Abstract
The extinct thylacine (Thylacinus cynocephalus) and the extant grey wolf (Canis lupus) are textbook examples of convergence between marsupials and placentals. Craniodental studies confirm the thylacine's carnivorous diet, but little attention has been paid to its postcranial skeleton, which would confirm or refute rare eyewitness reports of a more ambushing predatory mode than the pack-hunting pursuit mode of wolves and other large canids. Here we show that thylacines had the elbow morphology typical of an ambush predator, and propose that the ‘Tasmanian tiger’ vernacular name might be more apt than the ‘marsupial wolf’. The ‘niche overlap hypothesis’ with dingoes (Canis lupus dingo) as a main cause of thylacine extinction in mainland Australia is discussed in the light of this new information.
Keywords: Thylacinus cynocephalus, predatory behaviour, elbow joint, convergence, niche overlap, extinction
1. Introduction
The recently extinct thylacine (Thylacinus cynocephalus: Thylacinidae, Dasyuroidea, Marsupialia) was one of the largest known Australian marsupial carnivores (body mass approx. 25 kg), and the largest in historical times, termed the ‘marsupial wolf’ (owing to its superficially dog-like appearance) or the ‘Tasmanian tiger’ (owing to its striped coat). Thylacines were last known on the Australian mainland 3000 years ago [1], shortly following the appearance of dingoes (Canis lupus dingo) around 4000 years ago. The restriction of the thylacine to its historical range in Tasmania is often considered to be due to competition with this canid, although debate exists about the potential extent of niche overlap [2]. Unfortunately, the thylacine was extinct before its ecology and behaviour were well documented [3].
The thylacine is one of the most cited examples of evolutionary convergence, proposed as the marsupial equivalent of the wolf (e.g. [4], and almost any biology textbook). The species name, ‘cynocephalus’ (from the Greek kyno- and kephàlè), means ‘dog head’, and the thylacine skull does indeed bear an extraordinary resemblance to canids such as dogs and wolves [5], while the body is rather dog-like and the claws non-retractile [3]. Craniodental studies indicate that thylacines probably fed exclusively on meat, in a similar fashion to extant large canids [3,6–9]. However, considerations of convergence in terms of predatory behaviour are rare, although some authors have noted that thylacine postcrania were not indicative of the cursorial abilities of a wolf-like pursuit predator. (e.g. [1,3,10,11]). Wroe et al. [7] concluded that thylacines specialized on smaller sized prey, leading them to suggest that thylacines did not purse large prey in packs. However, the postcranial anatomy of the thylacine has not been compared with that of placental carnivores in any quantitative fashion.
An established morphological indicator of predatory behaviour can be found in the elbow joint, specifically in the shape of the distal humerus. Pursuit carnivores have a restricted range of motion at the elbow, while less cursorial predators retain the ability to supinate the forearm, and may use the forelimb to grapple with their prey [12,13]. Here, we employ geometric morphometrics (GM) to explore the morphology of the distal humerus of placental carnivores of known predatory behaviour. The intent is to characterize the likely predatory mode of the thylacine to consider how this may impact hypotheses relating to competition with invasive dingoes.
2. Material and methods
We measured 103 humeri of adult individuals belonging to 32 species of mammals (see electronic supplementary material, table S1), including eight thylacines from Tasmania.
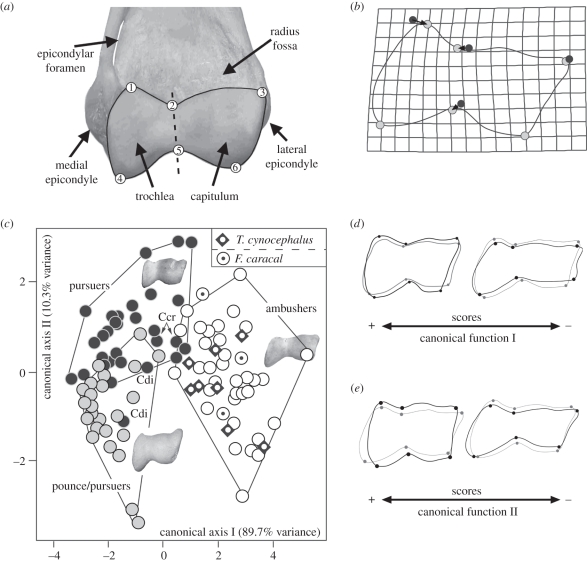
We employ the approach of Andersson [12,13] for capturing the shape of the distal humerus but using GM. Six landmarks of the posterodistal articular surface of the humerus were digitized on digital images (figure 1a). We used GM instead of traditional methods because: (i) the size of the specimens is removed by Procrustes (excepting those size effects related to allometry), (ii) GM allows the analysis of geometry about the relative positions of landmarks, and (iii) the results of multivariate analyses could be visualized as thin plate spline (TPS) diagrams.
