Abstract
Migratory land birds perform extreme endurance flights when crossing ecological barriers, such as deserts, oceans and ice-caps. When travelling over benign areas, birds are expected to migrate by shorter flight steps, since carrying the heavy fuel loads needed for long non-stop flights comes at considerable cost. Here, we show that great snipes Gallinago media made long and fast non-stop flights (4300–6800 km in 48–96 h), not only over deserts and seas but also over wide areas of suitable habitats, which represents a previously unknown migration strategy among land birds. Furthermore, the great snipes achieved very high ground speeds (15–27 m s−1), which was not an effect of strong tailwind support, and we know of no other animal that travels this rapidly over such a long distance. Our results demonstrate that some migratory birds are prepared to accept extreme costs of strenuous exercise and large fuel loads, even when stopover sites are available along the route and there is little tailwind assistance. A strategy of storing a lot of energy before departure, even if migration is over benign habitats, may be advantageous owing to differential conditions of fuel deposition, predation or infection risk along the migration route.
Keywords: avian migration, endurance exercise, geolocators, stopover ecology, wind assistance
1. Introduction
Many terrestrial birds fly several thousands of kilometres non-stop during migration when crossing ecological barriers, such as deserts, oceans and ice-caps [1–3], where they cannot find food for refuelling. But long non-stop flights come at some cost. In addition to the strenuous exercise itself, the huge fuel stores needed (up to 100 per cent of lean body mass in long-distance migrants [2,4,5]) have a negative effect on transport economy [6,7] and manoeuvrability [8] and may increase predation risk [9]. When flying over hospitable land, terrestrial birds, therefore, generally avoid long flights and migrate by shorter flight steps, carrying only small to moderate fuel loads [4].
Still, our understanding of the migration strategies of birds is rather limited because for the majority of species we cannot follow the annual movements of individuals. With minute geolocators [10], allowing the tracking of small individual birds [11], a new and exciting picture of avian flight capacity and migration strategies is emerging [11–14]. We used geolocators to unravel the complete annual migration of the great snipe Gallinago media, an enigmatic and endangered Eurasian inland shorebird with an unknown migration strategy.
2. Material and methods
(a). Fieldwork
In May 2009, 10 male great snipes (159–169 g) were captured in mistnets at a lek in Jämtland, Sweden (63° N, 12° E) and equipped with geolocators (Mk10, 1.1 g, British Antarctic Survey (BAS); electronic supplementary material, figure S1). In May 2010, three of the birds were recaptured.
(b). Data analysis
Times of sunrise and sunset were extracted (TransEdit, BAS) using a single light threshold value of 2. We calculated locations (BirdTracker, v. 1.0, BAS) using the length of the solar day (or night) to determine latitude and the time of local solar noon (or midnight) to determine longitude. For these calculations, we used a sun angle that minimized the difference in latitude between pre- and post-equinox periods when the birds were stationary (−4.0, −3.5 and −3.0 for individuals 1, 2 and 3, respectively). This procedure is based on the observation that the latitude error increases with increasing mismatch between light threshold value and inferred sun angle [15]. For the non-stop flights, data were smoothed twice [16] giving estimates for noon and midnight positions, which defined 12 h-segments. Travel distance is the sum of the length of individual segments, and the mean ground speed is the travel distance divided by the duration of the flight.
(c). Environmental data
Wind data were obtained from the NCEP/NCAR reanalysis project (NOAA/OAR/ESRL PSD, Boulder, CO, USA, http://www.esrl.noaa.gov/psd/). For every 12 h-segment, wind data were extracted for the start, mid- and endpoint, in which the midpoint was given twice as much weight during averaging. The airspeed and heading of the bird were calculated by subtracting the wind vector from the track vector. Subsequently, we calculated, for each long non-stop flight, the total distance the birds flew through the air and the corresponding mean airspeed. Flight altitudes of the birds were not recorded, so these calculations were repeated for different altitudes (750, 1500, 3000 and 5000 m). We also considered the scenario where the bird always chose the altitude with most profitable winds (‘min’ in figure 1c,d).
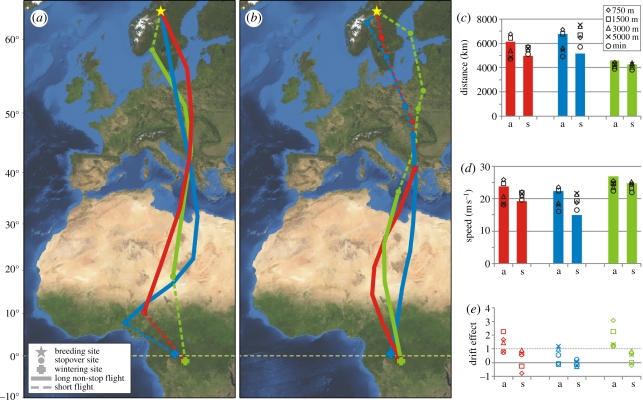
Figure 1.
