Abstract
Variation in baseline glucocorticoid (cort) levels can be attributed, at least in part, to differences in energetic demands confronting individuals. Elevated baseline cort levels are routinely interpreted as indicating individuals in poor condition, with low relative fitness. However, when greater reproductive effort increases energetic demands, individuals with high cort might paradoxically be those with the highest fitness. Here, we experimentally test the hypothesis that increased reproductive demand causes increases in baseline cort (the Cort-Adaptation hypothesis). We measured maternal baseline cort before and after experimentally enlarging and reducing brood sizes in tree swallows (Tachycineta bicolor). Females with experimentally enlarged broods had greater increases in baseline cort and fledged more offspring than females with reduced broods. Additionally, females with greater increases in baseline cort had higher offspring-provisioning rates than females with lower changes in cort. These findings demonstrate that increased reproductive demand can cause increased baseline cort. As yet, we do not know if these increases in cort cause increased allocation of resources towards reproduction, but the positive relationship between parental behaviour and cort suggests that increased cort does not always interfere with reproductive investment, and might instead facilitate it.
Keywords: brood size manipulation, Cort-Adaptation hypothesis, corticosterone, parental behaviour, tree swallow, Tachycineta bicolor
1. Introduction
Glucocorticoid (cort) levels are increasingly used as a metric to assess individual or population health. Elevated baseline cort levels are generally interpreted as indicating an individual or population in poor condition and with lower relative fitness than individuals or populations with lower cort (e.g. [1,2]). However, when the relationships between baseline cort and fitness are directly evaluated, findings do not always support this interpretation (reviewed in [3]). The Cort-Adaptation hypothesis asserts that increased reproductive demand can cause an increase in baseline cort and reproductive effort [3,4]. This hypothesis provides a plausible, but largely untested, explanation for some previous findings of positive relationships between cort and fitness [4–6]. Here, we experimentally test one of the central predictions of the Cort-Adaptation hypothesis that increased reproductive demand should cause within-individual increases in baseline cort, by experimentally manipulating brood size in tree swallows (Tachycineta bicolor).
Previous studies investigating the effects of brood size manipulation on parental cort levels have yielded inconsistent findings [7,8], with only one study reporting the predicted positive effect of increased brood size on parental cort [9]. Many prior studies did not look at baseline levels and did not sample females, and none investigated within-individual changes in cort. Thus, our understanding of the influences of experimentally altered reproductive effort on baseline cort levels is limited. We measured baseline cort in females both before and after experimental brood size manipulation, allowing us to determine a within-individual response to the manipulation. As considerable individual variation in hormone levels exists, controlling for this variation is key to understanding hormone–behaviour relationships.
If increased brood size causes an increase in parental energetic demands, it probably does so by influencing behaviour. For example, if parents adjust their rate of food provisioning according to the number of offspring in their brood, then parents with experimentally augmented broods should encounter higher energetic demands and have increased baseline cort. These increases in baseline cort might be both a response to increased reproductive effort (i.e. parental workload) and also a cue that promotes allocation of resources towards reproduction. We tested the prediction that increased baseline cort would be associated with increased parental effort by investigating relationships between experimentally manipulated brood size, parental behaviour and within-individual changes in cort.
2. Material and methods
(a). Study system
We conducted this study in May–July 2010 in a free-ranging population of box-nesting tree swallows at the Queen's University Biological Station (Ontario, Canada, 44°34′ N, 76°19′ W, 135 m elevation). The study site consists of seven grids of 10–35 nest-boxes in hay fields, with uniform inter-box spacing. We regularly monitor all nest-boxes in the population for evidence of breeding activity from the beginning of breeding in early May until all pairs have completed breeding in July.
(b). Bird capture and sampling
We captured all adult females twice during breeding either with trap-doors set on the nest-box entryways or by placing our hands over the entryway when the bird was inside the box. Method of capture did not influence cort levels (t-test of all 2010 female cort levels, n = 33 by hand, 54 by trap, t = −1.06, p = 0.29). To control for diel variation in cort, all captures occurred between 08.55 and 11.50 h. We collected samples before brood size manipulation on day 3 or 4 of the incubation period, and collected post-manipulation samples on day 11 or 12 of the nestling period. At each time point, we collected a small (approx. 120 µl) blood sample within 3 min of capture to ensure that measured hormone levels do not reflect the stress of capture [10], and painted females with a thin line of white acrylic paint across the wing flight feathers to permit differentiation of males and females in videotaped observations. We stored all blood samples on ice until transport to the laboratory.
