Summary
Haematopoietic stem cells (HSCs) are first detected in the floor of the embryonic dorsal aorta (DA) and here we investigate the signals that induce the HSC programme there. We show that while continued Hedgehog (Hh) signalling from the overlying midline structures maintains the arterial programme characteristic of the DA roof, a ventral Bmp4 signal induces the blood stem cell programme in the DA floor. This patterning of the DA by Hh and Bmp is the mirror image of that in the neural tube, with Hh favouring dorsal rather than ventral cell types, and Bmp favouring ventral rather than dorsal. With the majority of current data supporting a model whereby HSCs derive from arterial endothelium, our data identifies the signal driving this conversion. These findings are important for the production of HSCs from embryonic stem cells and establish a paradigm for the development of adult stem cells.
Introduction
Blood and endothelium develop in close association, possibly from bipotential precursors called haemangioblasts (reviewed in Dzierzak and Speck, 2008). Bmp is critical for haemangioblast formation, and Hedgehog (Hh), VEGF and Notch are required for the specification of arterial endothelium and adult HSCs in zebrafish (Gering and Patient, 2005; Lawson et al., 2002; McReynolds et al., 2007; Walmsley et al., 2002). Subsequent to its earlier role in arterial and HSC specification, Hh is later required for continued expression of the transcription factor, tbx20, in the DA roof, proximal to the source of Hh in the notochord (Gering and Patient, 2005; Murayama et al., 2006; Zhang and Rodaway, 2007). This dorsal restriction of tbx20 within the DA occurs at the same time as the initiation of definitive haematopoiesis in the ventral wall of the DA (Ahn et al., 2000; Gering and Patient, 2005) (Fig 1a), an observation which may reflect an antagonistic relationship between tbx20 and the haematopoietic programme, as seen during the earlier development of embryonic blood (Szeto et al., 2002). Thus the restriction of HSC formation to the DA floor may be a consequence of continued Hh signalling.
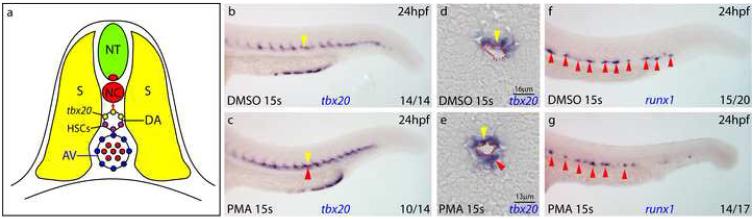
Figure 1. Ectopic Hedgehog signalling expands tbx20 expression into the ventral DA but does not suppress the initiation of HSC gene expression.
a) Diagram of transverse section illustrating DA polarisation in the zebrafish trunk at 24hpf. b-g) Embryos treated with DMSO or PMA from 15S and probed for tbx20 or runx1 at 24hpf. Dorsal and ventral DA expression (yellow and red arrows). PMA treatment expands tbx20 expression into the ventral DA (c, e compared to b, d) but runx1 is unaffected (f, g). AV, axial vein; DA, dorsal aorta; NC, notochord; NT, neural tube; PMA, purmorphamine; S, somite.
While the location of Hh protein has not been determined in zebrafish due to a lack of available antibodies, Hh expression has been demonstrated within the dorsally located notochord, but not the ventrally located endoderm, during the time of HSC emergence (Krauss et al., 1993; Lewis et al., 1999; Roy et al., 2001). Perhaps the best readout for cells actively receiving Hh signalling is the expression of the receptor, Patched (Ptc), and ptc1 is expressed within the dorsal wall of the DA, whilst ptc2 exhibits more diffuse expression throughout the trunk, encompassing the entire DA (Lewis et al 1999). The distinct functions of these receptors are not well understood, but it is highly likely that they will have different targets, based upon the disparate phenotypes observed in ptc1 and ptc2 mutants (Koudijs et al., 2008; Koudijs et al., 2005). Lewis et al’s data indicate that ptc1 expression requires a higher level of Hh signalling than ptc2, consistent with its expression in the dorsal wall of the DA, proximal to the source of midline Shh. Thus it is likely that Hh signalling in the dorsal wall of the DA is mediated by Ptc1. The expression of ptc2 within the ventral wall of the DA, more distal to the midline Shh source, implies that the ventral DA experiences a low level of Hh signalling. However, inhibition of Hh signalling at this time indicated that it is not required for HSC programming (Gering and Patient, 2005).
Here we demonstrate that distance from Hh signalling contributes to but is not solely responsible for the ventral localisation of HSCs. Using a conditional transgenic strategy, and in parallel, morpholino knock down, we provide in vivo functional evidence that bmp4 is required for the runx1-mediated emergence and maintenance of HSCs in the ventral wall of the DA.
Results
Ectopic Hedgehog signalling expands tbx20 expression into the ventral wall of the DA but does not suppress initiation of HSC gene expression
Because tbx20 and HSC gene expression are mutually exclusive, we tested the possibility that Hh might restrict HSC formation, by exposing the DA floor to continued Hh signalling. To expand the range of Hh signalling, embryos were treated with purmorphamine (PMA), which activates the Smoothened receptor (Sinha and Chen, 2006). Treated embryos exhibited morphological and gene expression alterations characteristic of Hh pathway activation (Fig S1) (Concordet et al., 1996; Ekker et al., 1995). In embryos treated from the 15-somite stage, when maintenance of tbx20 expression in the roof of the DA is Hh dependent, tbx20 expression was expanded into the DA floor (Fig 1c, e, red arrows). At the times studied, the endothelium of the DA consists of a single cell layer thus, since tbx20 staining directly abuts the DA lumen but also extends greater than one cell diameter ventrally (Fig 1e, red arrow), under the influence of PMA both endothelial cells of the DA and the ventral mesenchyme beneath were induced to express tbx20. Thus, elevated Hh signalling can induce tbx20 expression in the floor of the DA, making its absence there normally a likely consequence of distance from the notochord source of Hh (Fig 1a).
