Abstract
It is widely assumed that terrestrial food webs are built on a nitrogen-limited base and consequently herbivores must compensate through selection of high-protein foods and efficient nitrogen retention. Like many folivorous primates, gorillas' diet selection supports this assumption, as they apparently prefer protein-rich foods. Our study of mountain gorillas (Gorilla beringei) in Uganda revealed that, in some periods, carbohydrate-rich fruits displace a large portion of protein-rich leaves in their diet. We show that non-protein energy (NPE) intake was invariant throughout the year, whereas protein intake was substantially higher when leaves were the major portion of the diet. This pattern of macronutrient intake suggests that gorillas prioritize NPE and, to achieve this when leaves are the major dietary item, they over-eat protein. The concentrations of protein consumed in relation to energy when leaves were the major portion of the diet were close to the maximum recommended for humans and similar to high-protein human weight-loss diets. By contrast, the concentrations of protein in relation to energy when gorillas ate fruit-dominated diets were similar to those recommended for humans. Our results question the generality of nitrogen limitation in terrestrial herbivores and provide a fascinating contrast with human macronutrient intake.
Keywords: nutritional ecology, ape nutrition, primate diet, geometrical framework
1. Introduction
It is commonly accepted that terrestrial ecosystems are limited by the rate at which herbivores can acquire protein from plants [1–4]. Consistent with this tenet are several studies that interpret the predominance of protein-rich leaves in the diets of folivorous primates, including gorillas, to indicate that foraging by these primates is geared towards prioritizing the intake of protein [5–7]. However, this apparent preference for protein by mountain gorillas (Gorilla beringei) is puzzling because the gorilla diet, composed of high-protein terrestrial herbaceous vegetation, contains much higher protein concentrations [8,9] than are estimated to be required by gorillas in relation to food intake [10,11]. This is a contradiction that extends to many other folivorous primates [6,12].
To decipher this apparent ‘protein paradox’, we used the geometrical framework of nutrition [13] and took advantage of natural variation in the diets of wild mountain gorillas in Uganda. Geometrical analysis is a modelling approach used to determine which nutritional parameters animals maintain and which they allow to vary when faced with variation in food availability and composition (e.g. the intake of single nutrients, or balances among two or more nutrients) [13]. It thus enables the nutritional priorities of animals to be deciphered by comparing the extent to which the intake of different nutrients is defended against variation in food composition, in much the same way that thermoregulatory priorities of animals can be established by measuring the core body temperature in a variety of thermal environments. Geometrical analysis has been used to understand the foraging choices in a variety of animals [13–15], including humans [16].
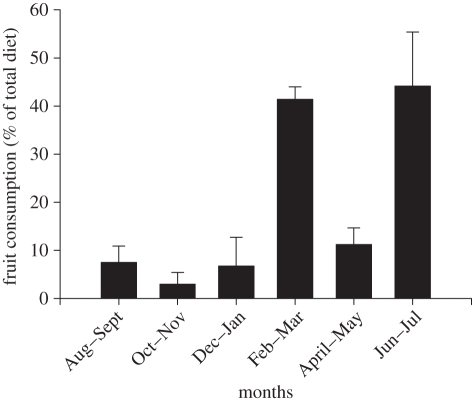
In Bwindi Impenetrable National Park, Uganda, the intake of energy-rich fruits by gorillas can seasonally exceed 40 per cent of the diet on a wet weight basis [17](figure 1). In other months, high-protein leaves compose the major portion of the diet, with fruits contributing less than 10 per cent. This seasonal disparity enabled us to infer from geometrical analysis the extent to which mountain gorillas prioritize protein over non-protein energy (NPE) and test whether protein is a limiting resource for gorillas.
Figure 1.
Mean (±s.d.) amount of fruit in the gorilla diet on a wet weight basis (replotted from Rothman et al. [17]).
