Abstract
Food processing is costly, potentially limiting the energy and time devoted to other essential functions such as locomotion or reproduction. In ectotherms, post-prandial thermophily, the selection of a warm environmental temperature after feeding, may be advantageous in minimizing the duration of this elevated cost. Although present in many vertebrate taxa, this behaviour had not previously been observed in invertebrates. Sanguivorous leeches ingest large blood meals that are costly to process and limit mobility until excess fluid can actively be expelled to reduce body volume. When presented with a temperature gradient from 10°C to 30°C, leeches select a temperature that is significantly warmer (24.3 ± 0.9°C, n = 6) than their acclimation temperature (Ta, 21°C). Unfed leeches preferred temperatures that were significantly cooler than ambient (12.8 ± 0.9°C, n = 6). This behavioural strategy is consistent with minimizing the time course of elevated post-feeding energy costs and reducing energy expenditure during fasting. Our observations raise the possibility that thermoregulatory behaviour of this type is an unrecognized feature of other invertebrate taxa.
Keywords: leech, thermoregulation, post-prandial, sanguivory, specific dynamic action
1. Introduction
For most animals, feeding comes at a cost. Food processing increases energy expenditure, an effect termed specific dynamic action (SDA) [1]. The costs can be substantial [2], potentially diverting energy from other processes such as growth, reproduction or locomotion [3]. Shortening the time course of SDA may therefore be selectively advantageous. Many ectothermic vertebrates achieve this through behavioural thermoregulation, selecting warmer environmental and, consequently, body temperatures after feeding to increase the rate of processing, a strategy termed post-prandial thermophily [4–8]. Although investigations of invertebrate temperature preference have a long history [9–12], adjustment of temperature preference in relation to feeding behaviour has not been clearly demonstrated in invertebrates.
This is surprising, as invertebrate SDAs can be substantial, particularly where there is a large relative meal size. Invertebrate sanguivores, for example, ingest some of the largest recorded meals relative to body mass [13,14]. Medicinal leeches can increase their body mass 1000 per cent during feeding [15], and the resulting SDA involves a 600 per cent increase in aerobic metabolism that can persist for several days [16]. The selection pressures for rapid meal processing should be particularly strong in this group, as in addition to the energy allocation problems posed by SDA, distension of the body reduces flexibility and limits the ability of the body wall musculature to power swimming or crawling [17]. This increases vulnerability to predation [18], and much of the increased energy expenditure is probably associated with increased urine production to expel excess fluid to reduce body volume and restore mobility [19].
We hypothesized that medicinal leeches would exhibit a post-prandial thermophilic response in order to minimize the time course of the SDA. In order to investigate their thermoregulatory behaviour, we exposed Hirudo verbana acclimated to 21°C (Ta) to an environmental temperature gradient (10–30°C) before and at five time points after feeding.
2. Material and methods
Medicinal leeches, H. verbana [20] (1.8 ± 0.52 g body mass, mean ± s.e.m., n = 7), were purchased from a commercial supplier (Leeches USA Ltd, New York, USA) and held in aquaria containing aquarium salt solution (0.75 g l−1 aquarium salt, Doc Wellfish, Chalfont, PA, USA) at 21°C. Leeches were acclimated to this temperature for a minimum of two weeks before data collection.
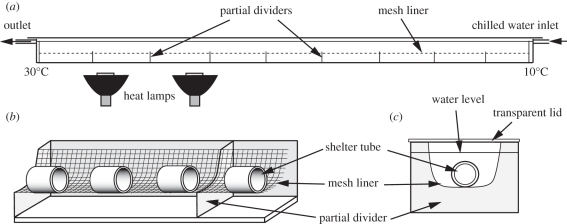
The design of the temperature gradient chamber is shown in figure 1. To avoid bias from starting location, leeches were introduced into the gradient chamber at a randomly selected shelter tube. Leech position was monitored by a camera (Canon Powershot S5IS, Canon Inc., Lake Success, NY, USA) above the tank that recorded an image every 5 min for 6 h (72 frames). All observations were made with a single leech in the gradient chamber at any one time.
Figure 1.
Schematic of the temperature gradient chamber. The apparatus consisted of an elongated plastic trough with a transparent lid (190 cm long × 6 cm deep × 8 cm wide). Chilled aquarium salt solution entered at 10°C and was warmed as it flowed through the chamber by an ambient temperature of 21°C and non-light emitting ceramic-element heat lamps, creating a linear temperature gradient reaching 30°C at the outlet. The warmed solution was recirculated to the inlet via a chiller. A mesh liner prevented direct contact between the leech and the walls of the tank. Partially dividing walls acted as flow baffles, preventing the formation of temperature laminae and convection currents. Tubes (5 cm long, 2 cm internal diameter) were placed at 2 cm intervals along the tank to provide shelter. (a) Overall arrangement of trough, flow and heat lamps. (b) Partial cutaway to show arrangement of dividing walls, mesh liner and shelter tubes. (c) Transverse section.
