Abstract
Ocean acidification is predicted to affect marine ecosystems in many ways, including modification of fish behaviour. Previous studies have identified effects of CO2-enriched conditions on the sensory behaviour of fishes, including the loss of natural responses to odours resulting in ecologically deleterious decisions. Many fishes also rely on hearing for orientation, habitat selection, predator avoidance and communication. We used an auditory choice chamber to study the influence of CO2-enriched conditions on directional responses of juvenile clownfish (Amphiprion percula) to daytime reef noise. Rearing and test conditions were based on Intergovernmental Panel on Climate Change predictions for the twenty-first century: current-day ambient, 600, 700 and 900 µatm pCO2. Juveniles from ambient CO2-conditions significantly avoided the reef noise, as expected, but this behaviour was absent in juveniles from CO2-enriched conditions. This study provides, to our knowledge, the first evidence that ocean acidification affects the auditory response of fishes, with potentially detrimental impacts on early survival.
Keywords: ocean acidification, auditory response, sensory behaviour, clownfish, reef noise
1. Introduction
Since the Industrial Revolution, approximately 142 billion tonnes of anthropogenic CO2 has been absorbed by the oceans, resulting in ocean acidification at a rate far faster than any time in the last 650 000 years [1], and causing the average pH of the ocean to drop by 0.1 units [2]. If global emissions continue on the current trajectory, atmospheric CO2, currently at 390 ppm, is predicted to reach 730–1020 ppm by 2100 [2,3], causing a further drop in ocean pH of 0.3–0.4 units [1,2]. Acidified conditions compromise the ability of marine calcifiers to build skeletons and shells [4], but the combined influences on non-calcifiers including fishes are far less understood [5]. Determining the effects of acidification on crucial behavioural responses in marine species is essential for predicting the ecosystem and societal effects of ocean acidification in the twenty-first century.
Following a planktonic larval phase in the open ocean, juvenile coral reef fishes use a suite of sensory capabilities to detect, orient towards, and discriminate between potential settlement sites [6]. Olfactory cues provide valuable information for settling fishes, but recent work has demonstrated that fishes reared in CO2-enriched conditions lose their ability to distinguish odours, preferring the odours of inappropriate habitats and failing to avoid predator cues [7]. Auditory cues from coral reefs, dominated by the clicks and chirps of resident crustaceans and fishes, are also important in guiding directional behaviour [8] and habitat preferences [9], and nocturnal reef sounds promote settlement [10] while daytime predator-rich noise is avoided [11,12]. Central to fish hearing is the inner ear, which includes dense carbonate otoliths (earbones). Otolith growth can be altered by elevated-CO2 conditions during development [13], suggesting that acidification could also affect the auditory response. A key question that emerges from recent work is whether elevated-CO2 conditions deleteriously affect the auditory response of reef fishes in their early life-history stages, confounding the effects of ocean acidification on other sensory modalities and further eroding orientation, habitat selection and predator avoidance behaviour.
Here, we used information from existing electrophysiological assessment of hearing in juvenile clownfish to set audible and naturally relevant levels of sound for playback in an auditory choice chamber. We studied the influence of CO2-enriched rearing and test conditions on the directional response of recently settled juvenile Amphiprion percula (orange clownfish, 17–20 days post-hatching) to a daytime recording of reef noise that was avoided by larvae in a previous experiment [11]. The CO2-conditions of our rearing and test environments were current-day ambient (∼390 µatm), and elevated-CO2 treatments (approx. 600, 700 and 900 µatm), consistent with the range of Intergovernmental Panel on Climate Change predictions for CO2 concentrations at the end of the twenty-first century [2].
2. Material and methods
(a). CO2-rearing environments
Amphiprion percula (orange clownfish) were reared using established techniques [7], and behavioural experiments were conducted in accordance with animal ethics guidelines at James Cook University. Larvae from a single clutch of eggs, thus controlling for genetic and parental condition effects, were divided on hatching into a series of identical rearing tanks with one of four different CO2 treatments ([2,3]: details in the electronic supplementary material).