To examine HSC programming, we monitored runx1 expression, which is essential for HSC development and is the first sign of HSC formation in zebrafish (Burns et al., 2005; Gering and Patient, 2005; Kalev-Zylinska et al., 2002). Despite the ectopic expression of tbx20 in the DA floor, runx1 expression in PMA treated embryos was indistinguishable from control embryos at 24hpf (Fig 1f-g, red arrows). Both the numbers of runx1+ cells and their levels of expression were unaffected. There was a similar lack of effect at 23hpf, when runx1 expression in the DA is first detectable, and also at 25hpf and 26hpf (data not shown). We therefore conclude that Hh affects neither the initiation of runx1 expression in the DA nor its maintenance.
It was not possible to study gene expression downstream of runx1, such as cmyb and ikaros (Gering and Patient, 2005; Thompson et al., 1998; Willett et al., 2001), since PMA treated embryos do not develop circulation due to loss of the axial vein (data not shown), and therefore the primitive blood expressing cmyb and ikaros remains in the ICM region, masking expression in the HSCs in the DA floor. Furthermore, it was not possible to assess derivatives of the HSCs in the thymus, for example rag1 expression (see Fig 2j-k & Fig 4k-l), because absence of circulation prevents HSC migration to this region (Kissa et al., 2008; Murayama et al., 2006). However, the initiation and maintenance of an additional HSC marker, gfi1, was also unaffected in the DA of PMA treated embryos, as seen for runx1 (Fig S2). Taken together, the undisturbed initiation of runx1 and gfi1 expression in PMA treated embryos indicates that distance from the Hh source cannot alone be responsible for the localisation of HSC emergence in the DA floor.
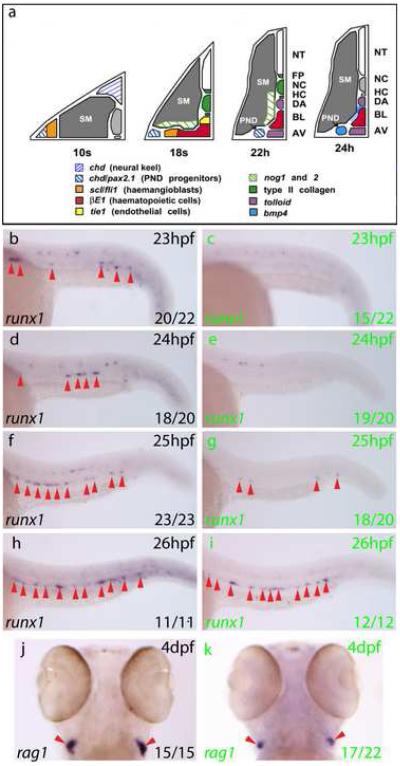
Figure 2. Bmp signalling is required for initiation of the HSC programme in the ventral DA.
a) Gene expression summary as progenitors migrate to the midline and form blood and DA/AV endothelium. DA region becomes conducive to Bmp signalling by 24hpf. b-i) Expression of runx1 (arrows) in embryos heat shocked at 21hpf. runx1 expression in the DA at 23-26hpf (GFP−, black letters) was lost in GFP+embryos (green letters) at 23 and 24hpf. Representative embryos are depicted and numbers with or without runx1 staining were scored. j, k) rag1 expression in thymi at 4dpf heat shocked at 21hpf. 17/22 GFP+ embryos had reduced rag1 expression compared to GFP−embryos, in which rag1 staining showed little variation. The remaining 5/22 GFP+ embryos exhibited wild type rag1 expression. AV, axial vein; BL, primitive blood; DA, dorsal aorta; HC, hypochord; NC, notochord; NT, neural tube; PND, pronephric duct; SM, somitic mesoderm.
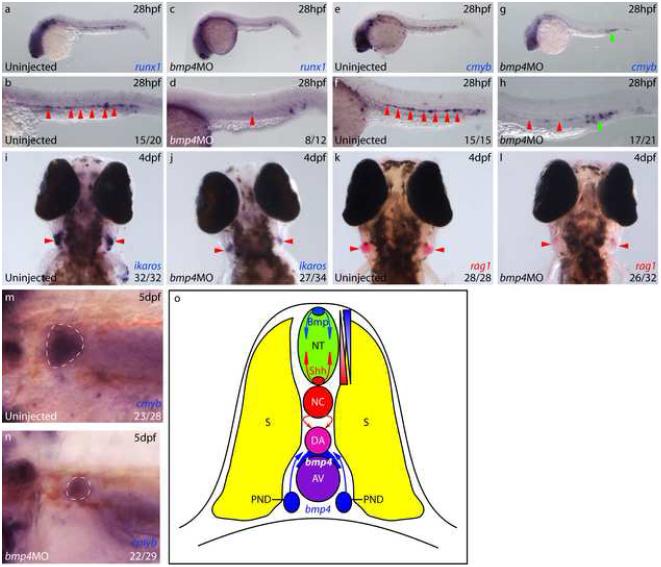
Figure 4. bmp4 is required for HSC emergence within the ventral DA.
