Abstract
Divergent selection through biotic factors like predation or parasitism can promote reproductive isolation even in the absence of geographical barriers. On the other hand, evidence for a role of adaptation to abiotic factors during ecological speciation in animals is scant. In particular, the role played by perpetual darkness in establishing reproductive isolation in cave animals (troglobites) remains elusive. We focused on two reproductively isolated ecotypes (surface- and cave-dwelling) of the widespread livebearer Poecilia mexicana, and raised offspring of wild-caught females to sexual maturity in a 12-month common-garden experiment. Fish were reared in light or darkness combined with high- or low-food conditions. Females, but not males, of the surface ecotype suffered from almost complete reproductive failure in darkness, especially in the low-food treatment. Furthermore, surface fish suffered from a significantly higher rate of spontaneous, stress-related infection with bacterial columnaris disease. This experimental evidence for strong selection by permanent darkness on non-adapted surface-dwelling animals adds depth to our understanding of the selective forces establishing and maintaining reproductive isolation in cave faunas.
Keywords: cave fauna, ecological speciation, life-history evolution, local adaptation, Poecilia mexicana
1. Introduction
A pivotal question in speciation research is how reproductive isolation between diverging populations is achieved and maintained, and theory distinguishes between pre-zygotic and post-zygotic isolation mechanisms [1]. During ecological speciation, reproductive isolation results from ecologically based divergent selection, and pre-zygotic isolation may arise as a byproduct of local adaptation if immigrants from ecologically divergent habitats are selected against [2]. This can be owing to natural selection, if immigrants show reduced viability [3,4], or sexual selection, if poorly adapted individuals have a disadvantage in mate competition [4,5].
While there is ample evidence that divergent selection through biotic factors like predation [3,6] and parasitism [7] can promote pre-zygotic isolation, the consequences of adaptation to abiotic habitat parameters have received surprisingly little attention in studies on animal speciation. Drastically different abiotic selection regimes occur, e.g. during the transition of diurnal, surface-dwelling organisms to permanently dark subterranean habitats. Cave organisms typically evolve enhanced non-visual sensory capacities to compensate for the lack of vision [8], which helps them find food in darkness [9].
However, the role of permanent darkness as a selective force establishing reproductive isolation is often overlooked, especially in systems where no geological barriers restrict migration between the surface and subterranean realm. In Tabasco, southern Mexico, Poecilia mexicana (Poeciliidae) are currently undergoing ecological speciation in a variety of adjacent benign and extreme (highly sulphidic and/or cave) habitats (see the electronic supplementary material). Despite the absence of physical barriers, gene flow between adjoining divergent habitats is low and there is no evidence for gene flow from neighbouring surface habitats into caves [4,10]. Predation [6], sexual selection and hydrogen sulphide-induced toxicity [4] act particularly strongly on males and are known to contribute to reproductive isolation in this system, but these forces do not fully explain the absence of immigration from surrounding surface habitats into either of the two caves (see the electronic supplementary material). Could darkness itself play a role as an isolating mechanism in adjoining surface/cave systems? Even though this question seems straightforward, virtually no study to date has tested it empirically.
We addressed this question by raising offspring of wild-caught individuals from the Cueva del Azufre [11], and two neighbouring surface populations to sexual maturity in a 12-month common-garden setting. Half of the fish were exposed to light, the other half to permanent darkness, and within each group, fish were reared either on a high food diet, or on a restrictive food regime. Based on the gene flow patterns discovered in nature [4,10], we expected to find decreased fitness of surface fish in darkness but not vice versa.
2. Material and methods
(a). Reproduction in darkness
Pregnant surface females were collected in January 2009 from Arroyo Bonita (AB) and Río Amatan (RA [10]); cave mollies stemmed from chamber V of the Cueva del Azufre (PSV [11]). At the University of Oklahoma, offspring of these field-collected fish were raised to reproductive maturity under established common-garden protocols (see the electronic supplementary material) in one of four treatments: treatments 1 and 2 involved a 12 L:12 D cycle coupled with low (treatment 1) or high food availability (treatment 2). In treatments 3 and 4, fish were raised in perpetual darkness, once again under low (treatment 3) or high food (treatment 4). Complete metamorphosis in males and successful parturition in females were scored as ‘successful reproduction’ (see the electronic supplementary material).