(b). Choice chamber design and testing protocol
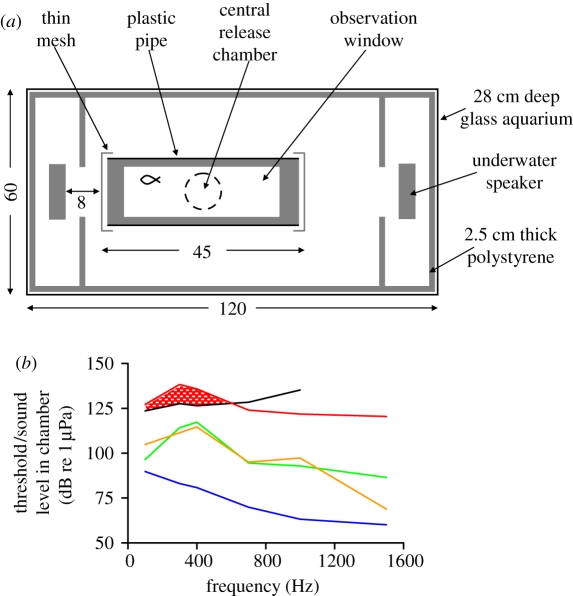
The auditory choice chamber consisted of an aquarium lined with polystyrene foam, with an underwater speaker (Electrovoice UW-30, Lubell Laboratories Inc., Columbus, OH, USA) at each end directed towards a tubular chamber with an observation window and acoustically transparent ends (figure 1a). Recently settled juvenile A. percula were tested for their response to a daytime recording of a high-quality reef in a marine-protected area (details of recording in the electronic supplementary material). The intensity for the sound playback was determined using the only available data on juvenile clownfish hearing (electrophysiological assessment of juvenile Amphiprion ephippium, details in the electronic supplementary material; hearing thresholds vary little between clownfish species [14]). According to the gradient of sound in the chamber, playback in the half of the chamber near to the speaker would be audible, whereas at the far end it would be below hearing threshold (figure 1b).
Figure 1.
Experimental arena and acoustic conditions during trials. (a) Acoustic choice chamber (measurements in centimetres). (b) Comparison of audiogram for Amphiprion ephippium (17–20 days post-hatching) with measurements of reef noise playback within the chamber. Shaded area indicates predicted audible component of sound near to the speaker. Mean sound levels within the chamber without playback are also shown; maximum s.e. between CO2-conditions was 1.2 dB (mean 0.6 dB) so error bars are not shown (black, 17–20 days post-hatching audiogram; red, near end of chamber; green, centre of chamber; yellow, far end of chamber; blue, no sound control).
At the start of each trial, a fish was transferred with a scoop to an acoustically transparent holding tube in the centre of the chamber. After 1 min acclimatization, sound was played for 1 min while the fish remained in the central holding tube, after which it was released and its position was recorded every 5 s for 2 min. Fish from each treatment group were tested in the same pH conditions in which they had been reared. Between trials, the chamber was flushed and rotated to prevent olfactory gradients, and half way through each series of trials the speaker providing the playback was switched to the opposite end of the tank. The potential for extraneous cues to bias directional swimming behaviour of larvae was tested using the experimental fish more than 24 h prior to the acoustic playback experiments, using the same protocol but without playback.
(c). Statistical analysis
Arcsine square-root transformations (appropriate for proportional data) were used to ensure normalized datasets in all eight conditions (sound/silent × 4 CO2 treatments; Kolmogorov–Smirnov Z tests; p > 0.28 in all cases), and only time spent by each fish in the half of the chamber near the active or focal speaker was tested. One-sample t-tests compared the time spent by each treatment group near to the active (or, in no-playback controls, the designated) speaker with H0 = 0.5. The effect of CO2 treatment on the response of fish between treatment groups was tested using ANOVA and Tukey HSD tests.
3. Results
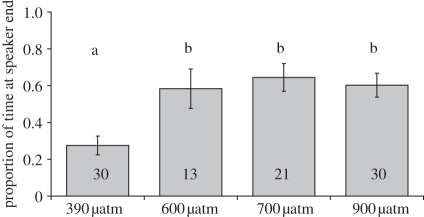
Recently settled juvenile A. percula reared and tested in ambient CO2-conditions (∼390 µatm) significantly avoided the predator-rich daytime recording of a reef during playback (one-sample t-test, arcsine square-root transformed data, H0 = 0.5, t29 = −4.6, p < 0.001), spending, on average, 73 per cent of the time in the half of the chamber away from the speaker (figure 2). By contrast, no avoidance behaviour was detected in larvae reared and tested in CO2-enriched conditions (600 µatm: t12 = 0.8, p = 0.43; 700 µatm: t20 = 1.8, p = 0.08; 900 µatm: t29 = 1.7, p = 0.10), with groups spending between 58 and 64 per cent of the time near to the speaker. A significant effect of CO2 treatment between the groups was detected, with the response in all of the elevated CO2 groups being significantly different to the ambient group (ANOVA: F3,90 = 7.3, p < 0.001; Tukey LSD test, p < 0.01: ambient ≠ (600, 700, 900 µatm)). No deviation from random movement was observed in the trials without playback sound: the mean proportion of time spent by each group towards the speaker ranged from 0.49 to 0.57 (one-sample t-tests, arcsine square-root transformed data, H0 = 0.5, n = 13–30, p > 0.37 throughout).
