating of in-dark selected plants (in-D seed) germinated to a much greater degree than did the seeds produced by the intermating of in-light selected plants (in-L seed). At 25 °C with light, the in-D seeds germinated at between 1.7 and 2.0× the level of the in-L samples. Differences were even more notable in tests run at 20/30 °C, where in-D selection led to more than a 3× increase in germination. When germination of the 2010 in-D seed on day 12 after germination initiation was compared with 2002 tests of their corresponding parental seed lots under 20/30 °C conditions, the increases were striking for both PI 631276 at 2.7× (60/22) and for PI 631322 at 8× (40/5) (Table 1, also see Fig. 1 and 2). No comparative test could be conducted for the 2010 seeds of PI 649036 at 20/30 °C, because no data had been collected under these conditions for the parental seed lot of this accession due to the limited number of seeds initially obtained.
Table 2.
Germination comparison (in %) among seed lots of three Echinacea pallida accessions.
| Treatment | Accession | Seed Lot
|
|||
|---|---|---|---|---|---|
| 2000 (parent) | 2004 (parent) | 2010 in-Dz | 2010 in-Ly | ||
| Light, 25 °C | PI 631276 | 76 bx | 93 a | 50 c | |
| PI 631322 | 67 bx | 84 a | 42 c | ||
| PI 649036 | 65 aw | 46 b | 29 c | ||
| Dark, 25 °C | PI 631276 | 37 ax | 25 a | 0.3 b | |
| PI 631322 | 31 ax | 36 a | 4 b | ||
| PI 649036 | 20 ax | 4 b | 2 b | ||
| Light, 20/30 °C | PI 631276 | 73 *** | 23 | ||
| PI 631322 | 54 *** | 14 | |||
Seeds from parent plants grown from in-dark (in-D) selected seeds.
Seeds from parent plants grown from in-light (in-L) selected seeds.
Within row mean separation by Duncan’s multiple range test,
P<0.0001 and
P<0.01.
Within row mean separation by t-test, P<0.001.
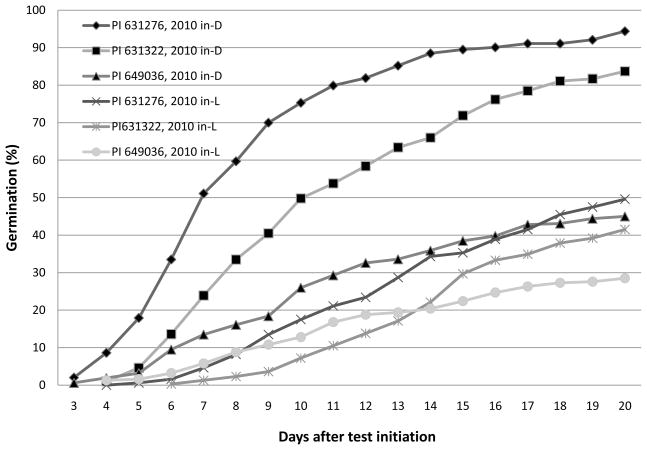
Fig. 1.
Daily cumulative germination of 2010 in-dark (in-D) and in-light (in-L) seed lots of Echinacea pallida accession PI 631276, PI 631322, and PI 649036 at 25 °C with light.
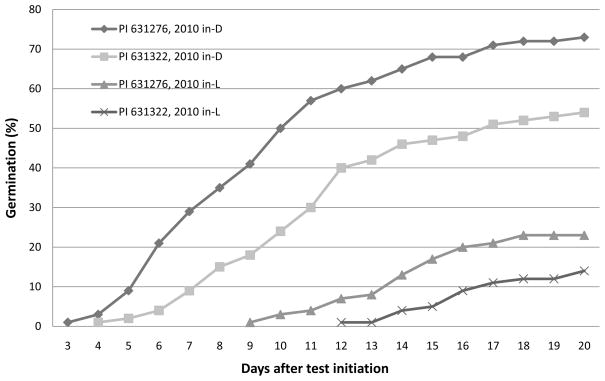
Fig. 2.
Daily cumulative germination of 2010 in-dark (in-D) and in-light (in-L) seed lots of Echinacea pallida accession PI 631276 and PI 631322 at 20/30 °C with light.
The marked increase in seed germination for the 2010 in-D seeds over the in-L seeds indicates that a single cycle of in-dark germination selection can be effective in selecting genotypes with reduced dormancy under light. This strongly suggests that this trait is highly heritable in these accessions of E. pallida, although perhaps to varying degrees.