a-d) runx1 expression was absent from the DA (arrows) in 8/12 bmp4 morphants at 28hpf (c, d), whilst the remaining 4/12 (a, b) exhibited reduced expression compared with uninjected controls. e-h) 17/21 bmp4 morphants exhibited absent or substantially downregulated cmyb expression in the DA (red arrows) at 28hpf. i, j) At 4dpf, 27/34 bmp4 morphants exhibited substantial downregulation of ikaros expression within the developing thymi, (arrows). The remaining 7/34 exhibited wild type expression. k, l) At 4dpf, 26/32 bmp4 morphants exhibited substantially reduced expression of rag1 in developing thymi (arrows), of which 7 exhibited no rag1 expression. The remaining 6/32 were wild type. m, n) Dorsolateral views of the developing pronephros at 5dpf depicting substantial downregulation of cmyb expression (outlined) in 22/29 embryos, of which 4/29 exhibited no expression. The remaining 7/29 were wild type. o) Diagram of zebrafish trunk depicting polarisation of the DA by dorsal Hedgehog and ventral Bmp signalling, mirroring the neural tube.
Bmp signalling is required for initiation and maintenance of runx1 expression in the ventral wall of the DA
Analysis of gene expression in the zebrafish trunk revealed that Bmp antagonists surround the pocket in which blood and endothelium differentiate, but that just prior to HSC emergence, the environment is transformed into one favouring Bmp signalling ventral to the DA (Fig 2a). Thus, the pocket in which the blood and endothelium differentiate is surrounded by tissues expressing chordin, noggin2 and type II collagen up to 22hpf (Furthauer et al., 1999; Yan et al., 1995) (and Fig S3). However, by 24hpf, while noggin1 remains expressed in the ventral somite distal to the DA, the pronephric ducts (PNDs) in the anterior, and the ventral mesenchyme in the posterior, express bmp4 (Chin et al., 1997) (and Fig S3a-b). In addition, the major vessels, including the DA, continue to express tolloid (from 21hpf), which cleaves residual Chordin (Connors et al., 1999). Furthermore, bmpr2a, alk1, alk3, alk8 and smad5 are all expressed in the DA (Monteiro et al., 2008). Thus, immediately prior to HSC emergence, the environment switches from anti- to pro-Bmp signalling, suggesting a potentially direct inductive role for Bmp in HSC emergence within the DA. Similar, localised expression of bmp4 has been observed in human, mouse, chick and Xenopus embryos (A. Ciau-Uitz and RP, unpublished; Marshall et al., 2000; Pimanda et al., 2007; Suonpaa et al., 2005).
To functionally test such a role for Bmp signalling, we used a transgenic zebrafish line expressing a temperature-inducible, truncated Bmp receptor (tBR), which acts as a dominant-negative mutant (Pyati et al., 2006; Pyati et al., 2005). Because tBR is fused to GFP, fluorescent embryos expressing tBR can be separated after heat shock from control embryos derived from the same transgenic heterozygous parent. Embryos were heat shocked at 21hpf so that Bmp signalling would be inhibited by 23hpf when runx1 expression initiates in the DA. Immunohistochemistry for phosphorylated Bmp receptor Smads confirmed that Bmp signalling was inhibited by 23hpf and recovering by 26hpf (Fig S4).
Embryos fixed between 23hpf and 26hpf were assayed for runx1 expression (Fig 2b-i). At 23hpf, 20/22 GFP−embryos displayed runx1+cells in the DA, whereas 15/22 GFP+embryos possessed no DA staining. In both GFP+and GFP−embryos, runx1 staining was detected equivalently in other areas of the embryo such as the olfactory placode and the Rohon-Beard neurons, demonstrating that the loss of runx1 expression was DA specific (Fig S5). The runx1 reduction in the DA persisted to 25hpf in the GFP+embryos, but by 26hpf runx1 expression was recovering (Fig 2h-i). Thus, the loss of runx1 in the DA correlates with downregulation of Bmp signalling at 23-25hpf, and recovery of runx1 at 26hpf correlates with the turnover of tBR and the recovery of phosphorylated Smad activity (Fig S4).
The first clearly identifiable derivatives of the definitive lineage in zebrafish embryos are the rag1/2 and ikaros expressing thymocytes in the bilateral thymi at 4dpf (Kissa et al., 2008; Willett et al., 1997), and we could test for the presence of these cells since all embryos possessed normal circulation. Consistent with a loss of HSCs in the DA, rag1 expression in the same batch of embryos at 4dpf was reduced in GFP+embryos compared with GFP−embryos (Fig 2j-k). Furthermore, although analysis of additional HSC markers such as cmyb, ikaros or cd41 was not possible since these genes are expressed in the overlying primitive blood at the time of HSC emergence (Gering and Patient, 2005; Lin et al., 2005; Thompson et al., 1998), the expression of gfi1, which is restricted to the definitive lineage, behaved in an identical manner to runx1 following Bmp inhibition (Fig S6). Taken together, these results demonstrate that Bmp signalling is required for the initiation of the HSC programme in the DA and for normal levels of HSC descendants.
