Abstract
Host manipulation by parasites not only captures the imagination but has important epidemiological implications. The conventional view is that parasites face a trade-off between the benefits of host manipulation and their costs to fitness-related traits, such as longevity and fecundity. However, this trade-off hypothesis remains to be tested. Dinocampus coccinellae is a common parasitic wasp of the spotted lady beetle Coleomegilla maculata. Females deposit a single egg in the haemocoel of the host, and during larval development the parasitoid feeds on host tissues. At the prepupal stage, the parasitoid egresses from its host by forcing its way through the coccinellid's abdominal segments and begins spinning a cocoon between the ladybird's legs. Remarkably, D. coccinellae does not kill its host during its development, an atypical feature for parasitoids. We first showed under laboratory conditions that parasitoid cocoons that were attended by a living and manipulated ladybird suffered less predation than did cocoons alone or cocoons under dead ladybirds. We then demonstrated that the length of the manipulation period is negatively correlated with parasitoid fecundity but not with longevity. In addition to documenting an original case of bodyguard manipulation, our study provides the first evidence of a cost required for manipulating host behaviour.
Keywords: host–parasitoid interactions, bodyguard manipulation, manipulative costs, trade-off
1. Introduction
Parasites have evolved various strategies to exploit their hosts, and the manipulation of host behaviour is without doubt the one that most strongly captures our imagination. Phenotypic alterations displayed by parasitized hosts are extremely diversified, ranging from small changes in the frequency or duration of a given activity to the display of novel, and sometimes spectacular behaviours, physiologies and/or morphologies. The idea that elements of an animal's behaviour can be the extended phenotype of a parasite's genes [1] is not only fascinating but has important epidemiological implications [2,3], and could even help to address the complexity of mechanisms that orchestrate human behaviour [4].
There is a conventional wisdom that parasites face a trade-off between the benefits of manipulation and the direct costs to fitness-related traits (e.g. longevity, size, fecundity). Although a few empirical cases suggest the existence of manipulative costs [5,6], this trade-off hypothesis remains to be tested [7].
Dinocampus coccinellae Schrank (Hymenoptera: Braconidae) is a solitary endoparasitoid of the spotted lady beetle Coleomegilla maculata Lengi (Coleoptera: Coccinellidae). Following larval development within the host's abdomen (approx. 20 days at 25°C), the parasitoid larva egresses from the host (figure 1a), spins a cocoon between the ladybird's legs (figure 1b), and initiates pupation. Remarkably, D. coccinellae does not kill its host during development, an atypical feature for parasitoids (but see [8]). Both in the laboratory and under field conditions, we observed that throughout parasitoid pupation (approx. 7 days), the coccinellid, which is partially paralysed, displays a grasping behaviour on top of the cocoon and twitches at irregular intervals, especially when disturbed (electronic supplementary material, video 1). The proximate causes of this host manipulation remain unclear, but it presumably results from venoms left by the larva when egressing, since the behavioural changes begin at this moment. Active larval secretions have been shown to be involved in host regulation by parasitoids [9].
Figure 1.
The parasitoid Dinocampus coccinellae and its host the ladybird Coleomegilla maculata. (a) Parasitoid larva egressing from the ladybird (photograph by M. Bélanger Morin). (b) Ladybird attending a parasitoid cocoon (photograph by F. Maure).
We hypothesized that this attending behaviour results from host manipulation by the parasitoid to convert the ladybird into a bodyguard. Because parasitoids are vulnerable to predation and hyperparasitism at the pupal stage [10], we tested whether host attending behaviour protects parasitoid pupae from their natural enemies [11]. We then tested whether the pupae benefiting from this protection paid a cost in terms of their reproductive potential and/or longevity after emergence.