2. Material and methods
We studied gorillas inhabiting Bwindi Impenetrable National Park, Uganda (0° 53′–1° 08′ S, and 29° 35′–29° 50′ E). The study group included two adult males, six adult females, four male juveniles and two infants who were not observed. Observations were restricted by the Ugandan government to 4 h d–1. During these 4 h, J.M.R., along with field assistants, conducted 30 min rotating focal observations and examined food intake (g min–1). We collected 1044 focal samples over 319 days for 1318 h during 1 year, and partitioned the data into successive two-month ‘periods’ as outlined in Rothman et al. [17], where further details of the methods are described.
We collected 336 foods across different seasons in areas where they were consumed by gorillas [17] and analysed them for available protein (AP) and NPE either via standard methods (n = 326) [10] at Cornell University, USA, or near infrared spectroscopy using a Foss XDS Rapid Content Analyzer (n = 10) [18] at Hunter College, USA. To calculate AP, we subtracted acid detergent insoluble nitrogen from total nitrogen before multiplying nitrogen by 6.25 [10], and then converted this value to kilojoule equivalents. To calculate NPE, we summed the energetic contributions from adjusted ether extract [19], non-structural carbohydrates and neutral detergent fibre (NDF) [10]. Energy contributions from NDF were estimated using age-sex and period-specific NDF digestibility coefficients after determining fibre digestibility using faecal lignin as an internal marker [17]. To calculate individual mean daily intake of AP and NPE, we followed Rothman et al. ([17], eqn 3).
We used the geometrical framework, a state-space modelling approach in which each axis represents a different nutrient, to decipher patterns of NPE and AP intake [13]. We constructed a two-dimensional model, with AP on the x-axis and NPE on the y-axis. In this way, diet compositions are represented as Cartesian points (showing the amounts of AP and NPE in the diets) or as lines radiating from the origin (‘nutritional rails’) with a particular slope indicating AP to NPE balance. We made comparisons using repeated-measures ANOVA, including ‘period’ as a within-subjects factor and age-sex group a between-subjects factor (PASW Statistics, v. 18). Equality of covariance matrices and normality were verified using Box's test and the Ryan–Joiner test, respectively.
3. Results
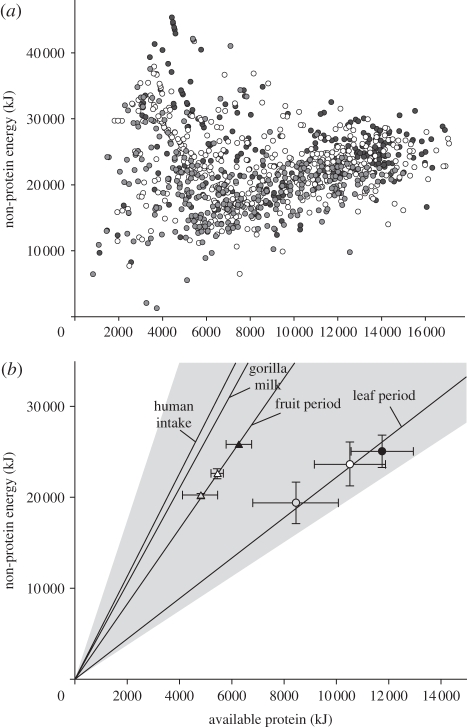
The daily intakes of AP and NPE by gorillas varied widely, with the ratio of AP to NPE spanning 0.06–2.9 (figure 2a). During high-fruit periods, mean daily macronutrient intakes for adult males, adult females and juveniles aligned along the AP to NPE nutritional rail (slope) of 0.3, and gorillas consumed 19 per cent of their total energy as protein (figure 2b). By contrast, when gorillas ate leaves, the balance of AP to NPE aligned along a nutritional rail of 0.5, and gorillas consumed 30 per cent of total energy as protein. The AP to NPE balance differed between periods (repeated-measures ANOVA; F1,9 = 453.5, p < 0.001), but did not differ among age-sex classes within periods (F2,9 = 0.175, p = 0.842), and nor did the interaction between period and age-sex class differ (F2,9 = 2.856, p = 0.110). In both fruit-rich and leaf-rich periods, overall macronutrient intake was highest in adult males, followed by adult females, with the lowest intakes in juveniles (F2,9 = 91.26, p < 0.001; figure 2b). Total macronutrient intake varied among periods (F1,9 = 52.296, p < 0.001), but there was no period by age-sex class interaction (F2,9 = 0.734, p = 0.506). Thus, gorillas had higher levels of total macronutrient intake in leaf-eating compared with fruit-eating periods, and this difference applied similarly to adult males, adult females and juveniles.