An initial control was performed to test for any leech location preference within the gradient chamber in the absence of a temperature gradient, but with water circulation maintained by the peristaltic pump. Control leeches (n = 7) initially explored the tank before settling close to one end, i.e. showing an ‘edge effect’. There was no preference for the inlet versus outlet ends of the tank, with three leeches finally settling at each end.
This protocol was repeated in unfed leeches with the temperature gradient in place (n = 7). Leeches were then fed to satiation on a container of warmed, defibrinated sheep blood (Hemostat Laboratories, Dixon, CA, USA), obtained by biting through a Parafilm-covered aperture [17]. Position monitoring was repeated placing leeches in the gradient at 0, 24, 48, 120 and 240 h after feeding (n = 6–7). Leeches were maintained at 21°C between temperature preference measurements. Temperature preferences were based on the final 3 h of position data in order to exclude initial exploratory behaviour.
Analysis of covariance was conducted with the general linear model in the application PASW Statistics (v. 18, IBM, Somera, NY, USA), to determine the preferred temperature in relation to feeding. Time interval (pre-feeding and 3, 27, 51, 123 and 143 h post-feeding) was included as a fixed factor in the model and the relative body mass (ratio of current body mass to pre-feeding body mass) as a covariate. Dunnett's procedure was used to make planned contrasts between the preferred temperature at each post-feeding interval and the pre-feeding preference. A one-sample t-test was used to test for significant differences between the preferred temperature at each time interval and the acclimation temperature (21°C). Dunnett's correction was applied to account for the use of multiple comparisons to a control value [21].
3. Results
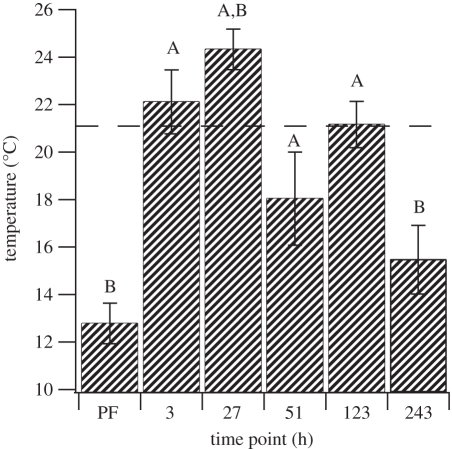
Temperature preference changed significantly with respect to time interval (figure 2, GLM, F5,33 = 3.7, p < 0.05). Before feeding, the preferred temperature was significantly lower than Ta (one-sample, two-tailed, t-test, t = −9.59, d.f. = 6, Dunnett's critical t-value = −3.49, p < 0.05). The preferred temperature remained significantly higher than the pre-feed preference for the first 5 days after feeding (planned contrasts, Dunnett's procedure, p < 0.05). Twenty-seven hours after feeding, the preferred temperature was also significantly higher than Ta (one-sample, two-tailed, t-test, t = 3.85, d.f. = 6, Dunnett's critical t-value = 3.49, p < 0.05). The preferred temperature after 10 days was statistically indistinguishable from the pre-feed preference (planned contrasts, Dunnett's procedure, p < 0.05), and significantly lower than Ta (one-sample, two-tailed, t-test, t = 3.83, d.f. = 5, Dunnett's critical t-value = −3.62, p < 0.05).
Figure 2.
Preferred temperature of the medicinal leech (H. verbana) before and after feeding. The horizontal dashed line shows the acclimation temperature (Ta, 21°C). A, significantly different from the pre-feeding preferred temperature (ANCOVA, planned contrasts, Dunnett's procedure, p < 0.05) (n = 7 for PF, 3, 27 and 51 h, n = 6 for 123 and 243 h). B, significantly different from Ta (one-sample, two-tailed, t-test, p < 0.05 after applying Dunnett's correction). Error bars indicate 1 s.e.m.
4. Discussion
We show for the first time that an invertebrate, the medicinal leech (H. verbana), exhibits feeding-related behavioural thermoregulation similar to that seen in ectothermic vertebrates. The preference for cooler temperatures while unfed (figure 1) is similar to that observed in some ectothermic vertebrates [22–24]. Leeches may wait many months between meals [15], and a cooler temperature preferendum would minimize energy expenditure during the interim [25]. The increase in metabolism immediately after feeding is probably associated with increased urine production to expel plasma from the ingested blood, a process reliant on the active transport of ions [19]. This rapidly reduces body volume and restores mobility [17]. Increased body temperature during this period would accelerate this process and ensure earlier cessation of the associated SDA.