When comparing test results for seed lots harvested in 2010 with 2009 test results for the corresponding 2000 and 2004 parental seed lots under 25 °C conditions with light, the 2010 in-D seed germinated at 122% and the 2010 in-L seed at 66%, respectively, of the parental seed lot of PI 631276, at 125% and 63%, respectively, for PI 631322, and at 71% and 56%, respectively, for PI 649036 (Table 2).
The comparison of germination of the 2010 seeds with the 2009 results of the parent seed lots may seem puzzling, especially for PI 649036, for which the in-D seed germinated at an even lower percentage than did the parental seed lot (Table 2). However, the relatively high germination of parental seed lots in the 2009 test was very likely due to dormancy breaking by after-ripening mechanisms (Bazin et al., 2011; Finch-Savage and Leubner-Metzger, 2006; Finkelstein et al., 2008) during storage. This is regularly observed in Echinacea and accessions of other Asteraceae, such as Calendula (Widrlechner, 2007), conserved at the NCRPIS. In this case, a reduction in dormancy during the course of storage is well supported by historical germination test results conducted over the lives of these parental seed lots.
Shortly after harvest, the germination was only 56% for PI 631276, 50% for PI 631322, and 39% for PI 649036 after the seeds were treated with ethephon to promote seed germination (Table 1). The effectiveness of ethephon in promoting E. pallida and E. angustifolia seed germination has been well documented (Qu et al., 2004; Sari et al., 2001). Germination tests conducted on these same seed lots of PI 631322 and 631276 at 20/30 °C conditions under light without using ethephon, about 18 months after harvest, resulted in values of 5% and 22%, respectively (Table 1). These percentages are much lower than the 2010 seed lots (40% for 631322 and 60% for PI 631276) under similar germination conditions (Fig. 2). Therefore, the 2009 test results have little direct value in evaluating the effectiveness of selection, other than to provide information on dormancy reduction of the parental seeds during storage. No further comparison to the 2009 germination results will be given herein.
Under 20/30 °C conditions, the 2010 in–L seeds germinated even less than did the parental seed lots on day 12 after test initiation: 7% vs. 22% for PI 631276 and 1% vs. 5% for PI 631322 (see Fig. 2). This indicates that our late, in-light selection of seeds with strong dormancy is very effective.
3.2. In-dark germination
Under in-dark test conditions, differences between the 2010 in-D and in-L seeds were even greater than observed when tested in light. There was a two-fold increase in germination rates for PI 649036 to more than 9 and 80-fold increases for PI 631322 and PI 631276, respectively (Table 2).
The increase of seed germination under darkness was a major goal when we designed these experiments. Because long storage prior to testing can also significantly increase in-dark germination as discussed earlier, we initially had concerns that selection effectiveness could be impaired due to the early germination of seeds that otherwise would not germinate quickly if newly harvested seeds were used. Although we cannot partition the differences that we observed among these different factors, the results demonstrate that our selection method is extremely promising for the in-dark germination trait, and its effects may be further enhanced by using freshly harvested seeds for parent plant selection.
3.3. Comparison of overall germination under different conditions
Both the 2010 in-D and in-L seeds germinated better at 25 °C than they did at 20/30 °C (Table 3). The constant 25 °C tests increased overall germination by 20 to 30%. We compared these two regimens primarily because the historical germination tests had been conducted at 20/30 °C, the AOSA (2010) standard Echinacea temperature regimen. The higher germination of the 2010 seed lots under 25 °C and the larger germination difference between in-D and in-L seeds under 20/30 °C than under 25 °C condition (Table 2) not only indicate that 25 °C is a more suitable temperature for E. pallida seed germination than is the 20/30 °C regimen, but also demonstrate that the in-D seed tolerate this unfavorable germination environments (20/30 °C) better than do the in-L seed. It is unclear at this time whether the lower (20 °C) or the higher (30 °C) temperature, or the interaction of both, led to suboptimal germination results in our experiment.
Table 3.
Germination comparison (in %) between 2010 in-dark (in-D) and in-light (in-L) seed lots of E. pallida PI 631276 and PI 631322 under different temperature regimens.
| Seed lot | Accession | Treatment
|
|
|---|---|---|---|
| Lt, 25 °C | Lt, 20/30 °C | ||
| 2010 in-D | PI 631276 | 92 * | 73 |
| PI 631322 | 84 ** | 54 | |
| 2010 in-L | PI 631276 | 50 ** | 23 |
| PI 631322 | 42 *** | 14 | |
Within row mean separation by t-test:
P<0.05,
P<0.01, and
P<0.001.