2. Material and methods
(a). Host, parasitoid and predator
Adult C. maculata (more than 4000) were collected in 2009 at Saint-Mathieu-de-Beloeil, Québec, Canada. Ladybirds were reared in plastic mesh boxes (946 ml, Ziploc), provided with moistened paper strips (multi-purpose paper, Staples) and fed ad libitum with pollen and aphids (Aphis glycines Matsumura and Acyrthosiphon pisum Harris (Hemiptera Aphididae)). Emerging parasitoids were used to start a laboratory colony. Adult C. maculata were daily exposed in a mesh cage (35.5 cm × 20 cm × 19 cm in height) to female D. coccinellae at a 2 : 1 ratio for 24 h. Parasitized C. maculata were transferred into plastic mesh boxes and fed as described above until parasitoid egression. Emerging D. coccinellae adults were fed with 20 per cent sugar water and droplets of pure honey.
Third instar green lacewing larvae Chrysoperla carnea (Neuroptera: Chrysopidae; approx. 8 mm in length) were used for the predation test. Lacewings are polyphagous predators that are frequently associated with aphid colonies. They were purchased from Plant Prod Québec, reared in a plastic mesh box (2.25 l, Ziploc), and fed with A. glycines and water.
All insects were reared in a growth chamber (Conviron E15) at 24 ± 1°C, 50 per cent relative humidity and 16 L : 8 D photoperiod.
(b). Predation tests
We tested the bodyguard hypothesis by examining the susceptibility of parasitoid cocoons to predation using the following three treatments: (i) parasitoid cocoons alone (the ladybirds were removed), (ii) parasitoid cocoons attended by a living ladybird (the ladybirds were fixed by the parasitoid on the cocoon), and (iii) parasitoid cocoons covered by an experimentally killed ladybird. For this last treatment, the coccinellid head capsule was manually crushed with heated pliers, taking care to maintain the ladybird in its original position on the cocoon. Parasitoid cocoons were less than 1 day old. Only cocoons that were naturally attached by the cocoon silk to a support substrate (leaf or paper strips) were used. Cocoons on their support substrate were fixed to a Petri dish (9 cm in diameter) with non-toxic white glue (Lepage BondFast) to be exposed to predators following the three treatments described above. Twenty-four hours before predation tests, lacewings were isolated, placed in a Petri dish (9 cm in diameter) on humid filter paper and not fed. The predator was then introduced into the Petri dish containing a parasitoid cocoon and the experiment began when the predator contacted the cocoon. The test lasted for 15 min, since preliminary tests revealed that this period was sufficient to record contacts. Predation success on the parasitoid cocoon was recorded at the end of the experiment. Each predator and cocoon was used only once, and the experiment was replicated 20–25 times per treatment. After each test, predators were measured (head capsule size); we found no significant difference in predator size among treatments (ANOVA: F2,57 = 1.5846, p = 0.2139).
(c). Trade-off tests
To test for the existence of a phenotypic trade-off between host manipulation effort and parasitoid reproduction and longevity, adult (male and female) C. maculata (2–15 days old) were randomly chosen, parasitized by D. coccinellae (1–15 days old), transferred to individual Petri dishes (9 cm in diameter) and fed ad libitum on A. glycines aphids and water. Following parasitoid egression and emergence, we measured the following parameters for parasitoids: pupal developmental time, size (right hind tibia length), fecundity and longevity. Pupal developmental time was calculated as the time between egression and adult emergence (observed twice a day). Larval developmental time was not considered here (e.g. as a possible estimator of the host exploitation rate by the wasps), since this variable is also known to be significantly influenced by superparasitism [12], a variable difficult to control here. Emerging parasitoids were randomly divided into two cohorts, one to estimate fecundity and the other longevity. Because conditions of resource limitation are favourable to the detection of trade-offs [13], fecundity was measured upon emergence (n = 27) and longevity was estimated while female parasitoids were supplied with water only (n = 15; observed twice a day). Because D. coccinellae is a synovigenic species (i.e. females can produce eggs throughout their life), potential fecundity, used as a proxy of total fecundity [14], was estimated as the number of mature eggs present in the parasitoid female at emergence after dissection using a stereomicroscope (7.5×–112.5×magnification). We used a stereomicroscope to measure the right hind tibia lengths of D. coccinellae and C. maculata, and the sex of C. maculata was determined by observation of genitalia. Ladybird survival on the cocoons was recorded to calculate the duration (in days) of the bodyguard manipulation.