(c). Brood size manipulation
We conducted the brood size manipulation with 4-day-old nestlings. We paired broods that hatched within 24 h of each other, and randomly assigned each nest to one of three experimental treatments: control (n = 10), where two nestlings were removed and replaced with two nestlings from a paired control nest; reduced (n = 14), where two nestlings were removed and translocated into a paired enlarged nest, and enlarged (n = 14), where two nestlings were introduced from reduced nests. Because of mortality, sample sizes for hormone and fledging success analyses were reduced to eight controls, 10 reduced and seven enlarged broods. Mean-manipulated brood size was 3.10 (reduced), 5.13 (control) and 7.29 (enlarged) nestlings.
(d). Behavioural observations
We videotaped parental behaviour at 16 nests (n = 7 control, 4 reduced and 5 enlarged broods) for a minimum of 3.9 h (mean 4.4 h) on day 10 of the nestling period between 06.30 and 19.15 h using cameras set at a minimum of 20 m from the nest-box. We conducted recordings of paired broods at the same time on the same day when possible (i.e. when nestlings from both nests of the pair survived to the day of recording). We scored the hourly rate of parental visitation to the nest-box (a proxy of offspring food provisioning rate) for males and females. To calculate provisioning rate, we considered the duration of the recording from the time of the first parental visit to the nest-box to the time of the last visit, to minimize effects of the disturbance associated with set-up and removal of the cameras.
(e). Hormone measurement
We centrifuged blood samples within 8 h of collection to separate plasma, which was then stored at −20°C until assay. We quantified plasma levels of total corticosterone (the primary cort in birds) in each sample in duplicate through direct radioimmunoassay, following extraction with re-distilled dichloromethane (see [11] for details). Within-assay variation among replicate known-concentration standard samples was 13 per cent (six standards).
(f). Statistical analyses
To test the hypothesis that increased reproductive effort causes an increase in maternal baseline cort levels, we used a generalized linear model (GLM) with change in a female's cort level (calculated as post-manipulation cort divided by pre-manipulation cort, log-transformed for normality) as the dependent variable and experimental group (control, reduced or enlarged), date of the post-manipulation sample and an interaction term as factors. For all of our analyses, we identified the best-fit model using Akaike's Information Criterion [12]. Thus date and the interaction term were dropped from the final model, as they did not significantly improve the model fit. Once the best model was obtained, we used post hoc t-tests to compare mean log-transformed change in cort among groups.
To determine what effect our manipulation had on reproductive success, we used a GLM with a Poisson distribution, with number of offspring fledged as the dependent variable and experimental group, egg-laying date and an interaction term as factors. We also conducted a similar analysis with proportion of offspring fledged (number of offspring fledged divided by number of offspring in the brood following the experimental manipulation) as the dependent variable, again using a GLM with a Poisson distribution. In both analyses, the best-fit model only included experimental group. We used post hoc t-tests of significant effects to compare the mean numbers of offspring fledged among groups.
Finally, to test the hypothesis that increased cort levels would be associated with increased parental effort, we ran a GLM with log-transformed maternal offspring-provisioning rate as the dependent variable and change in maternal cort, paternal provisioning rate, experimental group, time of day, date of recording and brood size as factors. The best-fit model only included change in maternal cort.
3. Results
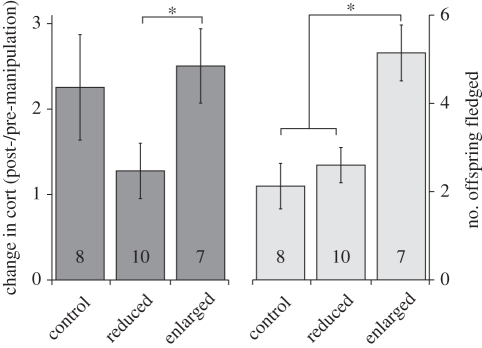
Females with experimentally enlarged broods had baseline cort levels that increased more than females with reduced broods. Changes in cort in females with control-manipulated broods were intermediate, and not significantly different from females with reduced or enlarged broods (figure 1; GLM, experimental group χ2 = 6.04, p = 0.04; post hoc t-tests: enlarged versus reduced t = 2.50, p = 0.03, enlarged versus control t = 0.64, p = 0.53, reduced versus control t = −1.63, p = 0.12).
Figure 1.
Female tree swallows with experimentally enlarged broods had greater increases in baseline cort (mean ± s.e., post-manipulation cort divided by pre-manipulation cort; dark-shaded bars on left) than females with reduced broods. Additionally, females with experimentally enlarged broods had higher fitness (mean ± s.e., number of offspring fledged; light-shaded bars on right) than females with reduced or control broods. Asterisks indicate statistically significant differences among groups. Sample sizes are provided within the bars.