Figure 1.
(a) Humeral trochlea of T. cynocephalus (AMNH-35866) showing the landmarks used in the morphometric analysis. (b) Transformation grid from the mean marsupial shape (excluding T. cynocephalus) to the average shape of T. cynocephalus (black circles, mean elbow shape; grey circles, T. cynocephalus). (c) Morphospace depicted from the first two canonical functions obtained from CVA. (d) Elbow shape variation accounted for by the first canonical function. (e) Elbow shape variation accounted for by the second canonical function. We show also the position of Felis caracal as an example of a species with similar variability to T. cynocephalus in the canonical axes scores, and some other species are shown for clarity. Abbreviations: Ccr, Crocuta crocuta; Cdi, Canis lupus dingo.
All the specimens were aligned using Procrustes (e.g. [14]). TPS functions were interpolated for deriving the uniform and non-uniform components of shape. The partial warp scores were calculated giving equal weights to all spatial scales (α = 0).
We performed a canonical variates analysis (CVA) from the partial warp scores to determine the features that best distinguish among different types of predatory behaviours. Following Van Valkenburgh [15], we classified extant species as follows: (i) ambush predators usually stalk their prey and may pursue them over short distances, and the forelimbs may be used to grapple with large prey, (ii) pounce/pursuit predators usually hunt small prey using either a pounce or short chase, and rarely grapple with their prey, (iii) pursuit predators usually chase their prey for a long distance (greater than 30 m), and may hunt cooperatively to bring down large prey, but do not grapple with their prey. However, these hunting types are usually correlated with prey size. For example, both ambush and pursuit predation can be directly linked to forearm mobility and elbow function in the case of large prey, but a predator will not ‘pounce’ on large prey nor ‘pursue’ small prey. We follow Ewer [16] in considering the cheetah (Acinonyx jubatus), the only highly cursorial felid, to be a pursuit predator. As there is debate about this classification of the cheetah, we repeated the analysis in two different ways: considering the cheetah as an ambusher and excluding cheetah from the analysis. In both cases, the results were similar to those presented below (electronic supplementary material, figures S1 and S2). Additionally, a TPS diagram was computed to illustrate the shape change of the average elbow shape in T. cynocephalus from the mean shape of other marsupials.
The reliability of the discrimination among the groups compared and the group assignation of the thylacine (introduced as an unknown) were assessed by the leave-one-out cross-validation method (e.g. [17]) and from the value of Wilks' lambda statistic (λ). All GM procedures were performed using TPSdig v. 1.4 and TPSRelw v. 1.49 [18,19] and the CVA were performed with the SPSS v. 15.0.
3. Results
Figure 1b shows how the thylacine elbow is shaped relative to other marsupials, in order to address any uncertainties about possible marsupial-biased phylogenetic effects. Figure 1c shows the bivariate plot depicted from the two canonical functions obtained from elbow shape. The first canonical function (figure 1c) mainly separates out ambush predators according to one set of morphological traits (figure 1d). In contrast, the second canonical function mainly separates pursuit predators from pounce/pursuit predators in accordance with a different set of morphological traits (figure 1e). Wilks' lambda statistic is significant for both the first (λ = 0.140; χ2 = 173.716; d.f. = 16; p < 0.0001) and second (λ = 0.691; χ2 = 32.745; d.f. = 7; p < 0.0001) functions. The percentage of correct assignments was 80.0 per cent, using the leave-one-out method. All the thylacines clustered with the pantherine cats, and they were also classified as ambush predators by CVA, with an average probability of 95 per cent [17].