Figure 2.
Mean intake of available protein (AP) and non-protein energy (NPE) by gorillas. (a) This illustrates the mean daily intakes based on 1044 focal samples. (b) A geometrical plot of mean daily intakes (±s.d.) during fruit (triangles) and leaf periods (circles). Nutritional rails indicate AP to NPE balance in fruit and leaf periods (derived from data in (a), weighted by successive two-month intervals over 1 year [17]), the balance of AP to NPE in mid-lactation gorilla milk [20], and the recommended balance of AP to NPE for humans to maintain healthy diets [21]. The grey area represents the upper and lower limits of AP to NPE suggested for humans. (a) Black circles, adult males; white circles, adult females; grey circles, juveniles and (b) black-filled triangles, adult male (fruit periods); grey triangles, adult female (fruit periods); white triangles, juvenile (fruit periods); black circles, adult male (leaf periods); grey circles, adult female (leaf periods); white circles, juvenile (leaf periods); continuous lines, nutritional rails.
4. Discussion
During fruit periods, the balance of AP to NPE in the diet was slightly higher than that of gorilla mid-lactation milk (AP comprises 16% total energy; figure 2b) [20], a nutritionally complete food source for supporting growth in infants. This balance is similar to the diets chosen by humans in free-choice experiments (AP comprises 17% total energy [16]) and close to the current recommendations of the American Heart Association (15%) [21,22]. By contrast, when leaves dominated gorilla diets, 31 per cent of total energy is consumed as protein, a value close to the upper limit recommended for humans, and similar to human high-protein weight-loss diets (26–29%) [21,22].
Contrary to the expectation that nitrogen is limiting for terrestrial herbivores [1–3], the above comparisons, together with our geometrical analysis, indicate that gorillas over-ingest protein when eating a leaf-based diet, rather than specifically targeting leaves to supplement a protein-limited diet. Leaves form the majority of the diet for most of the year since fruits are seasonally unavailable [17], suggesting that this gorilla population is physiologically adapted to eating and excreting excess nitrogen. Tannins, which may contribute 4 per cent of the diet on a dry matter basis, might reduce protein availability [23], but some staple leaves do not have tannins, yet many of the commonly eaten fruits do [8]. For those leaves that contain high-tannin levels, their protein-precipitating capacity might aid gorillas to void the ingested excesses. Studies on protein digestibility and urinary nitrogen excretion are needed to provide additional insight into the protein gain of gorillas and other primates. In the Virungas, fruit is scarce, but the concentrations of crude protein to available carbohydrate in gorilla diets are strikingly similar to those in Bwindi. The Virunga gorillas consume alternatives to fruit, such as carbohydrate-rich stems, to meet their energetic needs [9].
Geometrical analysis suggests that spider monkeys (Ateles chamek) in Bolivia prioritize AP, not NPE [15], as do humans in an experimental study [16]. In contrast to gorillas, spider monkeys are primarily frugivorous with rapid digestive transit rates [24]. To meet protein needs spider monkeys supplement fruit-based diets with daily small quantities of protein-rich young leaves. A future priority is to consider how ecology and phylogeny underlie primate nutritional strategies, and how the dietary patterns of our close relatives can help us to improve human health.
Acknowledgements
This study was approved by the Uganda Wildlife Authority and the Uganda National Council for Science and Technology, and complied with all regulations of the government of Uganda.