(a) The autumn and (b) spring migration of three great snipes travelling between the breeding site in Sweden (63° N, 12° E) and winter quarters in central Africa (1° S, 20° E). Bar graphs show (c) the distance and (d) ground speed for non-stop flights. Symbols depict distance and speed in relation to surrounding air, should the bird have flown at a given altitude. ‘min’ reflects the conditions for a bird that always selects the most profitable altitude. (e) Relationship between wind and directional changes (see main text). a, autumn; s, spring.
(d). Wind drift analysis
The effect of wind on the direction of movement was analysed by relating track direction (direction of a 12 h-segment) to the angle between track and heading direction. This angular difference is the angle of drift or compensation caused by wind. A value of 0 indicates no relationship between wind and course changes (complete compensation). Positive values indicate that the track direction is a function of the wind direction, i.e. the track direction is affected by the wind (wind drift). A value of 1 is the special case where the bird keeps a constant heading and is fully affected by wind (full drift). Negative values mean that the flight paths are directed into the crosswind component.
3. Results
In late August, three birds flew 6170, 6800 and 4620 km non-stop from the breeding area to tropical Africa (figure 1a,c). These flights lasted for 72, 84 and 48 h, respectively (electronic supplementary material, table S1). In spring, the birds flew 4980, 5180 and 4280 km non-stop (during 72, 96 and 48 h, respectively) from the equatorial winter quarters to the Balkan region (figure 1b,c). From there onwards, the birds made several short flights interspersed with short stopovers, until reaching the breeding grounds (figure 1b).
Travel speeds were very high for the non-stop flights: ground speeds were 22–27 m s−1 in autumn and 15–25 m s−1 in spring (figure 1d). Meteorological data indicated that in autumn tailwind support existed at high altitudes (3000–5000 m a.s.l.). However, for these altitudes calculated airspeeds were only 12–22% lower than corresponding ground speeds (figure 1d) indicating only a limited effect of wind. In spring, two birds even faced net headwinds at all altitudes. For high altitudes (where the birds had most tailwind support), and for the scenario where the bird always chooses the altitude with the most profitable winds, a positive relationship existed between the wind effect and the track direction, indicating lateral wind drift (figure 1e).
4. Discussion
Great snipe flights are remarkable for two reasons. First, it is surprising that the flights are very long. In autumn, the non-stop flights cover the whole temperate zone and part of the savannah, including 2500 km across areas where numerous other migrants with similar ecology (e.g. ruff Philomachus pugnax, wood sandpiper Tringa glareola) are known to stop and refuel in autumn [1,17]. Moreover, the great snipes we tracked made stopovers in these areas in spring (figure 1b), indicating that the temperate zone generally offers suitable stopover habitats for them. Also, in the spring a substantial part of the non-stop flights was over potential feeding habitat (at least 1300 km of savannah). To our knowledge, the flights of the great snipes are the longest non-stop flights ever recorded where a substantial part of the distance (especially in autumn) is over potentially benign areas (green areas in figure 1a,b).
Second, the non-stop flights were notably fast, and the ground speeds of great snipes (15–27 m s−1) were substantially higher than those recorded for three other shorebird species during similar non-stop flights for 3–8 days over 2500–11 700 km (7.4–18 m s−1) [2,18,19]. Winds were not or only slightly favourable during non-stop flights, and thus the high ground speeds of the great snipes are accounted for by high airspeeds (16–26 m s−1) rather than strong tailwinds. The great snipes presumably had high airspeeds because of a high wing loading (up to 77 N m−2 at the onset of the flight; electronic supplementary material, table S2) and flying at relatively high altitudes. Great snipes do not seem to be specifically adapted for long flights as they have relatively rounded wings, whereas pointed wings are associated with energy efficient flight (electronic supplementary material, figure S2 and table S2). However, the aspect ratio of great snipes' wings is somewhat higher than for the closely related, and less-migratory, common snipe Gallinago gallinago [20]. The curved shape of the flight courses (figure 1a,b) may be explained by lateral wind drift from a rather constant heading by varying high-altitude winds (figure 1e). Wind drift generally increases travel distance [21] and thus will contribute to high speeds.