(d). Statistics
Fisher's exact tests on table r × k and standard Fisher's exact tests were conducted to compare predation on parasitoid cocoons among treatments. To explore whether fecundity and/or longevity of the parasitoid is affected by the survival of the bodyguard, these two variables were separately modelled by linear models. The measured potential predictors were parasitoid size, host size, parasitoid pupal development time, host sex and the survival duration of the attending ladybird. Parasitoid fecundity, parasitoid size and host size were log-transformed to stabilize the variance. Because sample sizes were small, we were not able to test for normality or homoscedasticity. Interactions among predictors were systematically investigated and subsequently dropped from the models because they were not significant. Linear models were computed using R software, v. 2.10.1 [15].
3. Results
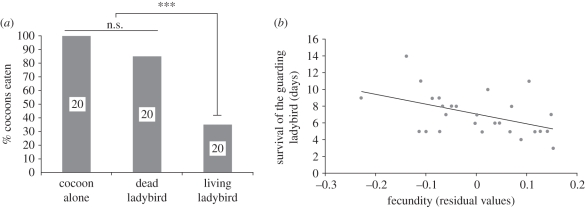
Parasitoid cocoons attended by living ladybirds suffered significantly less predation by lacewings than did cocoons alone or cocoons covered by dead ladybirds (Fisher's exact test on table r × k; p < 0.0001; figure 2a; electronic supplementary material, videos 2 and 3).
Figure 2.
(a) Percentage of Dinocampus coccinellae cocoons eaten by larval green lacewing, Chrysoperla carnea, when parasitoid cocoons were exposed alone, covered by a dead ladybird (Coleomegilla maculata), or attended by a living ladybird. Probabilities were obtained using the Fisher exact test, ***p < 0.0001. Numbers refer to sample sizes. (b) Relationship between the survivorship of attending Coleomegilla maculata ladybirds and the number of mature eggs at emergence of Dinocampus coccinellae parasitoids. Residuals correspond to fecundity data corrected by the size and the pupal development time of the parasitoid, the sex and the size of the ladybird. r2 = 0.219 and p = 0.0137.
There was a negative relationship between ladybird survivorship on the cocoon and the potential fecundity of the parasitoid (figure 2b), even when the other variables are considered (electronic supplementary material, tables 1 and 2). Furthermore, parasitoid pupal development displayed low variability (8.2 ± 0.10 days; mean ± s.e.) and was not correlated with bodyguard duration (linear regression, r2 = 0.45, p = 0.08). There was no relationship between the period of coccinellid survivorship and parasitoid longevity (r2 = 0.0239, p = 0.583).
4. Discussion
In addition to documenting a novel case of bodyguard manipulation by a parasite, our study provides the first evidence that the costs required to perform the manipulated behaviour must be considered when exploring the role of life-history trade-offs in the evolution of manipulative species.
Is the usurpation of ladybird behaviour by the parasitoid an effective strategy to protect developing parasitoid pupae from natural enemies? Our results provide support for the prediction that ladybirds reduce predation on pupating parasitoids, but this benefit is significant only when the ladybird is alive on the cocoon. Ladybirds therefore represent true bodyguards.
