Abstract
White-nose syndrome (WNS) is a disease responsible for unprecedented mortality in hibernating bats. First observed in a New York cave in 2006, mortality associated with WNS rapidly appeared in hibernacula across the northeastern United States. We used yearly presence–absence data on WNS-related mortality among hibernating bat colonies in the Northeast to determine factors influencing its spread. We evaluated hazard models to test hypotheses about the association between the timing of mortality and colony-level covariates, such as distance from the first WNS-affected site, colony size, species diversity, species composition and type of hibernaculum (cave or mine). Distance to origin and colony size had the greatest effects on WNS hazard over the range of observations; the type of hibernaculum and species composition had weaker effects. The distance effect showed a temporal decrease in magnitude, consistent with the pattern of an expanding epizootic. Large, cave-dwelling bat colonies with high proportions of Myotis lucifugus or other species that seek humid microclimates tended to experience early mortality. Our results suggest that the timing of mortality from WNS is largely dependent on colony location, and large colonies tend to be first in an area to experience high mortality associated with WNS.
Keywords: bat, colony size, Cox proportional hazards model, epizootic, hibernacula, white-nose syndrome
1. Introduction
White-nose syndrome (WNS), a disease of hibernating bats, was first observed in 2006 in Howes Cave, a commercial cave in upstate New York (figure 1). Shortly thereafter, WNS appeared in hibernacula across the northeastern United States (US) leading to unprecedented declines in hibernating bat populations [1]. Mortality rates in WNS-affected colonies frequently exceed 90 per cent, and regional extinction of a previously common species has been predicted [1].
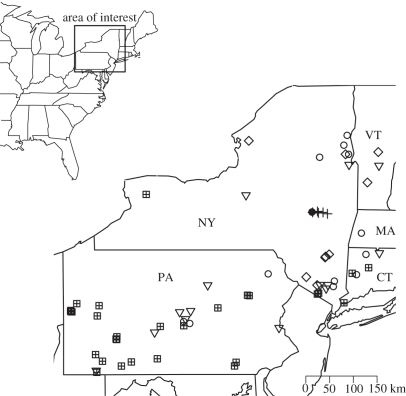
Figure 1.
Hibernating bat colonies in hazard models of WNS in the northeastern US. Filled circle, origin; plus symbols, 2007; diamonds, 2008; open circles, 2009; inverted triangles, 2010; sub-divided squares, unaffected, indicates the winter year that WNS first occurred in the colony.
WNS is associated with a cold-adapted fungus, Geomyces destructans, that invades exposed skin of bats, and grows maximally at temperatures typical of bat hibernacula (5–10°C) [2]. WNS is characterized by cutaneous fungal infection, depleted fat reserves, damaged wing tissue and abnormal behaviour [2]. The fungus infects all six hibernating bat species in the northeastern US, probably with differential effects [3]. Geomyces destructans has also been identified from bat species throughout Europe with no evidence of mass mortality, and was probably present in Europe well before its discovery in the USA [4].
Using data on the timing of WNS-related mortality (hereafter ‘WNS’) in hibernacula in the northeastern US (figure 1), we assessed ecologically plausible hazard models of WNS emergence patterns among mixed-species colonies of hibernating bats. Based on a survival distribution, in this case the time until WNS occurs in colonies, hazard models examine the influence of covariates on the instantaneous rate of arrival of WNS to colonies (i.e. the hazard rate). Covariate coefficients represent the multiplicative effect on the hazard rate of an increase in the covariate [5,6].