4. Discussion
The CVA of the shape of the distal humerus clearly distinguishes among pursuers, pounce/pursuers and ambushers, demonstrating the utility of the elbow shape as a morphological indicator of predatory behaviour in carnivorans. The elbow morphology reflects the fact that cursorial locomotion and manual manipulation are conflicting functions [12,13]; the price paid by pursuit predators (and, to a lesser extent, by pounce/pursuit predators) for limbs adapted for speed and locomotor efficiency is the restriction of the range of joint motion to the parasagittal plane, and hence the loss of the ability to supinate the forearm, which is essential for grappling with prey.
As shown in figure 1b, the thylacine plots within the range of the elbow shape variation of living ambushers. Some spotted hyaenas (pursuit predators) were plotted close to some thylacines; however, given that all the thylacines were classified as ambushers, this does not provide evidence that the elbow of the thylacine is close to that of pursuers. Rather, this may be an example of behaviour preceding morphology in the case of the spotted hyaena.
This positioning of the thylacine does not necessarily reflect a specialized felid-like predatory behaviour of grappling with the prey, especially as thylacines lack the retractile claws of felids. Rather it shows the retention of the ability to supinate the forelimb, unlike the condition seen in pursuit predators. Thus, the anatomy of the distal humerus of the thylacine shows that it was most likely to have been an ambusher of some sort, and very unlikely to have had canid-like pursuit predatory behaviour. However, it is possible that all marsupials are constrained to retain the capacity to pronate/supinate the elbow owing to constraints on the forelimb morphology necessary for the neonate to crawl to the pouch (e.g. [20]). Thus, the marsupial reproductive strategy might actually limit the capacity of marsupials to become cursorially-adapted pursuit predators.
Despite the canid-like skull, the postcrania of the thylacine more closely resemble the generalized felid condition than the more specialized canid one, and the elbow morphology bears even less resemblance to that of known extant pounce/pursuit predators than to pursuit predators. Additionally, the dentition of T. cynocephalus, as in all felids, is that of a true hypercarnivore, dominated by vertical shear and lacking the versatility in tooth morphology typical of most canids.
In summary, we propose that the thylacine was more of an ambush predator than the living grey wolf, which is often considered as its ecological counterpart. We provide quantitative support to the suspicions of earlier researchers that the thylacine was not a pursuit predator, thus bringing into question the degree of ecomorphological convergence between thylacines and wolves. In fact, the predatory behaviour of the thylacine was probably closer to that of ambushing felids than to that of large pursuit canids. Consequently, at least in terms of the postcranial anatomy, the vernacular name of ‘Tasmanian tiger’ may be more apt than that of ‘marsupial wolf’.
If there is not as great an ecomorphological convergence between the thylacine and large placental canids, particularly wolves or dingoes, as has usually been assumed, the reliability of the ‘niche overlap hypothesis’ with immigrant canids (i.e. dingoes) as a potential cause for the extinction of the thylacine in mainland Australia is not so evident. Dingoes are pursuit or pounce/pursuit predators [21], thus probably employing a different predatory mode from thylacines. Consequently, the ‘niche overlap hypothesis’ as an explanation for the mainland thylacine extinction could have been overstated. However, the social behaviour of dingoes, and the possibility of competition for the same prey, albeit with a differing predatory mode, may still have played a role in excluding thylacines from the mainland, as evidenced by the survival of thylacines in dingo-free Tasmania.
Acknowledgements
We thank Eileen Westwig and Louisse Tomsett for providing access to specimens, and Alberto Martín-Serra, Sergio Vizcaíno, Marcos Ercoli and Pancho Prevosti for additional data. This study was supported by a Fulbright Postdoctoral grant (FU2009-0184) to B.F. and a Bushnell Foundation grant to C.J.
