Abstract
Following encoding, memory remains temporarily vulnerable to disruption. Consolidation refers to offline time-dependent processes that continue after encoding and stabilize, transform or enhance the memory trace. Memory consolidation resulting from sleep has been reported for declarative and non-declarative memories in humans. We first investigated the temporal course of memory retrieval in chimpanzees, bonobos and orangutans. We found that the amount of retrieved information was time dependent: apes' performance degraded after 1 and 2 h, stabilized after 4 h, started to increase after 8 and 12 h and fully recovered after 24 h. Second, we show that although memories during wakefulness were highly vulnerable to interference from events similar to those witnessed during the original encoding event, an intervening period of sleep not only stabilized apes' memories into more permanent ones but also protected them against interference.
Keywords: memory, time, sleep, interference, great apes
1. Introduction
The long-lasting trace of an experience is not completely fixed nor consolidated at the time of the experience [1–3]. Consolidation requires time to reorganize the memory traces so that they become more stable. An understanding of memory requires the comprehension of the time-dependence of storage processes. In humans, there is evidence that this process of reorganization can take several hours, and subjects' performance in non-declarative and declarative memory tasks can improve substantially after delays of 8 h or more [3–5]. Research on memory in great apes has mainly focused on the type of information that can be retrieved and for how long it can be remembered [6,7]. Great apes are able to remember information (i.e. food locations) for intervals shorter than 30 min and longer than 24 h. However, not much research has focused on how great apes' memory traces evolve within 24 h after encoding. We addressed this question in experiment 1.
2. Experiment 1: the role of time (i)
(a). Material and methods
We tested five chimpanzees, three bonobos and one orangutan in a memory task that consisted of remembering the location of a food reward placed under one of three cups on a platform after one of four different retention intervals (RIs: 2 min, 1, 2 and 24 h). After baiting had taken place, subjects left the testing room and were involved in other activities. Food retrieval took place after the RI had elapsed and subjects returned to the testing room (see the electronic supplementary material for details).
(b). Results and discussion
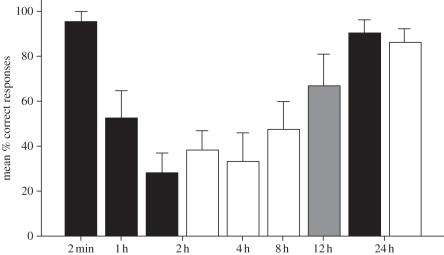
Apes performed unevenly across RIs (Friedman test, 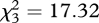 , p = 0.001, n = 9; figure 1). Wilcoxon post hoc tests revealed that subjects' performance was significantly better after 2 min than 1 h (z = 2.40, p = 0.016, n = 9) or 2 h (z = 2.55, p = 0.011, n = 9), but not after 24 h (z = 1.73, p = 0.083, n = 9). Additionally, subjects performed significantly better after 24 h than 2 h (z = 2.26, p = 0.024, n = 9), but not after 1 h (z = −1.91, p = 0.055, n = 9). Apes remembered the baited location significantly above chance (i.e. 33.3%) in the 2 min (Wilcoxon test: z = 2.88, p = 0.004, n = 9) and 24 h RIs (Wilcoxon test: z = 2.73, p = 0.006, n = 9), but not in the other intervals (Wilcoxon tests—1 h: z = 1.73, p = 0.08, n = 9; 2 h: z = 0.37, p = 0.70, n = 9).
, p = 0.001, n = 9; figure 1). Wilcoxon post hoc tests revealed that subjects' performance was significantly better after 2 min than 1 h (z = 2.40, p = 0.016, n = 9) or 2 h (z = 2.55, p = 0.011, n = 9), but not after 24 h (z = 1.73, p = 0.083, n = 9). Additionally, subjects performed significantly better after 24 h than 2 h (z = 2.26, p = 0.024, n = 9), but not after 1 h (z = −1.91, p = 0.055, n = 9). Apes remembered the baited location significantly above chance (i.e. 33.3%) in the 2 min (Wilcoxon test: z = 2.88, p = 0.004, n = 9) and 24 h RIs (Wilcoxon test: z = 2.73, p = 0.006, n = 9), but not in the other intervals (Wilcoxon tests—1 h: z = 1.73, p = 0.08, n = 9; 2 h: z = 0.37, p = 0.70, n = 9).