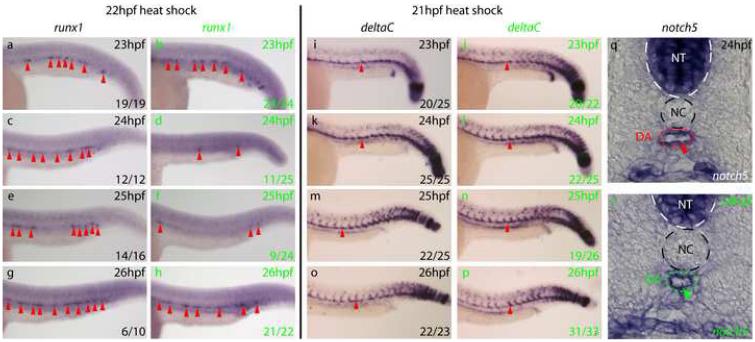
To determine if Bmp signalling is required for maintenance as well as initiation of the definitive haematopoietic programme, we performed a heat shock at 22hpf, and assayed for runx1 expression (Fig 3a-h). GFP+(24/24) and GFP−(19/19) embryos both possessed strong runx1 staining in the DA at 23hpf. However, at 24hpf, a strong downregulation of runx1 expression was observed, which persisted until 26hpf. These results demonstrate that Bmp signalling is required both for the initiation and maintenance of runx1 expression in the floor of the DA.
Figure 3. Bmp signalling is required for maintenance of runx1 expression in the ventral DA but not for maintenance of the arterial programme.
a-h) runx1 expression (arrows) in embryos heat shocked at 22hpf. runx1 initiated normally, was lost by 24hpf in GFP+embryos (green letters) compared to GFP−embryos (black letters) but recovered by 26hpf. Representative embryos are shown. Embryos containing a few runx1+ cells were scored as negative.. i-p) deltaC expression in the DA (arrows) in embryos heat shocked at 21hpf was unaffected by inhibition of Bmp signalling. q, r) notch5 expression in the ventral wall of the DA (arrow) in sectioned embryos heat shocked at 21hpf was normal.
To determine if elevated Bmp signalling could expand the domain of runx1 expression dorsally, we analysed the expression of runx1 and gfi1 in dino mutants, which possess a genetic lesion at the chordin locus (Fig S7) (Schulte-Merker et al., 1997). Surprisingly, the initiation and maintenance of both markers in the DA were unaffected in dino embryos and neither gene exhibited ectopic expression in the DA (Fig S7a-j, red arrows); however, both were ectopically expressed in the posterior ICM as previously demonstrated (Leung et al., 2005). Arterial expression of tbx20 was unaffected, while its expression within a ventral population of cells under the yolk cell extension was substantially increased in dino mutants in comparison to wild-type embryos (Fig S7k-l, yellow and orange arrows, respectively), as expected for Bmp-dependent expression (Fig S8a-h, red arrows and Pyati et al., 2006). Preliminary experiments with a heat shock inducible Bmp2b transgenic zebrafish line (Chocron et al., 2007), support this lack of HSC gene expression in the dorsal DA (data not shown). We therefore conclude that Bmp levels may already be optimal ventrally and that dorsal cells may be unable to respond.
The arterial programme is unaffected by loss of Bmp signalling
Since arterial specification and HSC emergence are intimately linked (Burns et al., 2005; Gering and Patient, 2005), the loss of runx1 in the DA led us to examine the arterial programme in Bmp-inhibited embryos. Arterial markers such as notch5 and deltaC are expressed throughout the DA roof and floor from 22hpf, shortly before runx1 expression (Patterson et al., 2005). We therefore induced tBR expression via a 21hpf heat shock and assayed arterial gene expression alongside runx1. We found no difference between GFP+and GFP−embryos at any stage, even at stages when runx1 expression was lost (Fig 3i-r and data not shown). Thus, the loss of runx1 expression was not due to a loss of endothelium in the DA floor, but a loss of expression within those cells. Analysis of tbx20 expression also revealed no differences between the GFP+and GFP−embryos in expression or polarisation of tbx20 transcripts (Fig S8). Taken together, these observations demonstrate that Bmp signalling within the trunk prior to the onset of HSC gene expression is required for initiation of definitive haematopoiesis in the ventral wall of the DA but not the maintenance of ventral arterial marker expression in those cells.
bmp4 is required for HSC emergence within the ventral DA
Since induction of tBR produced only transient inhibition of Bmp signalling (Fig S4), which led to recovery of HSC gene expression (Fig 2b-i) and consequently only a moderate downregulation of rag1 at 4dpf (Fig 2j-k), we wished to determine if a more complete loss of Bmp signalling could create a more profound effect on the HSC programme. Given the presence of bmp4 transcripts proximal to the DA floor during the initiation of definitive haematopoiesis in zebrafish (Fig 2a and Fig S3a-b), and also in other vertebrates, together with the absence of bmp2b and bmp7 transcripts in zebrafish (data not shown), we knocked down bmp4 expression by morpholino (mo) injection (Chocron et al., 2007). Embryos injected with bmp4 morpholino displayed inappropriate expression of gata1 at 10s in the most ventral posterior mesoderm (Fig S9a-b), as previously demonstrated in a bmp4 mutant (Stickney et al., 2007). At 26hpf, bmp4 morphants exhibited complete loss of runx1 expression within the DA (Fig S10a-b), however, neural runx1 expression remained unaffected, demonstrating that bmp4 is required for the initiation of runx1 expression in the ventral wall of the DA. Furthermore, at 28hpf, runx1 expression remained absent from the DA in two thirds of bmp4 morphants (Fig 4a-d, arrows), while cmyb expression was also substantially downregulated in the DA of bmp4 morphants (Fig 4e-h, red arrows). Interestingly, cmyb expression within the erythromyeloid progenitors (EMPs) in the posterior blood island was unaffected in bmp4 morphants (Fig 4g-h, green arrows), supporting previous studies which indicate that EMPs arise independently of HSCs (Bertrand et al., 2007). While HSC gene expression was reduced, the expression of arterial markers was unaffected in bmp4 morphants (Fig S9c-j), showing that the bmp4 signal acts specifically on the definitive haematopoietic programme and not the maintenance of arterial gene expression within the DA.