We used a stepwise backwards binary logistic regression based on likelihood ratios to analyse the frequency of individuals that successfully reproduced within the first year of life implemented in PASW Statistics 18.0.3 (IBM Corporation). In this model ecotype (cave versus surface), light treatment (light : dark versus darkness), food treatment (high versus low food) and their interactions were included as independent variables.
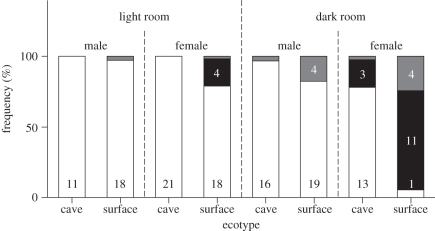
Some fish (n = 10) died within the first couple of weeks of having been introduced into their respective treatment. At the point of death, they were too young to reliably determine their sex. For visual representation, we scored half of these fish as males and half as females (grey in figure 1), but removed them from the dataset for the logistic regression.
Figure 1.
Percentage of surface- and cave-dwelling P. mexicana (n = 145) that successfully reproduced (white), failed to reproduce in their first year of life (black), or died as juveniles (grey). Half of the juveniles were scored as males, half as females for the purpose of this graph; thus, values in the second, fourth, fifth and seventh bar from the left represent ‘0.5 individuals’.
(b). Columnaris disease
One outcome that we did not predict a priori was the high incidence of spontaneous infection with fatal columnaris disease. Eleven of our test fish contracted columnaris disease, and the majority of these (81.1%) died within a few days after its discovery. This disease is caused by the ubiquitous Gram-negative bacterium Flavobacterium columnare, and is usually associated with stress, caused by poor environmental conditions [12]. Since we detected this disease among juveniles, males and females, we decided a posteriori to analyse the occurrence of columnaris disease in our experiment using the full dataset (n = 145) by conducting a similar logistic regression model as described above; this time, the dependent variable was ‘infection with columnaris (yes/no)’.
3. Results
(a). Rate of successful reproduction
All individuals that began male metamorphosis successfully completed maturation (n = 64). Since that means that there was no measurable variation in males (figure 1), we excluded all males from our planned analysis. When analysing successful female reproduction (n = 71), all covariates and most interactions were not significant and thus removed from the final model during the stepwise backwards approach (all p ≥ 0.75). Only the interaction of ‘ecotype by light treatment’ was retained as statistically significant (−2 log likelihood = 24.44, Nagelkerke r2 = 0.60; Wald = 8.22, d.f. = 1, p = 0.004). Permanent darkness had a much stronger negative effect on surface fish than on cave fish (figure 1).
(b). Stress-induced pathology: columnaris disease
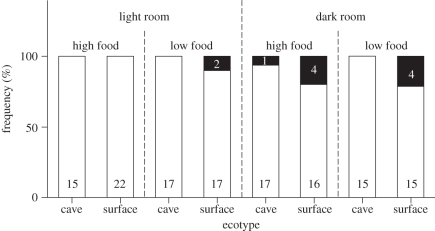
Light treatment (Wald = 4.33, d.f. = 1, p = 0.038) and the interaction of ‘ecotype by food treatment’ (Wald = 4.71, d.f. = 1, p = 0.030) significantly influenced the occurrence of columnaris (−2 log likelihood = 67.69, Nagelkerke r2 = 0.16), while all other covariates and interactions were removed during the stepwise backward approach (p ≥ 0.12). More fish contracted columnaris in permanent darkness (figure 2); however, the only cave molly infected with columnaris was raised in high food in the dark, while surface mollies in general were more likely to contract columnaris, especially under low-food conditions (figure 2).
Figure 2.
Percentage of surface- and cave-dwelling P. mexicana (n = 145) that remained healthy (white) or contracted columnaris disease (black).