Figure 1.
Mean percentage of correct responses for experiment 1, experiment 2 and no interference (day trials) in experiment 3 as a function of the RIs (error bars represent the standard error of the mean). Note that the sample composition varied between experiments (experiment 1 (black bars): five chimpanzees, three bonobos, one orangutan; experiment 2 (white bars): four bonobos, four orangutans; experiment 3 (grey bar): three bonobos, four orangutans).
Experiment 1 established that subjects' performance in the 1 and 2 h RIs deteriorated substantially. However, their performance in the 24 h RI recovered to levels comparable to the 2 min RI. This means that memories were not lost but became temporarily inaccessible. These data, however, are silent regarding the contribution of sleep [4,8,9] or temporal gradient [1,2,5,10] in memory consolidation. Experiment 2 further investigated the temporal gradient of memory recovery.
3. Experiment 2: the role of time (ii)
(a). Material and methods
We tested four bonobos and four orangutans in the same task as in experiment 1 using 2, 4, 8 and 24 h as RIs (table 1). Subjects always left the testing room after the baiting took place. Additionally, we recorded whether subjects slept during the 8 h RI (see the electronic supplementary material for details).
Table 1.
Name, gender, age (years) and study participation (nt, not tested).
| name | genus | age | experiment participation |
||
|---|---|---|---|---|---|
| experiment 1 | experiment 2a | experiment 3a | |||
| Joey | Pan paniscus | 28 | yes | yes | yes |
| Limbuko | P. paniscus | 15 | nt | nt | yes |
| Kuno | P. paniscus | 14 | nt | yes | yes |
| Ulindi | P. paniscus | 17 | yes | yes | nt |
| Yasa | P. paniscus | 13 | yes | yes | nt |
| Frodo | Pan troglodytes | 17 | yes | nt | nt |
| Patrick | P. troglodytes | 13 | yes | nt | nt |
| Tai | P. troglodytes | 8 | yes | nt | nt |
| Trudi | P. troglodytes | 17 | yes | nt | nt |
| Sandra | P. troglodytes | 17 | yes | nt | nt |
| Bimbo | Pongo pygmaeus | 20 | yes | yes | yes |
| Padana | Pongo pygmaeus | 13 | nt | yes | yes |
| Dokana | Pongo pygmaeus | 21 | nt | yes | yes |
| Pini | Pongo pygmaeus | 22 | nt | yes | yes |
aChimpanzees could not be tested in experiments 2 and 3 for logistical reasons concerning their housing arrangement.
(b). Results and discussion
Apes remembered the baited location significantly better than chance in the 24 h RI (Wilcoxon test: z = 2.59, p = 0.009, n = 8; figure 1), but not in the other RIs (Wilcoxon tests: z < 1.42, p > 0.14, n = 8, in all cases). However, it appeared that apes' performance improved with time because the 24 h RI did not significantly differ from the 8 h RI (Wilcoxon test: z = 1.91, p = 0.055, n = 8), but it still significantly differed from the 2 and 4 h RIs (Wilcoxon tests: z > 2.03, p < 0.05, n = 8, in both cases). We only recorded one occurrence in which one subject, Bimbo, lay down for more than 20 min with its eyes closed. Two other subjects closed their eyes for periods shorter than 1 min on less than five occasions on different days. Therefore, we can attribute the apparent improvement to the passage of time in the absence of sleep. Additionally, extrapolating the gain in performance between the 4 and the 8 h RIs (3% gain per hour) could explain the performance in the 24 h RI.
Nevertheless, sleep may have some beneficial effects. Research on sleep deprivation in rodents has shown that sleep benefits memory consolidation [11–13]. Studies with humans showed that sleep, compared with wakefulness, actively improved subjects' performance [8,9] and can shelter memory from interference during wakefulness [8,14]. Experiment 3 investigated the role of sleep and interference on memory consolidation. If sleep only prevents interference from happening, memories after a period of sleep should still be vulnerable to interference. However, if sleep can also play a protective role, memories after sleep should be more resistant to interference. Additionally, it tested whether subjects who slept remembered better than those who did not sleep.