To confirm that the morpholino-induced loss of runx1 expression in the DA truly reflected a loss of HSCs, we assayed rag1+, ikaros+ thymocytes at 4dpf. bmp4 morphants contained substantially reduced rag1 and ikaros expression within the thymus, while expression of foxn1 in the thymic epithelium was unaffected, demonstrating that the thymi were present but contained substantially fewer thymocytes (Figs 4i-l and S10c-f, arrows). To confirm that HSCs and not just T-cells were affected, we analysed the expression of cmyb within the developing kidney at 5dpf and found that bmp4 morphants exhibited a substantial downregulation or total absence of cmyb in comparison to controls (Fig 4m-n, outlined). Taken together with the downregulation of rag1 observed in embryos expressing tBR (Fig 2k-l), these data indicate that bmp4 induces HSCs in the DA floor. Thus, overall, the data presented here show that dorsoventral patterning of the DA is a mirror image of the patterning of the neural tube, whereby Bmp specifies ventral rather than dorsal fate and Hh specifies dorsal rather than ventral cell fates (Fig 4o).
Discussion
The first HSCs in developing embryos are clearly located in the floor of the DA, however whether they arise from the wall of the DA, the underlying mesenchyme or the embryonic blood has been a matter for some considerable debate (Dzierzak and Speck, 2008). However, evidence supporting an endothelial precursor for the HSC continues to accumulate, most recently using VE-Cadherin to label the cells (Chen et al., 2009; Zovein et al., 2008). Furthermore, both cell lineage tracing and genetic disruption of signalling pathways support independent development of embryonic blood and HSCs, and the shared signalling dependence of the arterial and HSC programmes also support the DA wall as the source of the HSCs (Ciau-Uitz et al, 2000; Burns et al., 2005; Gering and Patient, 2005). We have now identified two signals that differentially affect these two programmes, with Hh maintaining the dorsal arterial programme and Bmp inducing the ventral HSC programme.
In chick embryos, at the time of HSC emergence, the DA contains two different mesodermal contributions, with the floor derived from splanchnopleural mesoderm and the roof from somites (Pouget et al., 2006). It is formally possible therefore that the observed polarisation of the DA, with HSCs only arising in the floor, reflects cell lineage rather than local signalling. However, thus far in zebrafish, evidence has only been produced for a contribution to the DA from lateral (splanchnopleural) mesoderm (Zhong et al., 2001). In addition, more recent evidence from the chick indicates that even the roof of the DA initially derives from splanchnopleural mesoderm (T. Jaffredo, pers. comm.; Pouget et al., 2006). Thus, even in the chick the splanchnopleural mesoderm derived DA is likely to be patterned before its replacement by somitic endothelial cells. However, the failure to induce ectopic HSC gene expression in dino embryos, suggests that Bmp signalling may be optimal around the ventral DA and that the dorsal wall of the DA may not be competent to receive Bmp signalling.
The precocious differentiation of the posterior PLM into gata1+ primitive blood induced by tbx20 knockdown can be mimicked by morpholino knockdown of bmp4 (Fig S9a-b) ,since tbx20 is under the control of Bmp signalling in these cells (Pyati et al., 2006). Conversely, in dino mutant embryos, which exhibit increased Bmp signalling, tbx20 expression is expanded in the posterior PLM and gata1 expression is reduced (data not shown). Thus, bmp4 controls the timing of primitive haematopoietic differentiation in the posterior PLM via tbx20. However, since Hh-dependent tbx20 expression in the DA does not antagonise definitive haematopoiesis, its presence there is likely to reflect its role in vascular lumenisation and inter-somitic vessel sprouting (Szeto et al., 2002). Furthermore, in contrast to its role in controlling the timing of primitive haematopoietic differentiation in the posterior PLM, bmp4 expression around the DA is required to promote definitive haematopoietic specification by initiating the expression of runx1 in the ventral wall of the DA.
Requirements for Notch and Hh signalling have been demonstrated for the expression of runx1 within the DA, albeit at different times (Burns et al., 2005; Gering and Patient, 2005). Whilst a direct role for Hh signalling remains to be demonstrated, Notch signalling has been shown to be required directly for HSC gene expression in the DA at 36hpf (Burns et al., 2005). However, since we and others have shown that HSC gene expression is observed within the DA much earlier than this (Gering and Patient, 2005; Kalev-Zylinska et al., 2002), it remains to be determined whether Notch signalling acts directly in the initiation of definitive haematopoiesis within the DA. Therefore, it is formally possible that, whilst bmp4 is responsible for initiation and early maintenance of the HSC programme, Notch signalling may be important in the later maintenance of the programme; however, parallel inputs of both pathways cannot be excluded.