3.4. Rate of germination
Early, synchronized seed germination is a very favorable characteristic in crop production, since it reduces the opportunity for seeds to experience unfavorable environmental conditions in the field before germination (Bewley, 1997). It also leads to uniform stand establishment that better facilitates field management and the possibility of longer overall growing seasons (Taylor and Harman, 1990). In Echinacea, seed stratification under moist, cool conditions has been used to accelerate and synchronize germination (Romero et al., 2005) in addition to its use for dormancy breaking per se. Rapid, synchronized germination is also a beneficial outcome of our selection and breeding experiment. In addition to higher total germination, germination was significantly earlier for in-D seeds than for in-L seeds (Fig. 1 and 2). On day 12 after initiation, 81% of the PI 631276 in-D seeds germinated at 25 °C with light, but only 24% of the in-L seeds had germinated. For PI 631322, the numbers were 59% and 14%, respectively. The difference was even larger at 20/30 °C, at 60% and 7%, respectively, for the in-D seeds of PI 631276, and at 40% and 1% for the in-D seeds of PI 631322.
3.5. Rationale and prospects for future research
Recently, breeding for reduced post-harvest seed dormancy has been conducted in switchgrass (Panicum virgatum L.) with significant progress in reducing the degree of dormancy being reported after four cycles of selection (Burson et al., 2009). In the switchgrass research, parent plants were obtained by selecting the earliest germinating seeds under 12 h light. Although no specific underlying mechanisms were given, Qu et al. (2004) suggested that because Echinacea seeds in full dormancy cannot germinate either in light or darkness but that light can promote germination, so seed that can germinate in darkness should have no dormancy or that dormancy has been completely released. By this reasoning, if seeds can germinate in dark immediately after ripening, they have the lowest or no dormancy. Comparing to the parent seed lots, the very high germination achieved in this experiment with the in-D seeds, especially for PI 631276 and PI 631322, suggest that our method of selecting parental plants through choosing seeds that germinated early in darkness is very effective in E. pallida. Considering that the original seed lots used for this selection experiment were about nine years old and the reduced dormancy during storage might have deceased the power of selection, using fresh seed for selection may be even more effective.
As we look to refine and expand this line of research, we should also note that our selection intensity for selection during in-dark germination was 4.5% (9/200). If the very first germinated seeds can be effectively identified from even larger samples, increased selection intensity may further reduce dormancy and increase early germination. One can avoid generating too small a selected population via increased selection intensity [and the potential for future interference by inbreeding depression or self-incompatibility (Stephens, 2008)] by starting with even larger seed samples for germination. After a few more cycles of selection and breeding, following the general principles of recurrent selection (Fehr, 1987), this approach would be highly likely to generate genotypes of E. pallida with very low levels of seed dormancy.
Considering the ease of interspecific hybridization (McGregor, 1968), and evidence of little phylogenetic differentiation (Flagel et al., 2008) within the genus Echinacea, not to mention the success of unintentional selection in generating genotypes of E. purpurea (Qu et al., 2005) with little or no seed dormancy, we believe that our selection method with E. pallida will likely be successful when applied to other species, including the problematic, commercially important species, E. angustifolia. This selection method could also be extended to other crop species that have undesirably strong levels of seed dormancy whenever germination is promoted by exposure to light.
In conclusion, research on overcoming Echinacea seed dormancy typically focuses on finding effective treatment methods, which always require time and resources, and may not always produce consistent results. Ultimately, the development and use of genotypes that lack seed dormancy can effectively avoid these problems. Our results indicate that selection of the earliest seeds to germinate in darkness is a very effective strategy in E. pallida. Plants grown from these seeds produced seeds with significantly reduced dormancy under both light and dark conditions, suggesting high hertitability of this trait in Echinacea. This selection method, followed by seed production in isolation, is a simple procedure that can readily be used by both Echinacea growers and researchers.
Highlights.
Strong seed dormancy has been an obstacle for Echinacea field production.
E. pallida was our model species to reduce seed dormancy through selection.
Seedlings from in-dark germination produced seeds with much reduced seed dormancy.
Germination rates for in-dark seeds were increased under both light and darkness.
In-dark seeds also showed early, synchronized germination over in-light seeds.
