Abstract
Anthropogenic noise, now common to many landscapes, can impair acoustic communication for many species, yet some birds compensate for masking by noise by altering their songs. The phylogenetic distribution of these noise-dependent signal adjustments is uncertain, and it is not known whether closely related species respond similarly to noise. Here, we investigated the influence of noise on habitat occupancy rates and vocal frequency in two congeneric vireos with similar song features. Noise exposure did not influence occupancy rates for either species, yet song features of both changed, albeit in different ways. With increases in noise levels, plumbeous vireos (Vireo plumbeus) sang shorter songs with higher minimum frequencies. By contrast, grey vireos (Vireo vicinior) sang longer songs with higher maximum frequencies. These findings support the notion that vocal plasticity may help some species occupy noisy areas, but because there were no commonalities among the signal changes exhibited by these closely related birds, it may be difficult to predict how diverse species may modify their signals in an increasingly noisy world.
Keywords: acoustic communication, anthropogenic noise, masking, occupancy rate, signal change, vireo
1. Introduction
Acoustic communication is prevalent in numerous animal taxa, and environmental features, such as vegetation structure, play important roles in the evolution of acoustic signals [1]. Another salient environmental feature that influences signal transmission is background noise, and through industrialization and the dendritic network of transportation lines, human-generated noise (henceforth ‘noise’) can now be heard across vast landscapes [2]. Though acoustic signals may be adapted to natural sources of ambient sounds (e.g. wind, rain and other animal vocalizations) [1], noise is often louder, more continuous and can overlap frequencies used by many species for communication. Thus, for animals that rely greatly on acoustic communication, such as birds, noise represents a widespread and novel force that can influence signal structure [3].
Some birds have different signals in environments with high levels of noise relative to those in less noisy areas [4,5] and, for some, altered signal features represent short-term behavioural adjustments [6,7]. Yet, not all species change signals in noisy environments, and this apparent absence of noise-dependent signal adjustments may force some species to abandon noisy areas for environments where their signals transmit well [5]. Because this inability to communicate may explain decreases in avian community species richness and diversity in noisy areas [2,3,8], data on the phylogenetic distribution of noise-dependent behavioural responses are currently needed. However, perhaps more importantly, we still lack knowledge of whether closely related species respond to noise in the same manner, limiting our ability to use phylogenetic relationships to predict which species may be able to cope with noise.
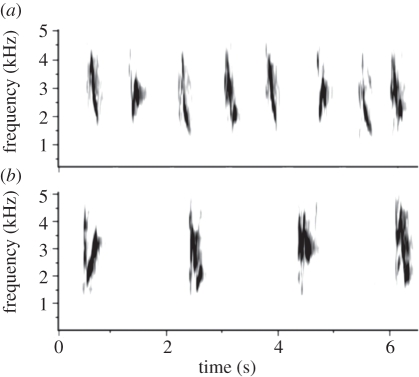
Here, we examine behavioural responses to noise in two congeneric songbirds with similar song features and overlapping habitat requirements. Plumbeous (Vireo plumbeus) and grey vireos (Vireo vicinior) inhabit arid coniferous woodlands and their songs are characterized by short phrases at similar frequencies (figure 1) [9]. Given their similar songs, noise should be an equally strong masking force for both species and, owing to their shared evolutionary history, we predict that they should use similar behavioural responses to masking by noise via noise-dependent changes to the same song features or by avoiding noisy areas. We first determine whether either vireo's habitat occupancy rate changes with background noise amplitude. We then investigate whether songs of either species change in response to noise and whether both species alter their signals in the same manner. We focus on song features shown to be important to signalling in noisy environments: the contrast between a signal and background noise, signal duration and signalling rate (reviewed in [10,11]). First, because human-generated noise may mask lower frequencies, we expect song frequency to increase in order to reduce acoustic overlap between the signal and noise and increase the contrast between the two. Second, signal detection in noisy conditions may improve with increases in temporal signal features; thus, we expect vireos to deliver longer signals (duration) or signal more frequently (rate) with increased noise exposure.