Although concerns have been raised about the interpretation of thermal gradient data [26], the thermoregulatory response is likely to be functionally and ecologically relevant in this species. Medicinal leeches are typically found in small bodies of fresh water [27,28]. These can provide a variety of thermal niches, as temperature can vary with depth, degree of sun exposure and amount of vegetation, allowing for temperature selection in the field [28,29].
Our observations raise the possibility that the thermoregulatory behaviour of this type is an unrecognized feature of other invertebrate taxa, with broad implications concerning their behavioural and physiological ecology. Furthermore, the neural architecture of the medicinal leech is uniquely well mapped [30], making this species a valuable new model for investigating the neural control of behavioural thermoregulation.
Acknowledgements
This work was supported by Wellesley College, the Brachman Hoffman fund and NSF 0715937 to D.J.E.
References
- 1.McCue C. M. 2006. Specific dynamic action: a century of investigation. Comp. Biochem. Physiol. A 144, 381–394 10.1016/j.cbpa.2006.03.011 (doi:10.1016/j.cbpa.2006.03.011) [DOI] [PubMed] [Google Scholar]
- 2.Secor S. 2009. Specific dynamic action: a review of the postprandial metabolic response. J. Comp. Physiol. B 179, 1–56 10.1007/s00360-008-0283-7 (doi:10.1007/s00360-008-0283-7) [DOI] [PubMed] [Google Scholar]
- 3.Alsop D., Wood C. 1997. The interactive effects of feeding and exercise on oxygen consumption, swimming performance and protein usage in juvenile rainbow trout (Oncorhynchus mykiss). J. Exp. Biol 200, 2337–2346 [DOI] [PubMed] [Google Scholar]
- 4.Dawson W. R. 1975. On the physiological significance of the preferred body temperatures of reptiles. In Perspectives in ecological ecology (eds Gates D. M., Schmerl R. B.), pp. 443–473 New York, NY: Springer [Google Scholar]
- 5.Slip D. J., Shine R. 1988. Thermophilic response to feeding of the diamond python, Morelia s. spilota (Serpentes: Boidae). Comp. Biochem. Physiol. A 89, 645–650 10.1016/0300-9629(88)90847-X (doi:10.1016/0300-9629(88)90847-X) [DOI] [Google Scholar]
- 6.Gatten R. E. 1974. Effect of nutritional status on the preferred body temperature of the turtles Pseudemys scripta and Terrapene ornata. Copeia 1974, 912–917 10.2307/1442590 (doi:10.2307/1442590) [DOI] [Google Scholar]
- 7.Kitchell J. F. 1969. Thermophilic and thermophobic responses of snakes in a thermal gradient. Copeia 1969, 189–191 10.2307/1441713 (doi:10.2307/1441713) [DOI] [Google Scholar]
- 8.Lang J. W. 1979. Thermophilic response of the American alligator and the American crocodile to feeding. Copeia 1979, 48–59 10.2307/1443728 (doi:10.2307/1443728) [DOI] [Google Scholar]
- 9.Gunn D. L. 1934. The temperature and humidity relations of the cockroach (Blatta orientalis). II. Temperature preference. Z. Vergl. Physiol. 20, 617–625 10.1007/BF00339156 (doi:10.1007/BF00339156) [DOI] [Google Scholar]
- 10.Herter K. 1928. Reizphysiologie und Wirtsfindung des Fischegels, Hemidepsis marginata. Zeit. f. vergl. Physiol. 8, 391–444 10.1007/BF00338967 (doi:10.1007/BF00338967) [DOI] [Google Scholar]
- 11.Herter K. 1929. Reizphysiologisches Verhalten und Parasitismus des Entenegels, Protoclepsis tesselata. Zeit. f. vergl. Physiol. 10, 272–308 10.1007/BF00340634 (doi:10.1007/BF00340634) [DOI] [Google Scholar]
- 12.Thomson R. W. 1938. The reactions of mosquitoes to temperature and humidity. Bull. Ent. Res 29, 125–140 10.1017/S0007485300026158 (doi:10.1017/S0007485300026158) [DOI] [Google Scholar]
- 13.Fielden L. J., Jones R. M., Goldberg M., Rechav Y. 1999. Feeding and respiratory gas exchange in the American dog tick, Dermacentor variabilis. J. Insect Physiol. 45, 297–304 10.1016/S0022-1910(98)00127-9 (doi:10.1016/S0022-1910(98)00127-9) [DOI] [PubMed] [Google Scholar]