Figure 2.
Effects of elevated-CO2 conditions on the directional response of recently settled juvenile clownfish (17–20 days post-hatching) to acoustic playback of daytime reef noise (r.m.s. intensity = 153 dB re 1 µPa at the speaker). Sample sizes, which varied owing to mortality during rearing, are given on bars, which indicate mean ± s.e.m.; significantly different responses are indicated by letters.
4. Discussion
We found a change in the directional response of recently settled juvenile A. percula to a predator-rich daytime reef recording, if reared in CO2-conditions predicted for the shallow ocean later this century. Daytime reef noise is usually avoided by free-swimming early-stage reef fishes [11,12] and, as expected, clownfish reared in ambient CO2-conditions avoided our playback, but this response was absent in larvae reared at 600 µatm CO2 or higher. On the current CO2 emissions trajectory, the average concentration of CO2 in the atmosphere and surface waters of the ocean is predicted to exceed 500 µatm by mid-century and could approach 1000 µatm by 2100 [2,3], suggesting that ocean acidification could compromise auditory behaviours crucial for survival.
Previous studies have demonstrated that A. percula reared in CO2-enriched conditions lose their innate avoidance response to olfactory predator cues, becoming attracted to the scent of predators and unable to distinguish predators from non-predators in olfactory y-mazes [15]. Our study demonstrates that the effects of CO2-enrichment on sensory behaviour are not limited to sensory modalities with external sensory receptors such as olfaction, but that a behavioural response from a sensory system with internal receptors is also affected, suggesting that responses to auditory stimuli are unlikely to compensate for the documented loss of olfactory capability.
Understanding the mechanisms by which sensory and behavioural responses to environmental cues become modified by exposure to CO2-enriched conditions is essential for predicting the detrimental impact of ocean acidification on fish behaviour. A previous study found increased growth of otoliths (earbones) in fishes reared in highly CO2-enriched concentrations [13], whereas at the less severe levels of CO2-enrichment used in this study, we did not see modification of otolith growth that could be attributed to the CO2 treatment (analysis of otolith morphology in the electronic supplementary material). This is consistent with another study, which found no effects on clownfish otoliths up to approximately 1050 µatm CO2 [16]. Likewise, no obvious modification was detected in the morphology of the nasal cavity of larval clownfish in an olfaction study [7]. Combined, these results point to disruption of behaviour either through deterioration of neural transmission, perhaps owing to modified internal acid-base balance, or through compromised processing of sensory information. Alternatively, it is possible that the performance of the sensory systems is not directly impacted, but instead state-dependent elicited behaviour is altered, perhaps owing to stress caused by acidification. These alternatives should be teased apart in future experiments combining neuronal electrophysiology with endocrinological manipulations. As the conditions simulated in this study will be reached in relatively few generations, the breakdown of behavioural responses to auditory stimuli, as well as previously documented changes to olfactory responses and other behaviours, suggest that marine organisms face a proximate era of severe evolutionary selection.
Acknowledgements
Experiments were conducted in accordance with animal ethics guidelines at James Cook University.
We thank Jeff Leis, Vince Rado, Geoff Endo and MARFU for logistic support, and Andy Radford, Marc Holderied and the Bristol BaBE Group for bioacoustics discussions. S.D.S. is funded by the Natural Environment Research Council and P.L.M. by the Australian Research Council.