The model proposed here for zebrafish seems likely to apply to other vertebrates since bmp4 is expressed ventral to the DA in human, mouse, chick and Xenopus embryos (A. Ciau-Uitz and RP, unpublished; Marshall et al., 2000; Pimanda et al., 2007; Suonpaa et al., 2005). Furthermore, Bmp receptor Smads have been shown to transactivate the runx1 promoter in a mouse myeloid cell line (Pimanda et al., 2007). Moreover, addition of Bmp4 to mouse AGM explants increases transplantable haematopoietic activity, although since in these experiments HSCs had already been formed, it was difficult to exclude an effect on expansion, as demonstrated for purified human haematopoietic progenitors (Bhardwaj et al., 2001; Durand et al., 2007). Thus, to the best of our knowledge our studies in developing zebrafish embryos represent the first demonstration that Bmp is required for HSC formation in vivo. Together with the observation that Hh is required for maintenance of the arterial programme in the dorsal wall of the DA, the Bmp-dependent emergence of HSCs ventrally in the DA indicates that patterning of the DA has parallels with that of the neural tube (Fig 4o).
Experimental Procedures
Zebrafish husbandry
Zebrafish (Danio rerio) embryos were obtained from a wild type strain and raised at 28.5°C (Westerfield, 1993). Hemizygous transgenic embryos expressing under heat shock control a dominant negative Bmp receptor fused to GFP Tg(hsp70l:dnBmpr-GFP) or Bmp2b Tg(hsp70l:bmp2b) were used (Chocron et al., 2007; Pyati et al., 2005).
Heat shock inductions
Batches of transgenic embryos were strictly staged as described (Kimmel et al., 1995) to ensure minimal developmental asynchrony and subjected to a 43°C heatshock for 30 minutes at 21hpf or 22hpf as described (Pyati et al., 2005).
Purmorphamine treatments
Purmorphamine (Chemistry Research Laboratory, South Parks Rd, Oxford, United Kingdom; stock solution in DMSO: 2.5mg/ml) was used in aquarium water at 20μM. Embryos were treated from before MBT and from the 15 somite stage until collection.
In situ hybridisation
Performed as described (Gering and Patient, 2005). RNA probes were labelled with digoxigenin (Roche) and detected using BM Purple (Roche).
Wax sectioning
Embryos were sectioned in paraffin wax (Walmsley et al., 2002).
Antibody staining of phosphorylated Smad1, 5, 8
Embryos were fixed in 4% PFA in phosphate buffered saline (PBS) at 4°C, washed in ethanol and endogenous peroxidase activity was blocked by incubation in 0.5% normal goat serum (NGS) (Sigma)/0.5% H2O2 for 30min at 25°C before blocking in 10% NGS in PBS Tween® (Sigma) for 2 hours at 25°C. Incubation with pSmad1/5/8 antibody (Cell Signalling Technology) (1:200) was overnight at 4°C. Incubation with secondary antibody (goat α rabbit – HRP 1:200) (Vectorlabs) was for 2 hours at 25°C. Staining was developed using 3, 3′-diaminobenzidine (DAB) (Vectorlabs SK4100) as per manufacturers instructions. Embryos were re-fixed at 4°C in 4% PFA.
bmp4 MO injections
0.5nl bmp4 morpholino (2ng/nl dissolved in distilled water) (Gene Tools) 5′-ggtgtttgattgtctgaccttcatg-3′ (Chocron et al., 2007).
Supplementary Material
Acknowledgements
We thank K. Crosier, M. Tada, J. Wittbrodt and L. Zon for reagents, F. Liu, R. Bahadori and S. Elworthy for experimental assistance and the reviewers for helpful comments. This work was supported by the Medical Research Council and the Leukaemia Research Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahn DG, Ruvinsky I, Oates AC, Silver LM, Ho RK. tbx20, a new vertebrate T-box gene expressed in the cranial motor neurons and developing cardiovascular structures in zebrafish. Mechanisms of development. 2000;95:253–258. doi: 10.1016/s0925-4773(00)00346-4. [DOI] [PubMed] [Google Scholar]
- Bertrand JY, Kim AD, Violette EP, Stachura DL, Cisson JL, Traver D. Definitive hematopoiesis initiates through a committed erythromyeloid progenitor in the zebrafish embryo. Development (Cambridge, England) 2007;134:4147–4156. doi: 10.1242/dev.012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhardwaj G, Murdoch B, Wu D, Baker DP, Williams KP, Chadwick K, Ling LE, Karanu FN, Bhatia M. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nature immunology. 2001;2:172–180. doi: 10.1038/84282. [DOI] [PubMed] [Google Scholar]
- Burns CE, Traver D, Mayhall E, Shepard JL, Zon LI. Hematopoietic stem cell fate is established by the Notch-Runx pathway. Genes & development. 2005;19:2331–2342. doi: 10.1101/gad.1337005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MJ, Yokomizo T, Zeigler BM, Dzierzak E, Speck NA. Runx1 is required for the endothelial to haematopoietic cell transition but not thereafter. Nature. 2009;457:887–891. doi: 10.1038/nature07619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin AJ, Chen J, Weinberg ES. Bone morphogenetic protein-4 expression characterizes inductive boundaries in organs of developing zebrafish. Development Genes and Evolution. 1997;207:107–114. doi: 10.1007/s004270050097. [DOI] [PubMed] [Google Scholar]