4. Experiment 3: the role of sleep and interference
(a). Material and methods
We tested three bonobos and four orangutans in the same task used in the previous experiments using a 12 h RI (table 1). We administered trials in two periods—day (start: 07.00 h; end: 19.00 h) and night (start: 19.00 h; end: 07.00 h). We presented subjects with three conditions: no interference, early interference (subjects received an interference task trial 2 min after the baiting event) and late interference (subjects received an interference task trial 2 min before the retrieval event; see the electronic supplementary material for details).
(b). Results and discussion
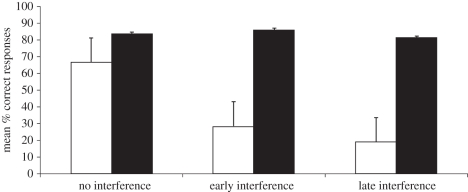
Figure 2 presents the mean percentages of correct trials in each condition. An ANOVA with period and interference as within-subject factors revealed a significant effect for period, F1,6 = 26.35, p = 0.002; interference, F1,6 = 8.48, p = 0.027 and period × interference, F1,6 = 6.58, p = 0.043. We analysed the effect of period on performance within each condition. There was no significant difference between test periods in the no interference condition (t(6) = 1.33, p = 0.23). In contrast, subjects performed better in the night compared with the day period both in the early interference condition (t(6) = 4.76, p = 0.003) and the late interference condition (t(6) = 4.59, p = 0.004).
Figure 2.
Mean percentages of correct responses for the test-task for the day (white bars) and night (black bars) trials as a function of the condition (presence or absence of interference task) (error bars represent the standard error of the mean).
Focusing on the interference task trials revealed that subjects performed above chance levels in both night (t(6) = 7.07, p < 0.001) and day (t(6) = 7.77, p < 0.001) late interference task trials, which is consistent with our previous findings from the 2 min trials of experiment 1. In early interference trials, subjects performed above chance levels in night interference task trials (t(6) = 4.53, p = 0.004) but not in day interference task trials (t(6) = 1.18, p = 0.279). Again, these data are consistent with the results of the test trials.
Experiment 3 provided additional support for the idea that time alone, without sleep, improved memory retrieval as shown by the subjects' success in the no interference condition during the day period. An analysis based on the data from figure 1 confirmed that time (RI) predicted performance with great accuracy (R2 = 0.984, F = 60.68, d.f. = 3, p = 0.003, Ŷ = −3.7781(t√t) + 36.9596t − 95.767√t + 113.300; see the electronic supplementary material, figure S3). Indeed, subjects' retrieval accuracy was significantly better in the 12 h RI than the 2 h RI (Wilcoxon test: z = 2.04, p = 0.041, n = 6). Furthermore, results from the late interference trials in the night period established that sleep actively strengthened apes' memories by rendering them resistant to interference.
5. General discussion
We found strong evidence that time and sleep play a crucial role in memory processing in great apes. Although there is evidence showing time-dependent processes in memory storage in rodents [15,16], most of these studies have either not shown the existence of a temporal gradient [17] or when they have shown the gradient [18,19], alternative explanations such as state-dependent memory or development of fear have not been ruled out [20,21]. In contrast, the observed U-shaped distribution of retrieval accuracy as a function of time in apes (figure 1) is difficult to explain by invoking either contextual or motivational factors. A mechanism like state-dependent memory, i.e. cues present at the time of encoding that later on trigger retrieval [22], could explain the high accuracy in the 24 h RI, but it cannot explain the interim decrease in retrieval accuracy. Similarly, time-of-day effects could explain the subjects' performance in the 24 h RI, but cannot explain why subjects' performance in the no interference condition did not differ between day and night periods. Results from the late interference task during the day trials and early interference task during the night trials also show that subjects remembered the location of the food, which indicates that time of the day cannot explain subjects' worse performance in the day trials. Additionally, subjects' better performance in the 12 h RI compared with the 2 h RI also rules out that explanation. Motivational states (i.e. hunger) throughout the day also fail to explain the differential performance between interference and non-interference trials. Moreover, the motivation hypothesis predicts that subjects should have performed best in the 4 and 8 h RI because these trials took place right before apes' main feeding times. But this was not the case. Finally, seeing the boxes during an extended time period prior to the test may have helped subjects improve their performance in the 24 h RI (experiment 1). However, this explanation cannot account for subjects' performance after 8 and 12 h RIs because they had no visual access to the boxes prior to the test.