Females with experimentally enlarged broods fledged more offspring than control or reduced-brood females (figure 1; GLM, experimental group χ2 = 11.43, p < 0.01; post hoc t-tests: enlarged versus reduced t = 3.39, p < 0.01, enlarged versus control t = 3.70, p < 0.01, reduced versus control t = 0.73, p = 0.48). Proportion of offspring fledged did not differ among groups (GLM, experimental group χ2 = 1.02, p = 0.60).
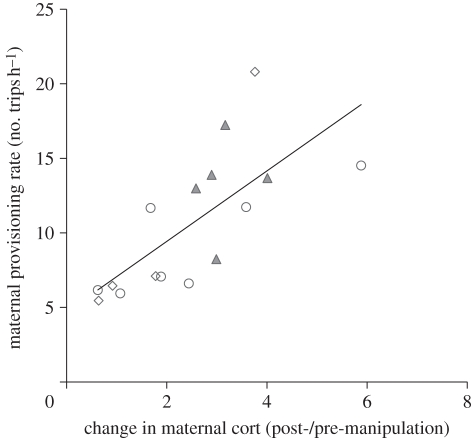
Females that provisioned their offspring at a higher rate had baseline cort levels that increased more than females that provisioned at a lower rate (figure 2; GLM, χ2 = 14.25, p < 0.001).
Figure 2.
Maternal offspring-provisioning rate (number of trips to the nest per hour) plotted against change in baseline cort (post-manipulation cort divided by pre-manipulation cort). Female tree swallows with greater increases in cort provisioned their offspring at a higher rate (GLM, χ2 = 14.25, p < 0.001). Reduced brood, open diamonds; control, open circles; increased, filled triangles.
4. Discussion
Here, we provide the first demonstration of within-individual increases in baseline cort levels in response to experimentally augmented reproductive demand. Female tree swallows with enlarged broods had cort levels on average more than twice their pre-manipulation cort levels, whereas females with reduced broods had cort levels similar to their pre-manipulation levels (figure 1). Our manipulation seemed to effectively push females in the two experimental groups away from the intermediate increase in cort seen in control females. These findings differ from several previous studies that failed to find increased baseline cort in response to increased brood size (e.g. [7,8]). No previous studies have investigated within-individual responses in baseline cort secretion, so we cannot determine whether the differences in our findings are due to this methodological difference or more fundamental differences among species. However, if we had not measured a within-female response, we would not have detected an effect of our manipulation on cort levels, underscoring the importance of these within-individual measures.
Interestingly, females with enlarged broods fledged more offspring (figure 1). The proportion of offspring fledged did not differ among treatment groups, suggesting that females with enlarged broods did not achieve increased fledging success solely because they had more offspring available owing to our manipulation, but because they were able to respond to the increased needs of their enlarged broods. Furthermore, females that had greater increases in baseline cort provisioned their offspring at a higher rate (figure 2). These findings follow the central prediction of the Cort-Adaptation hypothesis—that increased reproductive demand and parental workload cause increased baseline cort.
The increase in baseline cort of females in the enlarged brood group might have enabled them to respond to the increased needs of their broods. However, increases in cort are generally described as inhibitory to reproduction. Our findings, along with previous reports of positive associations between cort and reproductive effort (reviewed in [3]), challenge this view [13]. To explain this apparent paradox, we propose the Context-Dependent hypothesis, which asserts that when the fitness value of current reproduction is high, increased cort promotes reallocation of resources towards reproduction, whereas when the value of reproduction is low, increased cort has the commonly expected effect of reducing allocation to reproduction. In contrast to the Cort-Adaptation hypothesis, this hypothesis focuses on the context-dependent role that cort might play in resource allocation, and remains untested.
Elevated cort levels have traditionally been interpreted as indicating an individual or population in poor condition, and with low relative fitness [3]. However, when among-individual variation in cort levels is driven by variation in reproductive effort, individuals with the highest reproductive effort, and potentially higher relative fitness, have greater increases in cort. Such findings should serve as a caution, particularly for the use of cort as a measure of individual or population health in species of conservation concern.
Acknowledgements
We acknowledge funding from Queen's University's Summer Work Experience Programme, the U.S. National Science Foundation (ITM, IOS-0545735), and the Natural Sciences and Engineering Research Council of Canada (R.J.R.). We thank E. Dobson, C. Griffith, E. Murray, T. Willis and S. Zhang for assisting with data collection, members of the Martin and Bonier laboratories and two anonymous referees for comments on the manuscript, and the staff of the Queen's University Biological Station for logistical support.