Figure 1.
(a) Grey and (b) plumbeous vireo songs are short phrases of similar frequency (grey, mean peak frequency ≈3.1 kHz, range ≈ 1.8–4.5 kHz; plumbeous, mean peak frequency ≈ 3.1 kHz, range ≈ 1.7–5.3 kHz).
2. Material and methods
We conducted our study in Rattlesnake Canyon Habitat Management Area (RCHMA), located in NW New Mexico, USA. Study area, survey and song recording details can be found elsewhere [5,8]. Briefly, RCHMA is dominated by mixed-conifer woodlands and has a high density of natural gas wells. Many wells are coupled with compressors that run continuously and generate noise at high amplitudes (greater than 95 dB(A)). Compressor noise, like most anthropogenic noise, has more energy at lower frequencies, with diminishing energy towards higher frequencies [5,8]. Additionally, human activity at wells and vegetation surrounding wells were previously found not to differ between wells with (noisy treatment sites) and without compressors (quiet control sites), providing an opportunity to evaluate the influence of noise on vireo habitat occupancy rates and vocal behaviour [8].
We conducted point count surveys at 13–16 randomly selected locations in woodlands surrounding five treatment and eight control sites in 2007 [5]. Each location was visited twice and, because noise can severely bias bird detections [12], compressors were turned off during surveys. At each survey location, we measured A-weighted background noise amplitudes (equivalent continuous noise levels, fast response) with NIST-certified sound pressure meters (Casella CEL320/CEL1002 converter). This was completed on the second visit to each control site location, but because compressors were off during surveys at treatment sites, we returned a third time for these measurements when compressors were running. We modelled the influence of noise on habitat occupancy rates using generalized linear mixed models with binomial errors in the lme4 package in program R. We treated noise amplitude as a fixed effect and gas well site as a random effect. Occurrence at a point count location was defined as any positive detection within 60 m of the survey location during either visit.
Grey and plumbeous vireo vocalizations were recorded in woodlands surrounding 26 gas wells between 10 May and 2 July 2009 using a Marantz PMD 660 Digital recorder with an Audio-technica AT-815 directional shotgun microphone pointed directly at a singing individual, typically from a distance of 5–15 m. Immediately following the recording, we measured background noise amplitude as close as possible to where the individual had been vocalizing, using the same methods described above. From each recording (mean length = 143 s), we randomly selected five strophes and measured the minimum, maximum and peak frequencies (frequency with the maximum amplitude), plus singing rate and song duration. Peak frequency was measured automatically in RavenPro v. 1.3 and minimum and maximum frequencies were measured manually using spectrogram and power spectrum views (electronic supplementary material, figure S1). For all recordings, we used a sampling rate of 48 kHz, a Hamming window with a fast Fourier transformation length of 1024 and a frequency resolution of 47 Hz. Average values were calculated for each individual, and we used linear regression to examine the influence of noise amplitude on song features.
3. Results
Plumbeous vireos were less common and detected at 15 per cent of all survey locations (n = 190), and grey vireos were detected at 43 per cent of them. Increases in noise amplitude influenced neither grey nor plumbeous vireo occupancy rates (both, z < 0.71, p > 0.48). For signal features, peak frequency was uninfluenced by noise amplitude for both species (plumbeous, F1,39 = 1.15, r2 < 0.01, p = 0.29; grey, F1,19 = 2.44, r2 = 0.07, p = 0.13). However, across the range of noise exposure, plumbeous vireo minimum song frequency increased by over 700 Hz (F1,39 = 16.00, r2 = 0.27, p < 0.001), but grey vireo minimum frequency was unchanged (F1,19 = 0.002, p = 0.96; figure 2a). By contrast, plumbeous vireo maximum song frequency did not change with noise amplitude (F1,39 = 2.05, r2 = 0.03, p = 0.16), but grey vireo maximum frequency increased by over 900 Hz across the noise exposure range (F1,19 = 14.41, r2 = 0.40, p = 0.001; figure 2a). These changes resulted in a decrease in song frequency bandwidth by over 1.0 kHz for plumbeous vireos (F1,39 = 5.97, r2 = 0.11, p = 0.019), but an increase of 750 Hz in grey vireo frequency bandwidth (F1,19 = 5.53, r2 = 0.18, p = 0.030; figure 2a). Song duration was influenced by noise amplitude for both species, but the trends were opposite. Plumbeous vireo song duration decreased with an increase in noise (F1,39 = 7.99, r2 = 0.15, p = 0.007), yet grey vireo song duration was nearly 1.5 times longer in the noisiest areas compared with quiet areas (F1,19 = 13.13, r2 = 0.38, p = 0.002; figure 2b). For both species, song rate was uninfluenced by noise (plumbeous, F1,39 = 1.33, r2 < 0.01, p = 0.26; grey, F1,19 < 0.01, r2 < 0.01, p = 0.96).