- 14.Bradley T., Brethorst L., Robinson S., Hetz S. 2003. Changes in the rate of CO2 release following feeding in the insect Rhodnius prolixus. Physiol. Biochem. Zool. 76, 302–309 10.1086/367953 (doi:10.1086/367953) [DOI] [PubMed] [Google Scholar]
- 15.Lent C. M., Fliegner K. H., Freedman E., Dickinson M. H. 1988. Ingestive behavior and physiology of the medicinal leech. J. Exp. Biol. 137, 513–527 [DOI] [PubMed] [Google Scholar]
- 16.Zebe E., Roters F. J., Kaiping B. 1986. Metabolic changes in the medical leech Hirudo medicinalis following feeding. Comp. Biochem. Physiol. A 84, 49–55 10.1016/0300-9629(86)90041-1 (doi:10.1016/0300-9629(86)90041-1) [DOI] [Google Scholar]
- 17.Claflin S. B., Pien C. L., Rangel E. N., Utz K. E., Walther H. V., Wright A. N., Ellerby D. J. 2009. Effects of feeding on medicinal leech swimming performance. J. Zool. (Lond.) 277, 241–247 10.1111/j.1469-7998.2008.00534.x (doi:10.1111/j.1469-7998.2008.00534.x) [DOI] [Google Scholar]
- 18.Kutschera U., Roth M. 2005. Cannibalism in a population of the medicinal leech (Hirudo medicinalis L.). Biol. Bull. 32, 751–753 10.1007/s10525-005-0154-7 (doi:10.1007/s10525-005-0154-7) [DOI] [PubMed] [Google Scholar]
- 19.Zerbst-Boroffka I., Bazin B., Wenning A. 1997. Chloride secretion drives urine formation in leech nephridia. J. Exp. Biol. 200, 2217–2227 [DOI] [PubMed] [Google Scholar]
- 20.Siddall M. E., Trontelj P., Utevsky S. Y., Nkamany M., Macdonald K. S. 2007. Diverse molecular data demonstrate that commercially available medicinal leeches are not Hirudo medicinalis. Proc. R. Soc. B 274, 1481–1487 10.1098/rspb.2007.0248 (doi:10.1098/rspb.2007.0248) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunnet C. W. 1964. Tables for multiple comparisons with a control. Biometrics 20, 482–491 10.2307/2528490 (doi:10.2307/2528490) [DOI] [Google Scholar]
- 22.Javaid M. Y., Anderson J. M. 1967. Influence of starvation on selected temperature of some salmonids. J. Fish. Res. Board Can. 24, 1515–1519 [Google Scholar]
- 23.Lillywhite H. B., Licht P., Chelgren P. 1973. The role of behavioral thermoregulation in the growth energetics of the toad, Bufo boreas. Ecology 54, 375–383 10.2307/1934345 (doi:10.2307/1934345) [DOI] [Google Scholar]
- 24.Brown R. P., Griffin S. 2005. Lower selected body temperatures after food deprivation in the lizard Anolis carolinensis. J. Therm. Biol. 30, 79–83 10.1016/j.jtherbio.2004.07.005 (doi:10.1016/j.jtherbio.2004.07.005) [DOI] [Google Scholar]
- 25.McCue M. D. 2010. Starvation physiology: reviewing the different strategies animals use to survive a common challenge. Comp. Biochem. Physiol. A 156, 1–18 10.1016/j.cbpa.2010.01.002 (doi:10.1016/j.cbpa.2010.01.002) [DOI] [PubMed] [Google Scholar]
- 26.Wall M., Shine R. 2008. Post-feeding thermophily in lizards (Lialis burtonis Gray, Pygopodidae): laboratory studies can provide misleading results. J. Therm. Biol. 33, 274–279 10.1016/j.jtherbio.2008.02.005 (doi:10.1016/j.jtherbio.2008.02.005) [DOI] [Google Scholar]
- 27.Carena H. 1820. Monographie du genre Hirudo ou description des espéces de sangesues qui se trouvent ou qui sont en usage en piémont, avec des observations sur la génération, et sur d'autres points de l'histoire naturelle de quelques unes de ces espé ces. Mem. Reale Accad. Sci. Torino 25, 273–316 [Google Scholar]
- 28.Mann K. H. 1955. The ecology of the British freshwater leeches. J. Anim. Ecol. 24, 98–119 10.2307/1881 (doi:10.2307/1881) [DOI] [Google Scholar]
- 29.Beiswenger R. E. 1977. Diel patterns of aggregative behavior in tadpoles of Bufo americanus in relation to light and temperature. Ecology 58, 98–108 10.2307/1935111 (doi:10.2307/1935111) [DOI] [Google Scholar]
- 30.Friesen W. O., Kristan W. B. 2007. Leech locomotion: swimming, crawling and decisions. Curr. Opin. Neurobiol. 17, 704–711 10.1016/j.conb.2008.01.006 (doi:10.1016/j.conb.2008.01.006) [DOI] [PMC free article] [PubMed] [Google Scholar]