We predicted that if the spatial pattern of WNS is consistent with an expanding epizootic, hibernacula closest to the origin would initially have higher hazard rates than distant hibernacula, but over time the effect of distance on hazard rate would decrease. We also predicted that pathogen introduction, contact rates and thus hazard rates, increase with colony size. In the light of potential variation in susceptibility and hibernating behaviour among bat species [3,7,8], we tested the influence of species diversity, often important in prevalence of infectious disease [9] and the influence of proportions of individual species. Higher proportions of species that hibernate in dense clusters [8], or in relatively humid conditions ideal for fungal growth, may increase hazard rates [3]. We predicted that hazard rates may differ in caves and mines because of contrasting microclimatic regimes or potential differences in dispersal behaviours of bats in caves and mines [10]. We aimed to identify characteristics that affect colony-level risk of WNS, and determine the relative influence of these characteristics on the emergence of WNS in hibernacula across the northeastern US.
2. Material and methods
We used data on colony size and species composition from the most recent complete winter census of hibernating bats prior to WNS from 73 hibernacula in NY, PA, CT, VT and MA, USA (figure 1). Census methods are described elsewhere [1]. WNS was identified by the presence of abnormal numbers of bat carcases or substantial population decline, combined with WNS symptoms (fungal growth and aberrant behaviour [2]). WNS-affected sites were visited and confirmed unaffected prior to the year they experienced WNS-related mortality (see electronic supplementary material, S1 for further detail). Unaffected sites were considered to be unaffected in all previous years.
We used a Cox proportional hazards model to test the influence of the following covariates: geodesic distance to Howes Cave (‘distance to origin’), whether hibernacula were natural (‘cave’; n = 25) or non-natural (‘mine’; n = 48), log10 number of bats within hibernacula (‘colony size’), per cent of individuals of the four most commonly present species within hibernacula (Myotis lucifugus, Myotis septentrionalis, Perimyotis subflavus, Eptesicus fuscus), total per cent of species that typically hibernate in humid microclimates (M. lucifugus + M. septentrionalis + P. subflavus; ‘humid species’ [7]), total per cent of densely clustering species (M. lucifugus + M. sodalis; ‘clustering species’ [8]) and Simpson's reciprocal diversity index of bat species (‘SRD’ [11]).
We used Akaike's information criterion (AICC) to rank models. Because species composition covariates were highly correlated (electronic supplementary material, S2), they were included separately in competing models. Candidate models included all combinations of the covariates distance to origin, colony size and type of hibernaculum, and all these combinations with one species composition covariate (electronic supplementary material, S3). We estimated model-averaged coefficients using Akaike weights to account for model selection uncertainty (electronic supplementary material, S1; [12]). Influential data and nonlinearity were identified as described elsewhere [5]. Analyses were performed in R v. 2.11.0.
3. Results
Forty-four hibernacula experienced WNS-related mortality and 29 were unaffected at last observation (figure 1). Two models were equally parsimonious (ΔAICC < 2; table 1). Distance to origin, distance to origin × year and colony size had strong support. Support was somewhat weaker for hibernaculum type, and was equivocal for per cent humid species and per cent M. lucifugus (table 2).
Table 1.
Cox proportional hazards models of WNS among hibernating bat colonies in the northeastern US (AICC weight > 0.05), with degrees of freedom (d.f.), Akaike's information criterion (AICC), difference from lowest AICC score (ΔAICC), and AICC weights (w).
| covariates | d.f. | AICC | ΔAICC | w |
|---|---|---|---|---|
| distance to origin + distance to origin × year + colony size + hibernaculum type + % humid species | 5 | 226.066 | 0 | 0.228 |
| distance to origin + distance to origin × year + colony size + hibernaculum type + % M. lucifugus | 5 | 226.703 | 0.637 | 0.166 |
| distance to origin + distance to origin × year + colony size + % M. lucifugus | 4 | 228.349 | 2.283 | 0.073 |
| distance to origin + distance to origin × year + colony size + hibernaculum type + SRD | 5 | 228.406 | 2.340 | 0.071 |
| distance to origin + distance to origin × year + colony size + hibernaculum type | 4 | 228.697 | 2.631 | 0.061 |
| distance to origin + distance to origin × year + colony size + SRD | 4 | 228.918 | 2.856 | 0.055 |
Table 2.