4. Discussion
Females usually invest more in gametes than males, and female investment is even higher in viviparous animals owing to gestation [13,14], so males should require less energy for somatic maintenance and reproduction than females. Poecilia mexicana females in the wild spend considerably more time feeding than males [15], and males are usually lighter than same-sized females [16,17]. Hence, one would expect the impact of darkness to be aggravated in females, and indeed, a sex-specific effect was found in our present study: darkness hampered the maturation of surface females but not males. While it is possible that some surface molly females would have become reproductively active after the termination of the experiment, permanent darkness, nonetheless, has the characteristics of a chronic stressor that apparently forces organisms to divert resources from somatic maintenance, growth and reproduction to (re-)establish homeostasis [18]. If darkness is coupled with low levels of resource availability—as is likely the case for most caves [8]—these effects will be aggravated, as evidenced by the fact that even some cave mollies failed to reproduce in permanent darkness in the low-food treatment. Also, several surface fish that were raised under low-food conditions or in darkness contracted columnaris disease, a typical stress response in fish [18].
Previous research has shown that low rates of gene flow exist in the Cueva del Azufre system but are unidirectional from the inside of the caves towards the outside [4,10] (see also the electronic supplementary material). Our experimental data are congruent with this pattern, as darkness selected against female immigrants from the surface, while we found no evidence for selection through light acting against migrants from cave to surface habitats.
Translocation experiments in the field have demonstrated a major role of the second powerful selection factor in this system (toxic H2S) affecting the short-term survival of immigrant fish, while mechanisms shaping genetic differentiation along the light/dark ecological gradient remained elusive [4]. In particular, what selective forces besides predation [6] could explain the lack of gene flow between sulphidic surface habitats and the sulphidic cave (Cueva del Azufre), or the non-sulphidic surface habitats and the non-sulphidic cave (Cueva Luna Azufre)? Our present study provides evidence that darkness also acts as a strong selective force effectively preventing gene flow between diverging populations—albeit over a longer time frame—and thus, has the potential to establish reproductive isolation between diverging populations.
As of yet, we do not have any direct data on the underlying mechanisms behind our results. Surface mollies in darkness appeared to be able to consume their daily rations (based on left-over food particles and growth rates; R. Riesch 2010, unpublished data). Nonetheless, it seems possible that cave mollies, with their improved sensory capacities (i.e. improved mechanosensory lateral line system) [19], spent less time swimming in search of food [9], leading to reduced daily energy expenditure.
The cave molly is an example of a visually oriented species that has successfully colonized a cave and achieved reproductive isolation despite the close connection to surface ecosystems harbouring surface-dwelling conspecifics. Obligate cave fauna usually represents a non-random sub-sample of the species communities occurring in the surrounding surface habitats, with species that rely predominantly on visual orientation being under-represented [8]. Based on surveys of existing cave fauna, this has traditionally been ascribed not only to the inability of visually oriented species to find and explore food sources in permanent darkness, but also to the inability to successfully complete their life cycle and reproduce in the absence of light [8]. To our knowledge, our data provide the first experimental evidence for the strong negative selection by permanent darkness on visually oriented surface fish and therefore might help explain why most cave-adapted species have non-visually oriented ancestors.
Acknowledgements
The experiments comply with the current laws on animal experimentation of the United States of America (AUS-IACUC approved protocol: R06-026).
We would like to thank T. Colston and F. J. Gracía de León for help in the field, and J. Curtis and A. Makowicz for help during the experiment. D. N. Reznick graciously provided the common-garden protocols upon which this experiment is based. Funding came from the National Science Foundation of America (DEB-0743406).