Most studies on ape memory have used shorter or longer RIs than the ones presented here [6,7], which means that they could have potentially missed the temporal window when information was inaccessible. A study using the same procedure as the one reported here showed that apes did remember the location of a reward after 1 or 2 h RIs [23]. However, unlike the subjects tested here, those subjects remained in the testing room during the RI, which may have minimized interference by other activities in making memories temporarily inaccessible [9,24]. Although the effect of engaging in other activities during the RI on memory retrieval has been tested before in insects and rodents, studies have found conflicting results [25,26] and none of these studies has reported a recovery after 24 h.
Experiment 3 showed that sleep both facilitated the recovery of memories whose retrieval was disrupted before sleep and rendered memories resistant to interference. Therefore, we argue that, as in humans, sleep played an active role in great apes' memories by facilitating consolidation [8,14,24]. The lack of interfering activities during sleep alone cannot explain our results because subjects also performed well in the no interference condition during the day period.
We conclude that, following the encoding of the food location, there is a phase of several hours during which the memory trace is vulnerable to interference from other unrelated activities such that it thus becomes temporarily inaccessible. Poor memory retrieval can be ameliorated either by the reduction of unrelated activities during the RI or the mere passage of time. This study also suggested that sleep was involved in great apes' memory consolidation. Sleep not only made memory more resistant to the detrimental effects of interference during wakefulness but also protected memories from interference during the subsequent day.
Acknowledgements
G.M.-O. was supported by a post-doctoral fellowship of the ‘Programa de Ayuda a la Movilidad del Ministerio de Ciencia e Innovacion (Micinn)’, Spain. We would like to thank M. Halina for helping with the data collection for the third experiment. The experiments comply with the current laws of the country in which they were performed.
References
- 1.Müller G. E., Pilzecker A. 1900. Experimentelle Beiträge zur Lehre vom Gedächtnis. Z. Psychol. Ergänzungsband 1, 1–300 [Google Scholar]
- 2.McGaugh J. L. 2000. Memory: a century of consolidation. Science 287, 248–251 10.1126/science.287.5451.248 (doi:10.1126/science.287.5451.248) [DOI] [PubMed] [Google Scholar]
- 3.Walker M. P. 2005. A refined model of sleep and the time course of memory formation. Behav. Brain. Sci. 28, 51–64 [DOI] [PubMed] [Google Scholar]
- 4.Vertes R. P. 2004. Memory consolidation in sleep: dream or reality. Neuron 44, 135–148 10.1016/j.neuron.2004.08.034 (doi:10.1016/j.neuron.2004.08.034) [DOI] [PubMed] [Google Scholar]
- 5.Brashers-Krug T., Shadmehr R., Bizzi E. 1996. Consolidation in human motor memory. Nature 382, 252–255 10.1038/382252a0 (doi:10.1038/382252a0) [DOI] [PubMed] [Google Scholar]
- 6.Hoffman M. L., Beran M. J. 2006. Chimpanzees (Pan troglodytes) remember the location of a hidden food item after altering their orientation to a spatial array. J. Comp. Psychol. 120, 389–393 10.1037/0735-7036.120.4.389 (doi:10.1037/0735-7036.120.4.389) [DOI] [PubMed] [Google Scholar]
- 7.Schwartz B. L. 2005. Do non-humans primates have episodic memory? In The missing link in cognition (eds Terrace H. S., Metcalfe J.), pp. 225–241 Oxford, UK: Oxford University Press [Google Scholar]
- 8.Ellenbogen J. M., Hulbert J. C., Stickgold R., Dinges D. F., Thompson-Schill S. L. 2006. Interfering with theories of sleep and memory: sleep, declarative memory, and associative interference. Curr. Biol. 16, 1290–1294 10.1016/j.cub.2006.05.024 (doi:10.1016/j.cub.2006.05.024) [DOI] [PubMed] [Google Scholar]