Figure 2.
(a) Plumbeous vireo minimum frequency (black circles and solid line) and grey vireo maximum frequency (open squares and dashed line) increased with noise exposure. Horizontal solid and dashed lines denote mean values for plumbeous vireo maximum frequency and grey vireo minimum frequency, respectively. Vertical lines illustrate the change in bandwidth with increased noise exposure (plumbeous, dashed grey lines; grey, solid grey lines). (b) Plumbeous vireo song duration decreased with increased noise and grey vireo song duration increased (symbols as in (a)).
4. Discussion
Our results suggest that habitat occupancy rates for both vireos were uninfluenced by noise amplitude. By contrast, song frequency and song duration changed with noise amplitude for both; however, differing from expectations, no single song change was similar between the two species. Although restricted to a comparison of two congeners, these findings suggest that closely related species may not use the same noise-dependent signalling strategies to overcome masking by noise and, besides increasing vocal amplitude (the Lombard effect [11]), there may not be a single widespread signalling strategy used by songbirds in response to noisy conditions.
Noise-dependent shifts in low-frequency song features have been documented for several songbirds [3–7], yet fewer studies have reported coupled noise-dependent changes in frequency and song duration. For example, great tit (Parus major) songs with higher minimum frequencies are also shorter [4], which was also the case for the plumbeous vireo in this study. Additionally, house finches (Carpodacus mexicanus) confronted with noise employ two strategies: they either sing shorter songs with a higher minimum frequency or sing longer syllables with no change in frequency [7]. In this study, grey vireo song increased in both maximum frequency and song duration with increased noise levels; both changes may improve signal detection by a receiver [10,11], although a frequency increase to an already high-frequency feature may not increase detection over greater distances [13]. This coupled increase is surprising given that previous studies have not found increases to both of these features simultaneously [4,7], but may even suggest that performance trade-offs might exist between them (i.e. an increase in minimum frequency at the cost of shorter songs). Nevertheless, because species differ in their reliance on particular song features when assessing threats by conspecific competitors or choosing mates [14], it should not be surprising if we are to find that noise-dependent signal changes are species-specific to balance signal transmission with reliable indicators used for recognition of species identity and individual condition.
Finally, it is important to recognize that our data are correlative. Growing evidence suggests that signal adjustments in response to noise are made at the individual level [6,7] and, although other possibilities exist, such as noise-dependent frequency shifts representing epiphenomena of singing louder (see discussion in [5,13]), it is likely that differences in vireo song also reflect individual behavioural adjustments. That habitat occupancy rates for both species were unchanged with increased noise, yet both appear to have noise-dependent vocal flexibility, supports the idea that signal change may help some species persist in noisy areas [4,5]. However, because the vireos' songs changed in different ways, this prompts important questions regarding the predictability of noise-dependent signal changes across species and the potential trade-offs associated with different signalling strategies that birds might use in an increasingly noisy world.
Acknowledgements
We thank our many research assistants. We also thank anonymous reviewers for useful comments on earlier versions of this paper. This work was supported by NSF (IOS-0910092), the United States Bureau of Land Management, ConocoPhillips, Williams Energy and the University of Colorado. C.D.F. was supported by the National Evolutionary Synthesis Center (NSF EF-0905606).