We thank our assistants for their hard work in the field, and the Uganda Wildlife Authority for permission to conduct research. We are grateful to K. Milton, A. Pell, M. Power and P. Van Soest for helpful discussions. The National Science Foundation under grant no. 0922709, Jane Engel and the Robert G. Engel Family Foundation, Cornell University Graduate School, NSERC, and National Research Centre for Growth and Development of New Zealand provided funding.
References
- 1.White T. C. R. 1993. The inadequate environment: nitrogen and the abundance of animals. Berlin, Germany: Springer [Google Scholar]
- 2.Mattson W. J. 1980. Herbivory in relation to plant nitrogen content. Ann. Rev. Ecol. Syst. 11, 119–161 10.1146/annurev.es.11.110180.001003 (doi:10.1146/annurev.es.11.110180.001003) [DOI] [Google Scholar]
- 3.Elser J. J., et al. 2000. Nutritional constraints in terrestrial and freshwater food webs. Nature 408, 578–580 10.1038/35046058 (doi:10.1038/35046058) [DOI] [PubMed] [Google Scholar]
- 4.Moe S. J., Stelzer R. S., Forman R., Harpole W. S., Daufresne T., Yoshida T. 2005. Recent advances in ecological stoichiometry: insights for population and community ecology. Oikos 109, 29–39 10.1111/j.0030-1299.2005.14056.x (doi:10.1111/j.0030-1299.2005.14056.x) [DOI] [Google Scholar]
- 5.Rogers M. E., Maisels F., Williamson E. A., Fernandez M., Tutin C. E. G. 1990. Gorilla diet in the Lope Reserve, Gabon: a nutritional analysis. Oecologia 84, 326–339 10.1007/BF00329756 (doi:10.1007/BF00329756) [DOI] [PubMed] [Google Scholar]
- 6.Chapman C. A., Chapman L. J. 2002. Foraging challenges of red colobus monkeys: influence of nutrients and secondary compounds. Comp. Biochem. Phys. A 133, 861–875 10.1016/S1095-6433(02)00209-X (doi:10.1016/S1095-6433(02)00209-X) [DOI] [PubMed] [Google Scholar]
- 7.Ganas J., Ortmann S., Robbins M. M. 2008. Food preferences of wild mountain gorillas. Am. J. Primat. 70, 927–938 10.1002/ajp.20584 (doi:10.1002/ajp.20584) [DOI] [PubMed] [Google Scholar]
- 8.Rothman J. M., Dierenfeld E. S., Molina D. O., Shaw A. V., Hintz H. F., Pell A. N. 2006. Nutritional chemistry of foods eaten by gorillas in Bwindi Impenetrable National Park, Uganda. Am. J. Primatol. 68, 675–691 10.1002/ajp.20243 (doi:10.1002/ajp.20243) [DOI] [PubMed] [Google Scholar]
- 9.Rothman J. M., Plumptre A. J., Dierenfeld E. S., Pell A. N. 2007. Nutritional composition of the diet of the gorilla (Gorilla beringei): a comparison between two mountain habitats. J. Trop. Ecol. 23, 673–682 10.1017/S0266467407004555 (doi:10.1017/S0266467407004555) [DOI] [Google Scholar]
- 10.National Research Council 2003. Nutrient requirements of nonhuman primates, 2nd edn. Washington, DC: The National Academic Press [Google Scholar]
- 11.Oftedal O. T. 1992. The nutritional consequences of foraging in primates: the relationship of nutrient intakes to nutrient requirements. Phil. Trans. R. Soc. Lond. B 334, 161–170 10.1098/rstb.1991.0105 (doi:10.1098/rstb.1991.0105) [DOI] [PubMed] [Google Scholar]