Acknowledgments
The authors wish to thank Jeffrey Carstens and Nathan Johnson for their assistance in managing plants in the greenhouse and field, and Maria Erickson and David Kovach for their assistance with seed germination tests. We are also grateful to Drs. Kathleen Delate, Candice Gardner, Robert Geneve, Hector Perez, and two anonymous reviewers for their useful critiques of an earlier draft of this report. This journal paper of the Iowa Agriculture and Home Economics Experiment Station, Ames, Iowa, Project No.1018, was supported by Hatch Act and State of Iowa funds, and the research described herein was supported by Award Number P5AT004155 from the National Center for Complementary & Alternative Medicine. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Complementary & Alternative Medicine or the National Institutes of Health. Mention of commercial brand names does not constitute an endorsement of any product by the U.S. Department of Agriculture or cooperating agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Association of Official Seed Analysts (AOSA) Rules for testing seeds. Association of Official Seed Analysts; Ithaca, NY: 2010. [Google Scholar]
- Barrett B, Brown R, Rakel D, Mundt M, Bone K, Barlow S, Ewers T. Echinacea for treating the common cold: A randomized trial. Ann Intern Med. 2010;153:769–777. doi: 10.7326/0003-4819-153-12-201012210-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskin JM, Baskin CC. A classification system for seed dormancy. Seed Sci Res. 2004;14:1–16. [Google Scholar]
- Bazin J, Batlla D, Dussert S, El-Maarouf-Bouteau H, Bailly C. Role of relative humidity, temperature, and water status in dormancy alleviation of sunflower seeds during dry after-ripening. J Exp Bot. 2011;62:627–640. doi: 10.1093/jxb/erq314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewley JD. Seed germination and dormancy. Plant Cell. 1997;9:1055–1066. doi: 10.1105/tpc.9.7.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishnoi UR, Willis JE, Mentreddy SR. Methods to improve seed germination of purple coneflower (Echinacea purpurea (L.) Moench) Agric Biol J Am. 2010;1:185–188. [Google Scholar]
- Bratcher CB, Dole JM, Cole JC. Stratification improves germination of five native wildflower species. HortScience. 1993;28:899–901. [Google Scholar]
- Burson BL, Tischler CR, Ocumpaugh WR. Breeding for reduced post-harvest seed dormancy in switchgrass: Registration of TEM-LoDorm switchgrass germplasm. Journal of Plant Registrations. 2009;3:99–103. [Google Scholar]
- Chicca A, Adinolfi B, Martinotti E, Fogli S, Breschi MC, Pellati F, Benvenuti S, Nieri P. Cytotoxic effects of Echinacea root hexanic extracts on human cancer cell lines. J Ethnopharmacol. 2007;110:148–153. doi: 10.1016/j.jep.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Feghahati SMJ, Reese RN. Ethylene-, light-, and prechill-enhanced germination of Echinacea angustifolia seeds. J Amer Soc Hort Sci. 1994;119:853–858. [Google Scholar]
- Fehr WR. Principles of cultivar development, vol. 1: Theory and technique. Macmillan; New York: 1987. [Google Scholar]
- Finch-Savage WE, Leubner-Metzger G. Seed dormancy and the control of germination. New Phytologist. 2006;171:501–523. doi: 10.1111/j.1469-8137.2006.01787.x. [DOI] [PubMed] [Google Scholar]
- Finkelstein R, Reeves W, Ariizumi T, Steber C. Molecular aspects of seed dormancy. Annu Rev Plant Biol. 2008;59:387–415. doi: 10.1146/annurev.arplant.59.032607.092740. [DOI] [PubMed] [Google Scholar]
- Flagel LE, Rapp RA, Grover CE, Widrlechner MP, Hawkins J, Grafenberg JL, Alvarez I, Chung GY, Wendel JF. Phylogenetic, morphological, and chemotaxonomic incongruence in the North American endemic genus Echinacea. Amer J Bot. 2008;95:756–765. doi: 10.3732/ajb.0800049. [DOI] [PubMed] [Google Scholar]
- Foster S. Echinacea: Nature’s immune enhancer. Healing Arts Press; Rochester, VT: 1991. [Google Scholar]
- Gao YP, Zheng GH, Gusta LV. Potassium hydroxide improves seed germination and emergence in five native plant species. HortScience. 1998;33:274–276. [Google Scholar]
- Kindscher K, Price DM, Castle L. Resprouting of Echinacea angustifolia augments sustainability of wild medicinal plant population. Econ Bot. 2008;62:139–147. [Google Scholar]
- Li TSC. Echinacea: Cultivation and medicinal value. HortTechnology. 1998;8:122–129. [Google Scholar]
- McGregor RL. The taxonomy of the genus Echinacea (Composite) Univ of Kansas Sci Bul. 1968;48:113–142. [Google Scholar]
- National Center for Biotechnology Information. [3 May 2011];PubMed database. 2011 Accessed at http://www.ncbi.nlm.nih.gov/pubmed/on.