References
- 1.Smith M. 1982. Review of the thylacine (Marsupialia, Thylacinidae). In Carnivorous marsupials (ed. Archer M.), pp. 237–253 Sydney, Australia: Royal Zoological Society of New South Wales, Surrey [Google Scholar]
- 2.Johnson C. N., Wroe S. 2003. Causes of extinction of vertebrates during the Holocene of mainland Australia: arrival of the dingo, or human impact? Holocene 13, 941–948 10.1191/0959683603hl682fa (doi:10.1191/0959683603hl682fa) [DOI] [Google Scholar]
- 3.Jones M. E., Stoddart D. M. 1998. Reconstruction of the predatory behaviour of the extinct marsupial thylacine (Thylacinus cynocephalus). J. Zool. (Lond.) 246, 239–246 10.1111/j.1469-7998.1998.tb00152.x (doi:10.1111/j.1469-7998.1998.tb00152.x) [DOI] [Google Scholar]
- 4.Simpson G. G. 1965. The geography of evolution. New York, NY: Chilton Books [Google Scholar]
- 5.Werdelin L. 1987. Jaw geometry and molar morphology in marsupial carnivores: analysis of a constraint and its macroevolutionary consequences. Paleobiology 13, 342–350 [Google Scholar]
- 6.Wroe S., McHenry C., Thomason J. 2005. Bite club: comparative bite force in big biting mammals and the prediction of predatory behaviour in fossil taxa. Proc. R. Soc. B 272, 619–625 10.1098/rspb.2004.2986 (doi:10.1098/rspb.2004.2986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wroe S., Clausen P., McHenry C., Moreno K., Cunningham E. 2007. Computer simulation of feeding behaviour in the thylacine and dingo as a novel test for convergence and niche overlap. Proc. R. Soc. B 274, 2819–2828 10.1098/rspb.2007.0906 (doi:10.1098/rspb.2007.0906) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wroe S., Milne N. 2007. Convergence and remarkably consistent constraint in the evolution of carnivore skull shape. Evolution 61, 1251–1260 10.1111/j.1558-5646.2007.00101.x (doi:10.1111/j.1558-5646.2007.00101.x) [DOI] [PubMed] [Google Scholar]
- 9.Goswami A., Milne N., Wroe S. In press Biting through constraints: cranial morphology, disparity and convergence across living and fossil carnivorous mammals. Proc. R. Soc. B. 10.1098/rspb.2010.2031 (doi:10.1098/rspb.2010.2031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keast A. 1982. The thylacine (Thylacinidae, Marsupialia): how good a pursuit carnivore? In Carnivorous marsupials (ed. Archer M.), pp. 675–684 Sydney, Australia: Royal Zoological Society of New South Wales [Google Scholar]
- 11.Wroe S., Lowry M. B., Anton M. 2008. How to build a mammalian super-predator? Zoology 111, 196–203 10.1016/j.zool.2007.07.008 (doi:10.1016/j.zool.2007.07.008) [DOI] [PubMed] [Google Scholar]
- 12.Andersson K., Werdelin L. 2003. The evolution of cursorial carnivores in the Tertiary: implications of elbow–joint morphology. Proc. R. Soc. Lond. B 270, S163–S165 10.1111/j.1096-3642.2004.00129.x (doi:10.1111/j.1096-3642.2004.00129.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andersson K. 2004. Elbow-joint morphology as a guide to forearm function and foraging behaviour in mammalian carnivores. J. Zool. (Lond.) 142, 91–104 10.1111/j.1096-3642.2004.00129.x (doi:10.1111/j.1096-3642.2004.00129.x) [DOI] [Google Scholar]
- 14.Dryden I., Mardia K. 1998. Statistical shape analysis. Chichester, UK: Wiley [Google Scholar]
- 15.Van Valkenburgh B. 1985. Locomotor diversity within past and present guilds of large predatory mammals. Paleobiology 11, 406–428 [Google Scholar]
- 16.Ewer R. F. 1973. The carnivores. London, UK: Weidenfeld and Nicolson [Google Scholar]
- 17.Lachenbruch P. A. 1967. An almost unbiased method of obtaining confidence intervals for the probability of misclassification in discriminant analysis. Biometrics 23, 639–645 10.2307/2528418 (doi:10.2307/2528418) [DOI] [PubMed] [Google Scholar]
- 18.Rohlf F. J. 2004. TpsDig, v. 2.11. Digitize landmarks and outlines (computer program and documentation). Stony Brook, NY: Department of Ecology and Evolution, State University of New York at Stony Brook [Google Scholar]
- 19.Rohlf F. J. 2010. TpsRel, v. 1.49. Relative warp analysis (computer program and documentation). Stony Brook, NY: Department of Ecology and Evolution, State University of New York at Stony Brook [Google Scholar]
- 20.Sears K. E. 2004. Constraints on the morphological evolution of marsupial shoulder girdles. Evolution 58, 2353–2370 10.1111/j.0014-3820.2004.tb01609.x (doi:10.1111/j.0014-3820.2004.tb01609.x) [DOI] [PubMed] [Google Scholar]
- 21.Corbett L. 1995. The dingo in Australia and Asia. Sydney, Australia: University of New South Wales Press [Google Scholar]