- 12.McKey D. B., Gartlan J. S., Waterman P. G., Choo G. M. 1981. Food selection by black colobus monkeys (Colobus satanas) in relation to plant chemistry. Biol. J. Linn. Soc. 16, 115–146 10.1111/j.1095-8312.1981.tb01646.x (doi:10.1111/j.1095-8312.1981.tb01646.x) [DOI] [Google Scholar]
- 13.Raubenheimer D., Simpson S. J. 1997. Integrative models of nutrient balancing: application to insects and vertebrates. Nutr. Res. Rev. 10, 151–179 10.1079/NRR19970009 (doi:10.1079/NRR19970009) [DOI] [PubMed] [Google Scholar]
- 14.Mayntz D., Raubenheimer D., Salomon M., Toft S., Simpson S. J. 2005. Nutrient-specific foraging in invertebrate predators. Science 307, 111–113 10.1126/science.1105493 (doi:10.1126/science.1105493) [DOI] [PubMed] [Google Scholar]
- 15.Felton A. M., Felton A., Raubenheimer D., Simpson S. J., Foley W. J., Wood J. T., Wallis I. R., Lindenmayer D. B. 2009. Protein content of diets dictates the daily energy intake of a free-ranging primate. Behav. Ecol. 20, 685–690 10.1093/beheco/arp021 (doi:10.1093/beheco/arp021) [DOI] [Google Scholar]
- 16.Simpson S. J., Batley R., Raubenheimer D. 2003. Geometric analysis of macronutrient intake in humans: the power of protein? Appetite 41, 123–140 10.1016/S0195-6663(03)00049-7 (doi:10.1016/S0195-6663(03)00049-7) [DOI] [PubMed] [Google Scholar]
- 17.Rothman J. M., Dierenfeld E. S., Hintz H. F., Pell A. N. 2008. Nutritional quality of gorilla diets: consequences of age, sex and season. Oecologia 155, 111–122 10.1007/s00442-007-0901-1 (doi:10.1007/s00442-007-0901-1) [DOI] [PubMed] [Google Scholar]
- 18.Rothman J. M., Chapman C. A., Hansen J. L., Cherney D. J., Pell A. N. 2009. Rapid assessment of the nutritional value of foods eaten by mountain gorillas: applying near-infrared reflectance spectroscopy to primatology. Int. J. Primatol. 30, 729–742 10.1007/s10764-009-9372-z (doi:10.1007/s10764-009-9372-z) [DOI] [Google Scholar]
- 19.Palmquist D. L., Jenkins T. C. 2003. Challenges with fats and fatty acid methods. J. Anim. Sci. 81, 3250–3254 [DOI] [PubMed] [Google Scholar]
- 20.Whittier C. A., Milligan L. A., Nutter F. B., Cranfield M. R., Power M. L. In press Proximate composition of milk from free-ranging mountain gorillas (Gorilla beringei). Zoo Biol. (doi:10.1002/zoo.20363) [DOI] [PubMed] [Google Scholar]
- 21.Food and Nutrition Board of the Institute of Medicine of the National Academy 2005. Dietary reference intakes for energy, carbohydrate, fiber, fat, fatty acids, cholesterol, protein, and amino acids. Washington, DC: National Academies Press; [DOI] [PubMed] [Google Scholar]
- 22.Saint Joer S. T., Howard B. V., Prewitt E., Bovee V., Bazzarre T., Eckel R. H. 2001. Dietary protein and weight reduction. Circulation 104, 1869–1874 10.1161/hc4001.096152 (doi:10.1161/hc4001.096152) [DOI] [PubMed] [Google Scholar]
- 23.Rothman J. M., Dusinberre K., Pell A. N. 2009. Condensed tannins in the diets of primates: a matter of methods. Am. J. Primat. 71, 70–76 10.1002/ajp.20623 (doi:10.1002/ajp.20623) [DOI] [PubMed] [Google Scholar]
- 24.Milton K. 1981. Food choice and digestive strategies of two sympatric primate species. Am. Nat. 117, 496–505 10.1086/283730 (doi:10.1086/283730) [DOI] [Google Scholar]