Coefficient estimates (β), standard errors (s.e.), 95% confidence intervals (95% CI), exponent of coefficients (exp(β)), and relative weights (w+(j)) of covariates from hazard models of WNS among hibernating bat colonies in the northeastern US. Coefficients were adjusted for model selection uncertainty using all models containing the respective covariate.
| covariate | β | s.e. | 95% CI | exp(β) | w+(j) |
|---|---|---|---|---|---|
| distance to origin | −0.040 | 0.009 | −0.058, −0.022 | 0.961 | 1.000 |
| distance to origin × year | 0.008 | 0.002 | 0.004, 0.013 | 1.008 | 1.000 |
| log10(colony size) | 0.734 | 0.268 | 0.210, 1.258 | 2.083 | 0.969 |
| hibernaculum type | 0.659 | 0.349 | −0.025, 1.343 | 1.933 | 0.666 |
| % humid species | 0.018 | 0.012 | −0.006, 0.042 | 1.018 | 0.278 |
| % M. lucifugus | 0.014 | 0.009 | −0.005, 0.033 | 1.014 | 0.246 |
| SRD | −0.666 | 0.497 | −1.640, 0.308 | 0.514 | 0.131 |
| % clustering species | 0.015 | 0.012 | −0.008, 0.039 | 1.016 | 0.095 |
| % E. fuscus | −0.024 | 0.033 | −0.088, 0.040 | 0.976 | 0.075 |
| % P. subflavus | −0.014 | 0.027 | −0.067, 0.039 | 0.986 | 0.045 |
| % M. septentrionalis | −4.86 × 10−4 | 0.018 | −0.036, 0.035 | 1.000 | 0.034 |
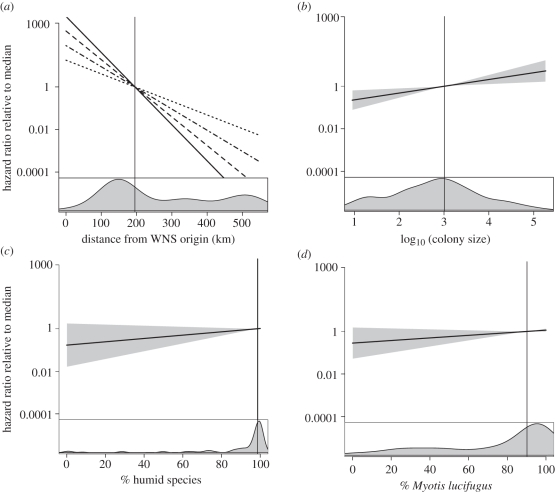
Distance had the greatest effect on hazard rate over the range of observations (figure 2). Each additional kilometre from the origin decreased the hazard rate by about 3.9 per cent in 2007 (exp(β) = 0.961); however, the effect of distance decreased with time (distance to origin × year exp(β) = 1.008; figure 2a). A 10-fold increase in colony size more than doubled the hazard rate (exp(β) = 2.463). The median WNS year for a large colony (more than 1000 bats) was 2009, whereas the median small colony (less than or equal to 1000 bats) remained unaffected until at least 2010. Mean distance to origin did not differ between small and large colonies (t-test, p = 0.566). Caves had nearly twice the hazard rate of mines (exp(β) = 1.933). The per cent humid species was weakly associated with WNS hazard, with each per cent humid species increasing the hazard rate by 1.8 per cent (exp(β) = 1.018). Each per cent M. lucifugus increased the hazard rate by 1.4 per cent (exp(β) = 1.014). Strong outliers and nonlinearity were not present in top models.
Figure 2.
Hazard ratios over the observed range of covariate values relative to the median (vertical lines) for, (a) distance from the origin of WNS in the northeastern US for winter years 2007–2010. Solid line, 2007; dashed line, 2008; dashed-dotted line, 2009; dotted line, 2010. (b) Colony size, (c) % humid species and (d) % Myotis lucifugus. Shading indicates 95% confidence intervals. See table 2 for distance to origin confidence intervals. Bottom panels are kernel density plots of covariate distributions.