Our results show that some migrants are prepared to carry out long-distance flights, even when stopover sites are available and there is little tailwind assistance, in spite of the costs of strenuous exercise [22] and carrying large fuel stores [4]. That great snipes deposit large fat stores in autumn is well exemplified by a historic report: ‘great snipes are so fat and heavy in autumn that their skin sometimes ruptures when the shot bird hits the ground’ ( [23], pp. 266–269). The over-flying of potential staging sites implies that great snipes minimize the time rather than the energy spent on migration [24], and that conditions in late summer are very favourable at the northerly latitudes in comparison with potential stopover sites further south in Europe with respect to fuel deposition, predation and/or infection risk [2,24–26]. In spring, the great snipes used several stopover sites in eastern Europe. This would allow them not only to refuel for further flights, but also to optimize the timing of arrival at the breeding grounds and to add extra stores for the start of the breeding period [2,27]. The long and fast flights of the great snipes across ecological barriers as well as benign habitats represent an unexpected and previously unknown strategy in bird migration, and indicate that we have not yet grasped the full range of variability in bird migration strategies.
Acknowledgements
The study was approved by the ethical committee in Malmö/Lund (M112-09).
We thank Johan Råghall and Hanna Eriksson for field assistance, and Staffan Bensch and two anonymous reviewers for useful comments. Financial support was received from the Swedish Research Council and the Centre for Animal Movement Research at Lund University (CAnMove, Linnaeus grant 349-2007-8690).
References
- 1.Alerstam T. 1990. Bird migration. Cambridge, UK: Cambridge University Press [Google Scholar]
- 2.Gill R. E., et al. 2009. Extreme endurance flights by landbirds crossing the Pacific Ocean: ecological corridor rather than barrier? Proc. R. Soc. B 276, 447–458 10.1098/rspb.2008.1142 (doi:10.1098/rspb.2008.1142) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox A. D., Glahder C. M., Walsh A. J. 2003. Spring migration and timing of Greenland White-fronted Geese: results from satellite telemetry. Oikos 103, 415–425 10.1034/j.1600-0706.2003.12114.x (doi:10.1034/j.1600-0706.2003.12114.x) [DOI] [Google Scholar]
- 4.Alerstam T., Lindström Å. 1990. Optimal bird migration: the relative importance of time, energy, and safety. In Bird migration: physiology and ecophysiology (ed. Gwinner E.), pp. 331–351 Berlin, Germany: Springer [Google Scholar]
- 5.Piersma T., Gill R. E., Jr 1998. Guts don't fly: small digestive organs in obese bar-tailed Godwits. Auk 115, 196–203 [Google Scholar]
- 6.Kvist A., Lindström Å., Green M., Piersma T., Visser H. 2001. Carrying large fuel loads during sustained bird flight is cheaper than expected. Nature 413, 730–732 10.1038/35099556 (doi:10.1038/35099556) [DOI] [PubMed] [Google Scholar]
- 7.Pennycuick C. J. 1989. Bird flight performance. Oxford, UK: Oxford University Press [Google Scholar]
- 8.Dietz M. W., Piersma T., Hedenström A., Brugge M. 2007. Intraspecific variation in avian pectoral muscle mass: constraints on maintaining manoeuvrability with increasing body mass. Func. Ecol. 21, 317–326 10.1111/j.1365-2435.2006.01234.x (doi:10.1111/j.1365-2435.2006.01234.x) [DOI] [Google Scholar]
- 9.Witter M. S., Cuthill I. C. 1993. The ecological costs of avian fat storage. Phil. Trans. R. Soc. Lond. B 340, 73–92 10.1098/rstb.1993.0050 (doi:10.1098/rstb.1993.0050) [DOI] [PubMed] [Google Scholar]
- 10.Croxall J. P., Silk J. R. D., Phillips R. A., Afanasyev V., Briggs D. R. 2005. Global circumnavigations: tracking year-round ranges of nonbreeding albatrosses. Science 307, 249–250 10.1126/science.1106042 (doi:10.1126/science.1106042) [DOI] [PubMed] [Google Scholar]