References
- 1.Creel S., Fox J. E., Hardy A., Sands J., Garrott B., Peterson R. O. 2002. Snowmobile activity and glucocorticoid stress responses in wolves and elk. Conserv. Biol. 16, 809–814 10.1046/j.1523-1739.2002.00554.x (doi:10.1046/j.1523-1739.2002.00554.x) [DOI] [Google Scholar]
- 2.Wasser S. K., Bevis K., King G., Hanson E. 1997. Noninvasive physiological measures of disturbance in the northern spotted owl. Conserv. Biol. 11, 1019–1022 10.1046/j.1523-1739.1997.96240.x (doi:10.1046/j.1523-1739.1997.96240.x) [DOI] [Google Scholar]
- 3.Bonier F., Martin P. R., Moore I. T., Wingfield J. C. 2009. Do baseline glucocorticoids predict fitness? Trends Ecol. Evol. 24, 634–642 10.1016/j.tree.2009.04.013 (doi:10.1016/j.tree.2009.04.013) [DOI] [PubMed] [Google Scholar]
- 4.Bonier F., Moore I. T., Martin P. R., Robertson R. J. 2009. The relationship between fitness and baseline glucocorticoids in a passerine bird. Gen. Comp. Endocrinol. 163, 208–213 10.1016/j.ygcen.2008.12.013 (doi:10.1016/j.ygcen.2008.12.013) [DOI] [PubMed] [Google Scholar]
- 5.Magee S. E., Neff B. D., Knapp R. 2006. Plasma levels of androgens and cortisol in relation to breeding behavior in parental male bluegill sunfish Lepomis macrochirus. Horm. Behav. 49, 598–609 10.1016/j.yhbeh.2005.12.003 (doi:10.1016/j.yhbeh.2005.12.003) [DOI] [PubMed] [Google Scholar]
- 6.Chastel O., Lacroix A., Weimerskirch H., Gabrielsen G. W. 2005. Modulation of prolactin but not corticosterone responses to stress in relation to parental effort in a long-lived bird. Horm. Behav. 47, 459–466 10.1016/j.yhbeh.2004.10.009 (doi:10.1016/j.yhbeh.2004.10.009) [DOI] [PubMed] [Google Scholar]
- 7.Saino N., Incagli M., Martinelli R., Möller A. P. 2002. Immune response of male barn swallows in relation to parental effort, corticosterone plasma levels, and sexual ornamentation. Behav. Ecol. 13, 169–174 10.1093/beheco/13.2.169 (doi:10.1093/beheco/13.2.169) [DOI] [Google Scholar]
- 8.Lendvai Á. Z., Giraudeau M., Chastel O. 2007. Reproduction and modulation of the stress response: an experimental test in the house sparrow. Proc. R. Soc. B 274, 391–397 10.1098/rspb.2006.3735 (doi:10.1098/rspb.2006.3735) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silverin B. 1982. Endocrine correlates of brood size in adult pied flycatchers, Ficedula hypoleuca. Gen. Comp. Endocrinol. 47, 18–23 10.1016/0016-6480(82)90078-8 (doi:10.1016/0016-6480(82)90078-8) [DOI] [PubMed] [Google Scholar]
- 10.Romero L. M., Reed J. M. 2005. Collecting baseline corticosterone samples in the field: is under 3 min good enough? Comp. Biochem. Physiol. 140, 73–79 10.1016/j.cbpb.2004.11.004 (doi:10.1016/j.cbpb.2004.11.004) [DOI] [PubMed] [Google Scholar]
- 11.Wingfield J. C., Vleck C. M., Moore M. C. 1992. Seasonal changes of the adrenocortical response to stress in birds of the Sonoran Desert. J. Exp. Zool. 264, 419–428 10.1002/jez.1402640407 (doi:10.1002/jez.1402640407) [DOI] [PubMed] [Google Scholar]
- 12.Burnham K. P., Anderson D. R. 2002. Model selection and multimodel inference: a practical information-theoretic approach. 2nd edn. New York, NY: Springer [Google Scholar]
- 13.Moore I. T., Jessop T. S. 2003. Stress, reproduction, and adrenocortical modulation in amphibians and reptiles. Horm. Behav. 43, 39–47 10.1016/S0018-506X(02)00038-7 (doi:10.1016/S0018-506X(02)00038-7) [DOI] [PubMed] [Google Scholar]