The most original finding of our study is the negative relationship between the duration of the active bodyguard period (i.e. when the ladybird remains alive on the cocoon) and the fecundity of the emerging wasp, which indicates the existence of a trade-off between manipulative and reproductive efforts in D. coccinellae. It is generally assumed that direct manipulative costs are likely to come from active interference with the host's neurochemistry that may involve the secretion and release of chemical substances by the parasite [16,17]. The development of specialized glands or tissues for the production of these chemicals must also be costly [7]. Presumably, proximate mechanisms involved in host manipulation by D. coccinellae would depend on venom(s) left by the parasitoid larvae before/at egression. However, our results suggest a more simple explanation. Parasitoids develop using the host's resources, but these resources are also needed to sustain the ladybird during the manipulation period, since it does not eat while on the cocoon. Because host manipulation and parasite development (including the production of eggs) are competing demands, both directly relying on the same resource, parasitoids cannot maximize these two traits. Further research would also be needed to determine whether parasitoids could compensate later in their life such a reduction in egg production at emergence. An alternative explanation of our results would be that some ladybirds resist host manipulation better than others. Such individuals would have both a higher probability of survival following parasitism and a better capacity to prevent the developing larva from using the host resources to increase parasitoid fecundity. However, this hypothesis is unlikely since it implies that the protection conferred by a living ladybird toward predators is a fortuitous coincidence.
The absence of a significant relationship between the duration of the active bodyguard period and the longevity of parasitoid females suggests that these two traits do not depend on the same resource. Without food at emergence, adult parasitoids died within a few days (F. Maure 2010, unpublished data), indicating that there has been no selection to derive supplementary resources from the host to circumvent a lack of food at emergence. Dinocampus coccinellae emerges in spring when floral nectar and honeydew are abundant in the environment. The search for sugar is among the first activities of emerging parasitoid wasps [18].
Another intriguing aspect of our study is that out of those manipulated ladybirds that remained alive until the parasitoid had completed its development and emerged, a substantial proportion of individuals (approx. 25%) recovered from parasitism; this is an atypical fate, since parasitoids usually kill their hosts. In spite of extensive research on manipulative parasites, reversible manipulations have been poorly studied [19,20]. The attributes of these recovering individuals clearly invite further exploration.
Acknowledgements
We thank Thierry Lefèvre, Simon Blanchet, Thierry Rigaud, Yannis Michalakis, Simon Daoust, Brian Mader, Jacqui Shykoff and two anonymous reviewers for helpful comments as well as Alexis Rutschmann for technical assistance. This work was funded by the FQRNT to J.B. and by the ANR project Blanc BODYGUARD.
References
- 1.Dawkins R. 1982. The extended phenotype. Oxford, UK: Oxford University Press [Google Scholar]
- 2.Lagrue C., Poulin R. 2009. Manipulative parasites in the world of veterinary science: implications for epidemiology and pathology. Vet. J. 184, 9–13 10.1016/j.tvjl.2009.01.015 (doi:10.1016/j.tvjl.2009.01.015) [DOI] [PubMed] [Google Scholar]
- 3.Prugnolle F., Lefèvre T., Renaud F., Møller A. P., Missé D., Thomas F. 2009. Infection and body odours: evolutionary and medical perspectives. Infect. Genet. Evol. 9, 1006–1009 10.1016/j.meegid.2009.04.018 (doi:10.1016/j.meegid.2009.04.018) [DOI] [PubMed] [Google Scholar]