- 11.Stutchbury B. J. M., Tarof S. A., Done T., Gow E., Kramer P. M., Tautin J., Fox J. W., Afanasyev V. 2009. Tracking long-distance songbird migration by using geolocators. Science 323, 896. 10.1126/science.1166664 (doi:10.1126/science.1166664) [DOI] [PubMed] [Google Scholar]
- 12.Shaffer S. A., et al. 2006. Migratory shearwaters integrate oceanic resources across the Pacific Ocean in an endless summer. Proc. Natl Acad. Sci. USA 103, 12 799–12 802 10.1073/pnas.0603715103 (doi:10.1073/pnas.0603715103) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Egevang C., Stenhouse I. J., Phillips R. A., Petersen A., Fox J. W., Silk J. R. D. 2010. Tracking of Arctic Terns Sterna paradisea reveals longest animal migration. Proc. Natl Acad. Sci. USA 107, 2078–2081 10.1073/pnas.0909493107 (doi:10.1073/pnas.0909493107) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Conklin J. R., Battley P. F., Potter M. A., Fox J. W. 2010. Breeding latitude drives individual schedules in a trans-hemispheric migrant bird. Nat. Comm. 1, 1– 6 10.1038/ncomms1072 (doi:10.1038/ncomms1072) [DOI] [PubMed] [Google Scholar]
- 15.Ekstrom P. A. 2004. An advance in geolocation by light. Mem. Natl Inst. Polar Res. Spec. Issue 58, 210–226 [Google Scholar]
- 16.Pütz K. 2002. Spatial and temporal variability in the foraging areas of breeding king penguins. Condor 104, 528–538 10.1650/0010-5422(2002)104[0528:SATVIT]2.0.CO;2 (doi:10.1650/0010-5422(2002)104[0528:SATVIT]2.0.CO;2) [DOI] [Google Scholar]
- 17.Fransson T., Österblom H., Hall-Karlsson S. 2008. Swedish bird ringing atlas, vol. 2 Stockholm, Sweden: Grouses-Woodpeckers [Google Scholar]
- 18.Minton C., Gosbell K., Johns P., Fox J. W., Afanasyev V. 2010. Initial results from light level geolocator trials on Ruddy Turnstones Arenaria interpres reveal unexpected migration route. Wader Study Group Bull. 117, 9–14 [Google Scholar]
- 19.Niles L. J., Burger J., Porter R. R., Dey A. D., Minton C. D. T., Gonzalez P. M., Baker A. J., Fox J. W., Gordon C. 2010. First results using light level geolocators to track Red Knots in the Western Hemisphere show rapid and long intercontinental flights and new details of migration pathways. Wader Study Group Bull. 117, 123–130 [Google Scholar]
- 20.Lockwood R., Swaddle J. P., Rayner J. M. V. 1998. Avian wingtip shape reconsidered: wingtip shape indices and morphological adaptations to migration. J. Avian Biol. 29, 273–292 10.2307/3677110 (doi:10.2307/3677110) [DOI] [Google Scholar]
- 21.Alerstam T. 1979. Wind as a selective agent in bird migration. Ornis Scand. 10, 76–93 10.2307/3676347 (doi:10.2307/3676347) [DOI] [Google Scholar]
- 22.Piersma T. 2011. Why marathon migrants get away with high metabolic ceilings: towards an ecology of physiological restraint. J. Exp. Biol. 214, 295–302 10.1242/jeb.046748 (doi:10.1242/jeb.046748) [DOI] [PubMed] [Google Scholar]
- 23.Nilsson S. 1858. Skandinavisk fauna. Foglarna andra bandet. Lund: Gleerups [Google Scholar]
- 24.Gudmundsson G. A., Lindström Å., Alerstam T. 1991. Optimal fat loads and long distance flights by migrating knots Calidris canutus, sanderlings C. alba and turnstones Arenaria interpres. Ibis 133, 140–152 10.1111/j.1474-919X.1991.tb04825.x (doi:10.1111/j.1474-919X.1991.tb04825.x) [DOI] [Google Scholar]
- 25.Jonker R. M., Eichhorn G., Van Langevelde F., Bauer S. 2010. Predation danger can explain changes in timing of migration: the case of the barnacle goose. PLoS ONE 5, e11369. 10.1371/journal.pone.0011369 (doi:10.1371/journal.pone.0011369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beekman J. H., Nolet B. A., Klaassen M. 2002. Skipping swans: fuelling rates and wind conditions determine differential use of migratory stopover sites of Bewick's Swans Cygnus bewickii. Ardea 90, 437–460 [Google Scholar]
- 27.Sandberg R., Moore F. R. 1996. Fat stores and arrival on the breeding grounds: reproductive consequences for passerine migrants. Oikos 77, 577–581 10.2307/3545949 (doi:10.2307/3545949) [DOI] [Google Scholar]