- 9.Stickgold R. 2005. Sleep-dependent memory consolidation. Nature 437, 1272–1278 10.1038/nature04286 (doi:10.1038/nature04286) [DOI] [PubMed] [Google Scholar]
- 10.Dudai Y. 2004. The neurobiology of consolidation, or, how stable is the engram? Annu. Rev. Psychol. 55, 51–86 10.1146/annurev.psych.55.090902.142050 (doi:10.1146/annurev.psych.55.090902.142050) [DOI] [PubMed] [Google Scholar]
- 11.Wilson M. A., McNaughton B. L. 1994. Reactivation of hippocampal ensemble memories during sleep. Science 265, 676–679 10.1126/science.8036517 (doi:10.1126/science.8036517) [DOI] [PubMed] [Google Scholar]
- 12.Pavlides C., Winson J. 1989. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. J. Neurosci. 9, 2907–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nadasdy Z., Hirase H., Czurko A., Csicsvari J., Buzsaki G. 1999. Replay and time compression of recurring spike sequences in the hippocampus. J. Neurosci. 19, 9497–9507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ellenbogen J. M., Payne J. D., Stickgold R. 2006. The role of sleep in declarative memory consolidation: passive, permissive, active or none? Curr. Opin. Neurobiol. 16, 716–722 10.1016/j.conb.2006.10.006 (doi:10.1016/j.conb.2006.10.006) [DOI] [PubMed] [Google Scholar]
- 15.Karni A., Tanne D., Rubenstein B. S., Askenasy J. J., Sagi D. 1994. Dependence on REM sleep of overnight improvement of a perceptual skill. Science 265, 679–682 10.1126/science.8036518 (doi:10.1126/science.8036518) [DOI] [PubMed] [Google Scholar]
- 16.McGaugh J. L. 1966. Time-dependent processes in memory storage. Science 153, 1351–1358 10.1126/science.153.3742.1351 (doi:10.1126/science.153.3742.1351) [DOI] [PubMed] [Google Scholar]
- 17.Akers K. G., Frankland P. W. 2009. Gradients graded: evidence for time-dependent memory reorganization in experimental animals. J. Exp. Neurosci. 2, 13–22 [Google Scholar]
- 18.Kamin L. J. 1957. The retention of an incompletely learned avoidance response. J. Comp. Physiol. Psychol. 50, 457–460 10.1037/h0044226 (doi:10.1037/h0044226) [DOI] [PubMed] [Google Scholar]
- 19.Duncan C. P. 1949. The retroactive effect of electroshock on learning. J. Comp. Physiol. Psychol. 42, 32–44 10.1037/h0058173 (doi:10.1037/h0058173) [DOI] [PubMed] [Google Scholar]
- 20.McGaugh J. L. 1972. The search for the memory trace. Ann. NY. Acad. Sci. 193, 112–123 10.1111/j.1749-6632.1972.tb27828.x (doi:10.1111/j.1749-6632.1972.tb27828.x) [DOI] [PubMed] [Google Scholar]
- 21.Gerber B., Menzel R. 2000. Contextual modulation of memory consolidation. Learn. Mem. 7, 151–158 10.1101/lm.7.3.151 (doi:10.1101/lm.7.3.151) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shettleworth S. J. 2010. Cognition, evolution, and behavior, 2nd edn New York, NY: Oxford University Press [Google Scholar]
- 23.Martin-Ordas G., Haun D., Colmenares F., Call J. 2010. Keeping track of time: evidence for episodic-like memory in great apes. Anim. Cogn 13, 331–340 10.1007/s10071-009-0282-4 (doi:10.1007/s10071-009-0282-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wamsley E. J., Stickgold R. 2010. Dreaming and offline memory processing. Curr. Biol. 23, R1010. 10.1016/j.cub.2010.10.045 (doi:10.1016/j.cub.2010.10.045) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Minami H., Dallenbach K. 1946. The effect of activity upon learning and retention in the cockroach, Periplaneta americana. Am. J. Psychol. 59, 1–58 10.2307/1416998 (doi:10.2307/1416998) [DOI] [PubMed] [Google Scholar]
- 26.Sangha S., McComb C., Lukowiak K. 2003. Forgetting and the extension of memory in Lymnaea. J. Exp. Biol. 206, 71–77 10.1242/jeb.00061 (doi:10.1242/jeb.00061) [DOI] [PubMed] [Google Scholar]