- 4.Lafferty K. D. 2006. Can the common brain parasite, Toxoplasma gondii, influence human culture? Proc. R. Soc. B 273, 2749–2755 10.1098/rspb.2006.3641 (doi:10.1098/rspb.2006.3641) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poulin R., Fredensborg B. L., Hansen E., Leung T. L. F. 2005. The true cost of host manipulation by parasites. Behav. Process. 68, 241–244 10.1016/j.beproc.2004.07.011 (doi:10.1016/j.beproc.2004.07.011) [DOI] [PubMed] [Google Scholar]
- 6.Seppälä O., Valtonen E. T., Benesh D. P. 2008. Host manipulation by parasites in the world of dead-end predators: adaptation to enhance transmission? Proc. R. Soc. B 275, 1611–1615 10.1098/rspb.2008.0152 (doi:10.1098/rspb.2008.0152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poulin R. 2010. Parasite manipulation of host behavior: an update and frequently asked questions. Adv. Stud. Behav. 41, 151–186 10.1016/s0065-3454(10)41005-0 (doi:10.1016/s0065-3454(10)41005-0) [DOI] [Google Scholar]
- 8.Karban R. 1998. Caterpillar basking behavior and nonlethal parasitism by tachinid flies. J. Insect. Behav. 11, 713–723 10.1023/A:1022350926720 (doi:10.1023/A:1022350926720) [DOI] [Google Scholar]
- 9.Quicke D. L. J. 1997. Parasitic wasps. London, UK: Chapman & Hall [Google Scholar]
- 10.Brodeur J., Boivin G. 2004. Functional ecology of immature parasitoids. Annu. Rev. Entomol. 49, 27–49 10.1146/annurev.ento.48.091801.112548 (doi:10.1146/annurev.ento.48.091801.112548) [DOI] [PubMed] [Google Scholar]
- 11.Brodeur J., Vet L. E. M. 1994. Usurpation of host behaviour by a parasitic wasp. Anim. Behav. 48, 187–192 10.1006/anbe.1994.1225 (doi:10.1006/anbe.1994.1225) [DOI] [Google Scholar]
- 12.Tunca H., Kilinçer N. 2009. Effect of superparasitism on the development of the solitary parasitoid Chelonus oculator Panzer (Hymenoptera: Braconidae). Turk. J. Agric. Forestry 33, 463–468 [Google Scholar]
- 13.Calow P. 1979. The cost of reproduction—a physiological approach. Biol. Rev. Camb. Phil. 54, 23–40 10.1111/j.1469-185X.1979.tb00866.x (doi:10.1111/j.1469-185X.1979.tb00866.x) [DOI] [PubMed] [Google Scholar]
- 14.Roitberg B. D., Boivin G., Vet L. E. M. 2001. Fitness, parasitoids, and biological control: an opinion. Can. Entomol. 133, 429–438 10.4039/Ent133429-3 (doi:10.4039/Ent133429-3) [DOI] [Google Scholar]
- 15.R Development Core Team. 2009. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (ISBN 3-900051-07-0, see http://www.R-project.org). [Google Scholar]
- 16.Thompson S. N., Kavaliers M. 1994. Physiological bases for parasite-induced alterations of host behaviour. Parasitology 109, 119–138 10.1017/S0031182000085139 (doi:10.1017/S0031182000085139) [DOI] [PubMed] [Google Scholar]
- 17.Thomas F., Adamo S. A., Moore J. 2005. Parasitic manipulation: where are we and where should we go? Behav. Process. 68, 185–200 10.1002/9780470114209.ch18 (doi:10.1002/9780470114209.ch18) [DOI] [PubMed] [Google Scholar]
- 18.Siekmann G., Tenhumberg B., Keller M. A. 2001. Feeding and survival in parasitic wasps: sugar concentration and timing matter. Oikos 95, 425–430 10.1034/j.1600-0706.2001.950307.x (doi:10.1034/j.1600-0706.2001.950307.x) [DOI] [Google Scholar]
- 19.Eberhard W. G. 2010. Recovery of spiders from the effects of parasitic wasps: implications for fine-tuned mechanisms of manipulation. Anim. Behav. 79, 375–383 10.1016/j.anbehav.2009.10.033 (doi:10.1016/j.anbehav.2009.10.033) [DOI] [Google Scholar]
- 20.Ponton F., Otálora-Luna F., Lefèvre T., Guerin P. M., Lebarbenchon C., Duneau D., Biron D. G., Thomas F. 2011. Water-seeking behavior in worm-infected crickets and reversibility of parasitic manipulation. Behav. Ecol. 22, 392–400 10.1093/beheco/arq215 (doi:10.1093/beheco/arq215) [DOI] [PMC free article] [PubMed] [Google Scholar]