- Parmenter GA. Chilling requirement of commercial Echinacea seed. New Zealand J Crop and Hort Sci. 1996;24:109–114. [Google Scholar]
- Pill WG, Haynes JG. Gibberellic acid during priming of Echinacea purpurea (L.) Moench seeds improves performance after seed storage. J Hort Sci. 1996;71:287–295. [Google Scholar]
- Qu L, Wang X, Chen Y, Scalzo R, Widrlechner M, Davis J, Hancock J. Commercial seed lots exhibit reduced seed dormancy in comparison to wild seed lots of Echinacea purpurea. HortScience. 2005;40:1843–1845. [PMC free article] [PubMed] [Google Scholar]
- Qu L, Wang X, Yang J, Hood E, Scalzo R. Ethephon promotes germination of Echinacea angustifolia and E. pallida in darkness. HortScience. 2004;39:1101–1103. [PMC free article] [PubMed] [Google Scholar]
- Romero F, Delate K, Hannapel DJ. The effect of seed source, light during germination, and cold-moist stratification on seed germination in three species of Echinacea for organic production. HortScience. 2005;40:1751–1754. [PMC free article] [PubMed] [Google Scholar]
- Ross SM. A standardized Echinacea extract demonstrates efficacy in the prevention and treatment of colds in athletes. Holist Nurs Pract. 2010;24:107–109. doi: 10.1097/HNP.0b013e3181d39b3f. [DOI] [PubMed] [Google Scholar]
- Sari AO, Morales MR, Simon JE. Ethephon can overcome seed dormancy and improve seed germination in purple coneflower species Echinacea angustifolia and E. pallida. HortTechnology. 2001;11:202–205. [Google Scholar]
- Schneider S, Reichling J, Stintzing FC, Messerschmidt S, Meyer U, Schnitzler P. Anti-herpetic properties of hydroalcoholic extracts and pressed juice from Echinacea pallida. Planta Med. 2010;76:265–272. doi: 10.1055/s-0029-1186137. [DOI] [PubMed] [Google Scholar]
- Shah SA, Sander S, White CM, Rinaldi M, Coleman CI. Evaluation of Echinacea for the prevention and treatment of the common cold: A meta-analysis. Lancet Infect Dis. 2007;7:473–480. doi: 10.1016/S1473-3099(07)70160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson GM. Seed dormancy in grasses. Cambridge University Press; New York: 1990. [Google Scholar]
- Smith-Jochum CC, Albrecht ML. Field establishment of three Echinacea species for commercial production. Acta Horticulturae. 1987;208:115–120. [Google Scholar]
- Sorenson JT, Holden DJ. Germination of prairie forb seed. J Range Mgt. 1974;27:123–126. [Google Scholar]
- Stephens LC. Self-incompatibility in Echinacea purpurea. HortScience. 2008;43:1350–1354. [Google Scholar]
- Taylor AG, Harman GE. Concepts and technologies of selected seed treatments. Ann Rev Phytopathol. 1990;28:321–339. [Google Scholar]
- Volker S, Rudolf H, Tyler VE. Rational phytotherapy: A physician’s guide to herbal medicine. Springer; Berlin: 2001. [Google Scholar]
- Wartidiningsih N, Geneve RL. Seed source and quality influence germination in purple coneflower [Echinacea purpurea (L.) Moench] HortScience. 1994;29:1443–1444. [Google Scholar]
- Wartidiningsih N, Geneve RL, Kester ST. Osmotic priming and chilling stratification improves seed germination of purple coneflower. HortScience. 1994;29:1445–1448. [Google Scholar]
- Widrlechner MP. While they were asleep: Do seeds after-ripen in cold storage? Experiences with Calendula. Combined Proc of the IPPS. 2007;56:377–382. [Google Scholar]
- Widrlechner MP, Abel CA, Wilson RL. Ornamental seed production in field cages with insect pollination. Combined Proc of the IPPS. 1997;46:512–516. [Google Scholar]
- Woelkart K, Linde K, Bauer R. Echinacea for preventing and treating the common cold. Planta Med. 2008;74:633–637. doi: 10.1055/s-2007-993766. [DOI] [PubMed] [Google Scholar]
- Zhai Z, Haney DM, Wu L, Solco AK, Murphy PA, Wurtele ES, Kohut ML, Cunnick JE. Alcohol extract of Echinacea pallida reverses stress-delayed wound healing in mice. Phytomedicine. 2009;16:669–678. doi: 10.1016/j.phymed.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]