4. Discussion
The pattern of spread of WNS-related mortality among hibernacula is consistent with an expanding epizootic. In 2007, sites near the origin had substantially higher hazard rates than distant sites, but over time as the infection spread across the region, distance offered less protection from WNS (figure 2a). Spatial expansion of WNS has been observed using information on WNS presence, but to our knowledge has not yet been quantitatively analysed using presence and absence data. This pattern of spread in combination with the high mortality of North American bats to WNS, the widespread distribution of G. destructans and lack of mass mortality of bats in Europe [4] suggest that this fungus may have been introduced to North America. New geographical foci do not confirm an exotic pathogen [13], however, and whether G. destructans was introduced from Europe or this epizootic results from an in situ pathogenic mutation remains an important unanswered question.
WNS hazard rate roughly doubled with each 10-fold increase in hibernating bats in a colony, and large colonies experienced earlier mortality than small colonies. If more bats prefer sites with optimal conditions for fungal growth, the effect of colony size may be indirect. Alternatively, more bats occupying a hibernaculum may increase the probability of pathogen introduction, or may increase host density and contact rates, thereby increasing the force of infection and reducing the time to substantial mortality [14]. The same principle may be expected for species that hibernate in dense clusters, but this trend was not detectable in colony-level timing of WNS (table 2). Variability in clustering behaviour among conspecifics, however, may limit species composition as an indicator of host density.
Our analysis suggests that colonies in caves had a higher hazard rate than those in mines. Mechanisms for this pattern are unclear, but two processes could contribute. First, there is some evidence that mines may be occupied by more localized bat populations than caves, possibly because mines are relatively new habitats [10]. More widely dispersing populations would be more likely to overlap with the distribution of a pathogen, thereby becoming exposed first; however, differential dispersal between bats in caves and mines has yet to be directly or systematically tested. Second, abiotic factors known to influence fungal growth such as relative humidity, roost substrate and ambient temperature may differ between caves and mines [15]. Future studies on dispersal distances and microclimatic regimes are needed to identify the causal mechanisms underlying these empirical observations.
We detected a weak positive association between WNS hazard rate and the proportion of bat species that seek humid microclimates, possibly because humid conditions are ideal for fungal growth. Alternatively, species that choose these conditions may be less able to cope with evaporative water loss from wing damage caused by infection [3]. Because humid species were dominated by M. lucifugus at our sites, and variability in species composition was low (figure 2c,d), we were unable to distinguish between models including one of these two covariates (table 1). Our results suggest that M. lucifugus may be particularly vulnerable to early mortality from WNS, possibly as a result of their preferred humid microclimate. Although species composition had relatively weak influence on the colony-level timing of WNS, it may markedly influence the severity of bat mortality within colonies. Future analyses comparing bat mortality rate across colonies and its relationship to species composition and other variables are critical for understanding disease dynamics and devising future conservation strategies.
Given that WNS-affected colonies of M. lucifugus experience mean first-year mortality of 85 per cent [1], the strong dependence of WNS on colony location relative to other factors (figure 2) does not bode well for populations within and near the expanding epizootic, as the largest colonies in a region are first to decline. These findings highlight the urgency of the threat to temperate insectivorous bat populations and the need for increased research and resources for the control of WNS.
Acknowledgements
We thank A. Hicks, G. Turner, C. Kocer, J. Dickson, T. French, S. Darling and R. Smith for data, and A. M. Kilpatrick, B. Fenton and an anonymous reviewer for comments. A.P.W. and W.F.F. thank ‘Training Workshops on the Ecology and Evolution of Infectious Diseases’ (NSF DEB-0722115, PI M. Antolin). Funding support includes, Morris Animal Foundation grant to T.H.K. and A.P.W., NSF Post-doctoral Fellowship to W.F.F., Woodtiger Fund to T.H.K., and BU CECB to K.E.L.