- Chocron S, Verhoeven MC, Rentzsch F, Hammerschmidt M, Bakkers J. Zebrafish Bmp4 regulates left-right asymmetry at two distinct developmental time points. Developmental biology. 2007;305:577–588. doi: 10.1016/j.ydbio.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Ciau-Uitz A, Walmsley M, Patient R. Distinct origins of adult and embryonic blood in Xenopus. Cell. 2000;102:787–96. doi: 10.1016/s0092-8674(00)00067-2. [DOI] [PubMed] [Google Scholar]
- Concordet JP, Lewis KE, Moore JW, Goodrich LV, Johnson RL, Scott MP, Ingham PW. Spatial regulation of a zebrafish patched homologue reflects the roles of sonic hedgehog and protein kinase A in neural tube and somite patterning. Development (Cambridge, England) 1996;122:2835–2846. doi: 10.1242/dev.122.9.2835. [DOI] [PubMed] [Google Scholar]
- Connors SA, Trout J, Ekker M, Mullins MC. The role of tolloid/mini fin in dorsoventral pattern formation of the zebrafish embryo. Development (Cambridge, England) 1999;126:3119–3130. doi: 10.1242/dev.126.14.3119. [DOI] [PubMed] [Google Scholar]
- Durand C, Robin C, Bollerot K, Baron MH, Ottersbach K, Dzierzak E. Embryonic stromal clones reveal developmental regulators of definitive hematopoietic stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:20838–20843. doi: 10.1073/pnas.0706923105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzierzak E, Speck NA. Of lineage and legacy: the development of mammalian hematopoietic stem cells. Nature immunology. 2008;9:129–136. doi: 10.1038/ni1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekker SC, Ungar AR, Greenstein P, von Kessler DP, Porter JA, Moon RT, Beachy PA. Patterning activities of vertebrate hedgehog proteins in the developing eye and brain. Curr Biol. 1995;5:944–955. doi: 10.1016/s0960-9822(95)00185-0. [DOI] [PubMed] [Google Scholar]
- Furthauer M, Thisse B, Thisse C. Three different noggin genes antagonize the activity of bone morphogenetic proteins in the zebrafish embryo. Developmental biology. 1999;214:181–196. doi: 10.1006/dbio.1999.9401. [DOI] [PubMed] [Google Scholar]
- Gering M, Patient R. Hedgehog signaling is required for adult blood stem cell formation in zebrafish embryos. Developmental cell. 2005;8:389–400. doi: 10.1016/j.devcel.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Kalev-Zylinska ML, Horsfield JA, Flores MV, Postlethwait JH, Vitas MR, Baas AM, Crosier PS, Crosier KE. Runx1 is required for zebrafish blood and vessel development and expression of a human RUNX1-CBF2T1 transgene advances a model for studies of leukemogenesis. Development (Cambridge, England) 2002;129:2015–2030. doi: 10.1242/dev.129.8.2015. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kissa K, Murayama E, Zapata A, Cortes A, Perret E, Machu C, Herbomel P. Live imaging of emerging hematopoietic stem cells and early thymus colonization. Blood. 2008;111:1147–1156. doi: 10.1182/blood-2007-07-099499. [DOI] [PubMed] [Google Scholar]
- Koudijs MJ, den Broeder MJ, Groot E, van Eeden FJ. Genetic analysis of the two zebrafish patched homologues identifies novel roles for the hedgehog signaling pathway. BMC developmental biology. 2008;8:15. doi: 10.1186/1471-213X-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koudijs MJ, den Broeder MJ, Keijser A, Wienholds E, Houwing S, van Rooijen EM, Geisler R, van Eeden FJ. The zebrafish mutants dre, uki, and lep encode negative regulators of the hedgehog signaling pathway. PLoS genetics. 2005;1:e19. doi: 10.1371/journal.pgen.0010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S, Concordet JP, Ingham PW. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993;75:1431–1444. doi: 10.1016/0092-8674(93)90628-4. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Developmental cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- Leung AY, Mendenhall EM, Kwan TT, Liang R, Eckfeldt C, Chen E, Hammerschmidt M, Grindley S, Ekker SC, Verfaillie CM. Characterization of expanded intermediate cell mass in zebrafish chordin morphant embryos. Developmental biology. 2005;277:235–254. doi: 10.1016/j.ydbio.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Lewis KE, Concordet JP, Ingham PW. Characterisation of a second patched gene in the zebrafish Danio rerio and the differential response of patched genes to Hedgehog signalling. Developmental biology. 1999;208:14–29. doi: 10.1006/dbio.1998.9169. [DOI] [PubMed] [Google Scholar]
- Lin HF, Traver D, Zhu H, Dooley K, Paw BH, Zon LI, Handin RI. Analysis of thrombocyte development in CD41-GFP transgenic zebrafish. Blood. 2005;106:3803–3810. doi: 10.1182/blood-2005-01-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CJ, Kinnon C, Thrasher AJ. Polarized expression of bone morphogenetic protein-4 in the human aorta-gonad-mesonephros region. Blood. 2000;96:1591–1593. [PubMed] [Google Scholar]
- McReynolds LJ, Gupta S, Figueroa ME, Mullins MC, Evans T. Smad1 and Smad5 differentially regulate embryonic hematopoiesis. Blood. 2007 doi: 10.1182/blood-2007-04-085753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro R, van Dinther M, Bakkers J, Wilkinson R, Patient R, ten Dijke P, Mummery C. Two novel type II receptors mediate BMP signalling and are required to establish left-right asymmetry in zebrafish. Developmental biology. 2008;315:55–71. doi: 10.1016/j.ydbio.2007.11.038. [DOI] [PubMed] [Google Scholar]