References
- 1.Coyne J. A., Orr H. A. 2004. Speciation. Sunderland, MA: Sinauer Associates [Google Scholar]
- 2.Rundle H. D., Nosil P. 2005. Ecological speciation. Ecol. Lett. 8, 336–352 10.1111/j.1461-0248.2004.00715.x (doi:10.1111/j.1461-0248.2004.00715.x) [DOI] [Google Scholar]
- 3.Nosil P. 2004. Reproductive isolation caused by visual predation on migrants between divergent environments. Proc. R. Soc. Lond. B 271, 1521–1528 10.1098/rspb.2004.2751 (doi:10.1098/rspb.2004.2751) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tobler M., Riesch R., Tobler C. M., Schulz-Mirbach T., Plath M. 2009. Natural and sexual selection against immigrants maintains differentiation among micro-allopatric populations. J. Evol. Biol. 22, 2298–2304 10.1111/j.1420-9101.2009.01844.x (doi:10.1111/j.1420-9101.2009.01844.x) [DOI] [PubMed] [Google Scholar]
- 5.Snowberg L. K., Benkman C. W. 2009. Mate choice based on a key ecological performance trait. J. Evol. Biol. 22, 762–769 10.1111/j.1420-9101.2009.01699.x (doi:10.1111/j.1420-9101.2009.01699.x) [DOI] [PubMed] [Google Scholar]
- 6.Tobler M. 2009. Does a predatory insect contribute to the divergence between cave- and surface-adapted fish populations? Biol. Lett. 5, 506–509 10.1098/rsbl.2009.0272 (doi:10.1098/rsbl.2009.0272) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacColl A. D. C., Chapman S. M. 2010. Parasites can cause selection against migrants following dispersal between environments. Funct. Ecol. 24, 847–856 10.1111/j.1365-2435.2010.01691.x (doi:10.1111/j.1365-2435.2010.01691.x) [DOI] [Google Scholar]
- 8.Culver D. C., Pipan T. 2009. The biology of caves and other subterranean habitats. New York, NY: Oxford University Press [Google Scholar]
- 9.Yoshizawa M., Gorički Š., Soares D., Jeffery W. R. 2010. Evolution of a behavioral shift mediated by superficial neuromasts helps cavefish find food in darkness. Curr. Biol. 20, 1631–1636 10.1016/j.cub.2010.07.017 (doi:10.1016/j.cub.2010.07.017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Plath M., Hermann B., Schröder C., Riesch R., Tobler M., García de León F. J., Schlupp I., Tiedemann R. 2010. Locally adapted fish populations maintain small-scale genetic differentiation despite perturbation by a catastrophic flood event. BMC Evol. Biol. 10, 256. 10.1186/1471-2148-10-256 (doi:10.1186/1471-2148-10-256) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon M. S., Rosen D. E. 1962. A cavernicolous form of the poeciliid fish Poecilia sphenops from Tabasco, México. Copeia 1962, 360–368 10.2307/1440903 (doi:10.2307/1440903) [DOI] [Google Scholar]
- 12.Riesch R., Plath M., Schlupp I. 2010. Toxic hydrogen sulfide and dark caves: life-history adaptations in a livebearing fish (Poecilia mexicana, Poeciliidae). Ecology 91, 1494–1505 10.1890/09-1008.1 (doi:10.1890/09-1008.1) [DOI] [PubMed] [Google Scholar]
- 13.Dumpala P. R., Gülsoy N., Lawrence M. L., Karsi A. 2010. Proteomic analysis of the fish pathogen Flavobacterium columnare. Proteome Sci. 8, 26. 10.1186/1477-5956-8-26 (doi:10.1186/1477-5956-8-26) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lande R. 1980. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34, 292–305 10.2307/2407393 (doi:10.2307/2407393) [DOI] [PubMed] [Google Scholar]
- 15.Charnov E. L. 1982. The theory of sex allocation. Princeton, NJ: Princeton University Press [Google Scholar]
- 16.Köhler A., Hildenbrand P., Schleucher E., Riesch R., Arias–Rodriguez L., Streit B., Plath M. In press Effects of male sexual harassment on female time budgets, feeding behavior, and metabolic rates in a tropical livebearing fish (Poecilia mexicana). Behav. Ecol. Sociobiol. (doi:10.1007/s00265-011-1161-y) [Google Scholar]
- 17.Riesch R., Plath M., Schlupp I. 2011. Toxic hydrogen sulphide and dark caves: pronounced male life-history divergence among locally adapted Poecilia mexicana (Poeciliidae). J. Evol. Biol. 24, 596–606 10.1111/j.1420-9101.2010.02194.x (doi:10.1111/j.1420-9101.2010.02194.x) [DOI] [PubMed] [Google Scholar]
- 18.Wendelaar Bonga S. E. 1997. The stress response in fish. Physiol. Rev. 77, 591–625 [DOI] [PubMed] [Google Scholar]
- 19.Parzefall J. 2001. A review of morphological and behavioural changes in the cave molly, Poecilia mexicana, from Tabasco, Mexico. Environ. Biol. Fish. 62, 263–275 10.1023/A:1011899817764 (doi:10.1023/A:1011899817764) [DOI] [Google Scholar]