References
- 1.Frick W. F., Pollock J. F., Hicks A. C., Langwig K. E., Reynolds D. S., Turner G. G., Butchkoski C. M., Kunz T. H. 2010. An emerging disease causes regional population collapse of a North American bat species. Science 329, 679–682 10.1126/science.1188594 (doi:10.1126/science.1188594) [DOI] [PubMed] [Google Scholar]
- 2.Blehert D. S., et al. 2009. Bat white-nose syndrome: an emerging fungal pathogen? Science 323, 277. 10.1126/science.1163874 (doi:10.1126/science.1163874) [DOI] [PubMed] [Google Scholar]
- 3.Cryan P., Meteyer C., Boyles J., Blehert D. 2010. Wing pathology of white-nose syndrome in bats suggests life-threatening disruption of physiology. BMC Biol. 8, 135. 10.1186/1741-7007-8-135 (doi:10.1186/1741-7007-8-135) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Puechmaille S. J., et al. 2011. Pan-European distribution of white-nose syndrome fungus (Geomyces destructans) not associated with mass mortality. PLoS ONE 6, e19167. 10.1371/journal.pone.0019167 (doi:10.1371/journal.pone.0019167) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox J. 2002. An R and S Plus companion to applied regression. Thousand Oaks, CA: Sage Publications [Google Scholar]
- 6.Cox D. H. 1972. Regressions models and life tables (with discussion). J. R. Stat. Soc. B Stat. Methodol. 34, 187–220 [Google Scholar]
- 7.Davis W. H. 1970. Hibernation: ecology and physiological ecology. In Biology of bats (ed. Wimsatt W. A.), pp. 265–300 New York, NY: Academic Press [Google Scholar]
- 8.Brack V. 2007. Temperatures and locations used by hibernating bats, including Myotis sodalis (Indiana bat), in a limestone mine: implications for conservation and management. Environ. Manag. 40, 739–746 10.1007/s00267-006-0274-y (doi:10.1007/s00267-006-0274-y) [DOI] [PubMed] [Google Scholar]
- 9.Keesing F., et al. 2010. Impacts of biodiversity on the emergence and transmission of infectious diseases. Nature 468, 647–652 10.1038/nature09575 (doi:10.1038/nature09575) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis W. H., Hitchcock H. B. 1965. Biology and migration of the bat, Myotis lucifugus, in New England. J. Mammal. 46, 296–313 10.2307/1377850 (doi:10.2307/1377850) [DOI] [Google Scholar]
- 11.Peet R. K. 1974. The measurement of species diversity. Annu. Rev. Ecol. Syst. 5, 285–307 10.1146/annurev.es.05.110174.001441 (doi:10.1146/annurev.es.05.110174.001441) [DOI] [Google Scholar]
- 12.Burnham K. P., Anderson D. 2003. Model selection and multi-model inference. New York, NY: Springer [Google Scholar]
- 13.Randolph S. E., Rogers D. J. 2010. The arrival, establishment and spread of exotic diseases: patterns and predictions. Nat. Rev. Microboil. 8, 361–371 10.1038/nrmicro2336 (doi:10.1038/nrmicro2336) [DOI] [PubMed] [Google Scholar]
- 14.Anderson R. M., May R. M. 1991. Infectious diseases of humans: dynamics and control. Oxford, UK: Oxford University Press [Google Scholar]
- 15.Stomeo F., Portillo M. C., Gonzalez J. M. 2009. Assessment of bacterial and fungal growth on natural substrates: consequences for preserving caves with prehistoric paintings. Curr. Microbiol. 59, 321–325 10.1007/s00284-009-9437-4 (doi:10.1007/s00284-009-9437-4) [DOI] [PubMed] [Google Scholar]