- Murayama E, Kissa K, Zapata A, Mordelet E, Briolat V, Lin HF, Handin RI, Herbomel P. Tracing hematopoietic precursor migration to successive hematopoietic organs during zebrafish development. Immunity. 2006;25:963–975. doi: 10.1016/j.immuni.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Patterson LJ, Gering M, Patient R. Scl is required for dorsal aorta as well as blood formation in zebrafish embryos. Blood. 2005;105:3502–3511. doi: 10.1182/blood-2004-09-3547. [DOI] [PubMed] [Google Scholar]
- Pimanda JE, Donaldson IJ, de Bruijn MF, Kinston S, Knezevic K, Huckle L, Piltz S, Landry JR, Green AR, Tannahill D, et al. The SCL transcriptional network and BMP signaling pathway interact to regulate RUNX1 activity. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:840–845. doi: 10.1073/pnas.0607196104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouget C, Gautier R, Teillet MA, Jaffredo T. Somite-derived cells replace ventral aortic hemangioblasts and provide aortic smooth muscle cells of the trunk. Development (Cambridge, England) 2006;133:1013–1022. doi: 10.1242/dev.02269. [DOI] [PubMed] [Google Scholar]
- Pyati UJ, Cooper MS, Davidson AJ, Nechiporuk A, Kimelman D. Sustained Bmp signaling is essential for cloaca development in zebrafish. Development (Cambridge, England) 2006;133:2275–2284. doi: 10.1242/dev.02388. [DOI] [PubMed] [Google Scholar]
- Pyati UJ, Webb AE, Kimelman D. Transgenic zebrafish reveal stage-specific roles for Bmp signaling in ventral and posterior mesoderm development. Development (Cambridge, England) 2005;132:2333–2343. doi: 10.1242/dev.01806. [DOI] [PubMed] [Google Scholar]
- Roy S, Qiao T, Wolff C, Ingham PW. Hedgehog signaling pathway is essential for pancreas specification in the zebrafish embryo. Curr Biol. 2001;11:1358–1363. doi: 10.1016/s0960-9822(01)00402-x. [DOI] [PubMed] [Google Scholar]
- Schulte-Merker S, Lee KJ, McMahon AP, Hammerschmidt M. The zebrafish organizer requires chordino. Nature. 1997;387:862–863. doi: 10.1038/43092. [DOI] [PubMed] [Google Scholar]
- Sinha S, Chen JK. Purmorphamine activates the Hedgehog pathway by targeting Smoothened. Nature chemical biology. 2006;2:29–30. doi: 10.1038/nchembio753. [DOI] [PubMed] [Google Scholar]
- Stickney HL, Imai Y, Draper B, Moens C, Talbot WS. Zebrafish bmp4 functions during late gastrulation to specify ventroposterior cell fates. Developmental biology. 2007;310:71–84. doi: 10.1016/j.ydbio.2007.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suonpaa P, Kohonen P, Koskela K, Koskiniemi H, Salminen-Mankonen H, Lassila O. Development of early PCLP1-expressing haematopoietic cells within the avian dorsal aorta. Scandinavian journal of immunology. 2005;62:218–223. doi: 10.1111/j.1365-3083.2005.01655.x. [DOI] [PubMed] [Google Scholar]
- Szeto DP, Griffin KJ, Kimelman D. HrT is required for cardiovascular development in zebrafish. Development (Cambridge, England) 2002;129:5093–5101. doi: 10.1242/dev.129.21.5093. [DOI] [PubMed] [Google Scholar]
- Thompson MA, Ransom DG, Pratt SJ, MacLennan H, Kieran MW, Detrich HW, 3rd, Vail B, Huber TL, Paw B, Brownlie AJ, et al. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Developmental biology. 1998;197:248–269. doi: 10.1006/dbio.1998.8887. [DOI] [PubMed] [Google Scholar]
- Walmsley M, Ciau-Uitz A, Patient R. Adult and embryonic blood and endothelium derive from distinct precursor populations which are differentially programmed by BMP in Xenopus. Development (Cambridge, England) 2002;129:5683–5695. doi: 10.1242/dev.00169. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book. A guide for the laboratory use of zebrafish (Danio rerio) Univ. of Oregon Press; Eugene: 1993. [Google Scholar]
- Willett CE, Cherry JJ, Steiner LA. Characterization and expression of the recombination activating genes (rag1 and rag2) of zebrafish. Immunogenetics. 1997;45:394–404. doi: 10.1007/s002510050221. [DOI] [PubMed] [Google Scholar]
- Willett CE, Kawasaki H, Amemiya CT, Lin S, Steiner LA. Ikaros expression as a marker for lymphoid progenitors during zebrafish development. Dev Dyn. 2001;222:694–698. doi: 10.1002/dvdy.1223. [DOI] [PubMed] [Google Scholar]
- Yan YL, Hatta K, Riggleman B, Postlethwait JH. Expression of a type II collagen gene in the zebrafish embryonic axis. Dev Dyn. 1995;203:363–376. doi: 10.1002/aja.1002030308. [DOI] [PubMed] [Google Scholar]
- Zhang XY, Rodaway AR. SCL-GFP transgenic zebrafish: in vivo imaging of blood and endothelial development and identification of the initial site of definitive hematopoiesis. Developmental biology. 2007;307:179–194. doi: 10.1016/j.ydbio.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Zhong TP, Childs S, Leu JP, Fishman MC. Gridlock signalling pathway fashions the first embryonic artery. Nature. 2001;414:216–220. doi: 10.1038/35102599. [DOI] [PubMed] [Google Scholar]
- Zovein AC, Hofmann JJ, Lynch M, French WJ, Turlo KA, Yang Y, Becker MS, Zanetta L, Dejana E, Gasson JC, et al. Fate tracing reveals the endothelial origin of hematopoietic stem cells. Cell stem cell. 2008;3:625–636. doi: 10.1016/j.stem.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.