References
- 1.The Royal Society 2005. Ocean acidification due to increasing atmospheric carbon dioxide. London, UK: The Royal Society; (Policy Document 12/05). [Google Scholar]
- 2.Meehl G. A., et al. 2007. Global climate projections. In Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (eds Solomon S., Qin D., Manning M., Chen Z., Marquis M., Averyt K. B., Tignor M., Miller H. L.), pp. 747–846 Cambridge, UK: Cambridge University Press [Google Scholar]
- 3.Raupach M. R., Marland G., Ciais P., Le Quere C., Canadell J. G., Klepper G., Field C. B. 2007. Global and regional drivers of accelerating CO2 emissions. Proc. Natl Acad. Sci. USA 104, 10 288–10 293 10.1073/pnas.0700609104 (doi:10.1073/pnas.0700609104) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orr J. C., et al. 2005. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature 437, 681–686 10.1038/nature04095 (doi:10.1038/nature04095) [DOI] [PubMed] [Google Scholar]
- 5.Ishimatsu A., Hayashi M., Kikkawa T. 2008. Fishes in high-CO2, acidified oceans. Mar. Ecol. Prog. Ser. 373, 295–302 10.3354/meps07823 (doi:10.3354/meps07823) [DOI] [Google Scholar]
- 6.Leis J. M. 2006. Are larvae of demersal fishes plankton or nekton? Adv. Mar. Biol. 51, 57–141 10.1016/S0065-2881(06)51002-8 (doi:10.1016/S0065-2881(06)51002-8) [DOI] [PubMed] [Google Scholar]
- 7.Munday P. L., Dixson D. L., Donelson J. M., Jones G. P., Pratchett M. S., Devitsina G. V., Doving K. B. 2009. Ocean acidification impairs olfactory discrimination and homing ability of a marine fish. Proc. Natl Acad. Sci. USA 106, 1848–1852 10.1073/pnas.0809996106 (doi:10.1073/pnas.0809996106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simpson S. D., Meekan M. G., Larsen N. J., McCauley R. D., Jeffs A. 2010. Behavioural plasticity in larval reef fish: orientation is influenced by recent acoustic experiences. Behav. Ecol. 21, 1098–1105 10.1093/beheco/arq117 (doi:10.1093/beheco/arq117) [DOI] [Google Scholar]
- 9.Radford C. A., Stanley J. A., Simpson S. D., Jeffs A. G. 2011. Juvenile coral reef fishes use sound to locate habitats. Coral Reefs 30, 295–305 10.1007/s00338-010-0710-6 (doi:10.1007/s00338-010-0710-6) [DOI] [Google Scholar]
- 10.Simpson S. D., Meekan M., Montgomery J., McCauley R., Jeffs A. 2005. Homeward sound. Science 308, 221. 10.1126/science.1107406 (doi:10.1126/science.1107406) [DOI] [PubMed] [Google Scholar]
- 11.Heenan A., Simpson S. D., Braithwaite V. A. 2009. Testing the generality of acoustic cue use at settlement in larval coral reef fish. In Proc. of the 11th Int. Coral Reef Symp., Fort Lauderdale, Florida, 7–11 July 2008, Session number 16, pp. 554–558 [Google Scholar]
- 12.Leis J. M., Sweatman H. P. A., Reader S. E. 1996. What the pelagic stages of coral reef fishes are doing out in blue water: daytime field observations of larval behavioural capabilities. Mar. Freshwater Res. 47, 401–411 [Google Scholar]
- 13.Checkley D. M., Dickson A. G., Takahashi M., Radich J. A., Eisenkolb N., Asch R. 2009. Elevated CO2 enhances otolith growth in young fish. Science 324, 1683. 10.1126/science.1169806 (doi:10.1126/science.1169806) [DOI] [PubMed] [Google Scholar]
- 14.Parmentier E., Colleye O., Mann D. 2009. Hearing ability in three clownfish species. J. Exp. Biol. 212, 2022–2025 10.1242/jeb.030270 (doi:10.1242/jeb.030270) [DOI] [PubMed] [Google Scholar]
- 15.Dixson D. L., Munday P. L., Jones G. P. 2010. Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol. Lett. 13, 68–75 10.1111/j.1461-0248.2009.01400.x (doi:10.1111/j.1461-0248.2009.01400.x) [DOI] [PubMed] [Google Scholar]
- 16.Munday P. L., Hernaman V., Dixson D. L., Thorrold S. R. 2011. Effect of ocean acidification on otolith development in larvae of a tropical marine fish. Biogeosci. Discuss. 8, 2329–2356 10.5194/bgd-8-2329-2011 (doi:10.5194/bgd-8-2329-2011) [DOI] [Google Scholar]