References
- 1.Slabbekoorn H., Smith T. B. 2002. Birdsong, ecology, and speciation. Phil. Trans. R. Soc. Lond. B 357, 493–503 10.1098/rstb.2001.1056 (doi:10.1098/rstb.2001.1056) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barber J. R., Crooks K. R., Fristrup K. M. 2010. The costs of chronic noise exposure for terrestrial organisms. Trends Ecol. Evol. 25, 180–189 10.1016/j.tree.2009.08.002 (doi:10.1016/j.tree.2009.08.002) [DOI] [PubMed] [Google Scholar]
- 3.Slabbekoorn H., Ripmeester E. A. 2008. Birdsong and anthropogenic noise: implications and applications for conservation. Mol. Ecol. 17, 72–83 10.1111/j.1365-294X.2007.03487.x (doi:10.1111/j.1365-294X.2007.03487.x) [DOI] [PubMed] [Google Scholar]
- 4.Slabbekoorn H., den Boer-Visser A. 2006. Cities change the songs of birds. Curr. Biol. 16, 2326–2331 10.1016/j.cub.2006.10.008 (doi:10.1016/j.cub.2006.10.008) [DOI] [PubMed] [Google Scholar]
- 5.Francis C. D., Ortega C. P., Cruz A. 2010. Vocal frequency change reflects different responses to anthropogenic noise in two suboscine tyrant flycatchers. Proc. R. Soc. B 277 10.1098/rspb.2010.1847 (doi:10.1098/rspb.2010.1847) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gross K., Pasinelli G., Kunc H. P. 2010. Behavioral plasticity allows short-term adjustment to a novel environment. Am. Nat. 176, 456–464 10.1086/655428 (doi:10.1086/655428) [DOI] [PubMed] [Google Scholar]
- 7.Bermúdez-Cuamatzin E., Rios-Chelen A. A., Gil D., Garcia C. M. 2010. Experimental evidence for real-time song frequency shift in response to urban noise in a passerine bird. Biol. Lett. 7, 36–38 10.1098/rsbl.2010.0437 (doi:10.1098/rsbl.2010.0437) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Francis C. D., Ortega C. P., Cruz A. 2009. Noise pollution changes avian communities and species interactions. Curr. Biol. 19, 1415–1419 10.1016/j.cub.2009.06.052 (doi:10.1016/j.cub.2009.06.052) [DOI] [PubMed] [Google Scholar]
- 9.Poole A. (ed.) 2005. The birds of North America online. Ithaca, NY: Cornell Lab of Ornithology; See http://bna.birds.cornell.edu/BNA/ [Google Scholar]
- 10.Wiley R. H. 2006. Signal detection and animal communication. Adv. Stud. Behav. 36, 217–247 10.1016/S0065-3454(06)36005-6 (doi:10.1016/S0065-3454(06)36005-6) [DOI] [Google Scholar]
- 11.Brumm H., Voss K., Köllmer I., Todt D. 2004. Acoustic communication in noise: regulation of call characteristics in a New World monkey. J. Exp. Biol. 207, 443–448 10.1242/jeb.00768 (doi:10.1242/jeb.00768) [DOI] [PubMed] [Google Scholar]
- 12.Pacifici K., Simons T. R., Pollock K. H. 2008. Effects of vegetation and background noise on the detection process in auditory avian point count surveys. Auk 125, 600–607 10.1525/auk.2008.07078 (doi:10.1525/auk.2008.07078) [DOI] [Google Scholar]
- 13.Nemeth E., Brumm H. 2010. Birds and anthropogenic noise: are urban songs adaptive? Am. Nat. 176, 465–475 10.1086/656275 (doi:10.1086/656275) [DOI] [PubMed] [Google Scholar]
- 14.Collins S. A. 2004. Vocal fighting and flirting: the function of birdsong. In Nature's Music. The science of birdsong (eds Marler P., Slabbekoorn H.), pp. 39–79 San Diego, CA: Elsevier Academic Press [Google Scholar]