Abstract
When choosing a mate, women are thought to face a trade-off between genetic and parental quality. Recent research suggests that this trade-off is influenced by environmental factors such as pathogen prevalence and resource scarcity, which affect the relative value of genetic and parental quality to offspring fitness. To further investigate these findings, the current study primed 60 women with pathogen prevalence, resource scarcity or an irrelevant threat, before administering a forced trade-off task that assessed mate preferences for traits thought to be indicative of genetic or parental quality. Women primed with pathogen prevalence revealed greater preferences for traits indicative of genetic quality at the expense of traits indicative of parental quality. The reverse was found for women primed with resource scarcity. These findings suggest that environmental factors may directly influence women's mate preferences owing to evolved plasticity, such that mate preferences are flexible in response to environmental factors.
Keywords: sexual selection, sexual strategy, environmental factors, priming, plasticity, behavioural ecology
1. Introduction
Not every man is made equal. Some are born with genetic advantages that improve their chance of survival and reproduction relative to others. To advertise their genetic advantages, it is thought that men possess honest signals of mate quality that are expensive to produce and are not easily faked [1]. These traits include mental markers, such as intelligence and creativity [2,3], but also behavioural and physical traits associated with exposure to high levels of testosterone during development (e.g. wide shoulders, strong jaw line and social dominance) [4]. While we will refer to these as ‘good-genes’ traits for brevity, it should be emphasized that much more direct evidence is required before any trait can be firmly accepted to indicate genetic quality.
Women are thought to prefer good-genes traits because they confer indirect (genetic) benefits to resulting offspring, who inherit the father's genetic quality and its associated survival and mating advantages [5]. However, indirect benefits of mate choice are as yet supported only by partial and indirect evidence, and traits reflecting genetic quality might also confer direct benefits to the woman or offspring, such as resourcefulness, protection from other males, increased social status or avoidance of pathogen transmission owing to immunocompetence thought to be associated with masculine traits [6]. As such, we do not speculate as to the relative importance of direct and indirect benefits of a mate with good-genes traits.
Despite the potential advantages of mating with a masculine man, studies have found that women have weak preferences for masculine traits [4] or even prefer feminine traits [7,8]. This suggests there are costs in choosing a masculine mate [8]. Indeed, masculinity and high levels of testosterone are associated with traits indicative of poor parental quality, such as a preference for short-term relationships and low faithfulness [9,10]. Conversely, feminine men may lack the immunocompetence required to support high levels of testosterone [6], but in turn have traits better suited for parenting, such as being more committed to a long-term relationship and caring for resulting offspring [9,10]. As such, it has been suggested that women face a trade-off between the potential benefits that could be gained between choosing a mate with good-genes traits, compared with a mate with ‘good-dad’ traits [11].
Previous research has hypothesized that this trade-off may be sensitive to the local environment, as the relative value of good-genes and good-dad traits to offspring fitness varies with differing environments. Accordingly, cross-cultural studies have found that women in countries with a high pathogen prevalence are likely to report greater preference for physical attractiveness [12] and masculine facial features [13,14]. However, being correlational in nature, cross-cultural research limits the current knowledge in two ways.
First, a causal relationship is uncertain, as other variables, such as differences in income, inequality and violence vary with pathogen prevalence and could underlie regional variation in mate preferences [15]. Second, assuming a direct relationship, correlational designs offer no insight into the mechanisms of the effect. It could be that environmental factors, via selection pressures, change the genetic component of preferences throughout a population. Alternatively, differences in preferences may be a product of evolved plasticity, whereby mate preferences change in direct response to perceived environmental factors.
Two recent experimental studies have found that women's facial preferences can be influenced towards masculine features by visual exposure to cues of pathogen contagions [16], or away when participants imagine themselves in a low-resource scenario [17]. These studies suggest flexible facial preferences that are calibrated to environmental cues. Preferences for masculinized or feminized faces have been assumed to reflect preferences for good-genes or good-dad traits more broadly [14], but it is important to test such trait preferences directly to generalize these findings.
Here, we present women with cues (primes) of pathogen prevalence, resource scarcity or an irrelevant threat (control condition) to test whether the salience of different environmental factors influences preferences for putative good-genes or good-dad traits. The priming technique involves exposing participants to an evocative stimulus and testing for behavioural consequences [18]. We hypothesize that women primed with pathogen prevalence should favour good-genes traits, whereas women primed with resource scarcity should favour good-dad traits.
2. Material and methods
(a). Participants
Participants were 65 females (mean = 18.59 years, s.d. = 1.57 years) enrolled in a 1st year psychology course at an Australian university who participated in return for course credit. Participants were unlikely to have been familiar with the theoretical framework of the study. Participation was conditional on being heterosexual and not currently in a long-term relationship.
(b). Design
We used an independent groups design. Participants were first randomly assigned to complete one of three questionnaires designed to prime them with an environmental threat. These were pathogen prevalence, resource scarcity or an irrelevant threat (control condition). Participants were then given a forced trade-off task to assess their mate preferences in terms of good-genes and good-dad traits. The primes and trade-off task are described below, and can be found in full in the electronic supplementary material.
(c). Primes
The priming questionnaires were all matched to contain 15 items that required participants to rate agreement to statements on a 7-point scale (1, strongly disagree; 7, strongly agree). To prime pathogen prevalence, we used the Perceived Vulnerability to Disease Questionnaire [19]. An example item includes, ‘In general, I am very susceptible to colds, flu and other infectious diseases’. To prime resource scarcity, participants completed the Financial Concerns Questionnaire, a purpose-designed questionnaire that included items such as, ‘I worry about the rising cost of food’. The Belief in the Paranormal Questionnaire [20] was chosen as the priming questionnaire for the irrelevant threat condition as supernatural threats should have no influence on mate preferences. It included items such as, ‘I firmly believe that ghosts and spirits do exist’.
(d). Forced trade-off measure
The forced trade-off paradigm for measuring mate preferences was based on the research design of Li et al. [7]. This involved assigning participants a limited number of ‘mate dollars’ which they could invest in traits in order to construct their ideal partner. Ten traits were listed in total, five each for both good-genes and good-dad traits. This effectively created a forced trade-off. Traits representing good-genes were those that have either been theorized to be indicators of genetic quality, which were ‘intelligence’ [2,3] and ‘creativity’ [2,3], or were associated with testosterone, masculinity, and, by extension, good immune functioning, which were ‘muscularity’ [21], ‘high social level’ [22] and ‘confidence’ [4,8]. Traits representing good-dad traits were those that were either directly related to resource attainment and parental investment, which were ‘high earning potential’ and ‘commitment’, or have been shown to be perceived as good parental qualities, which were ‘emotionally warm’ [23], ‘kind’ [22] and ‘nurturing’ [22,23]. Participants were assigned 25 mate dollars and traits were presented in a randomized order.
3. Results
Since differing ‘budgets’ can influence spending patterns for various traits desired in a mate [7], participants who failed to adhere to the 25 mate dollar budget were removed from analysis. This reduced the sample to 60 participants. Univariate tests showed that the data were normally distributed.
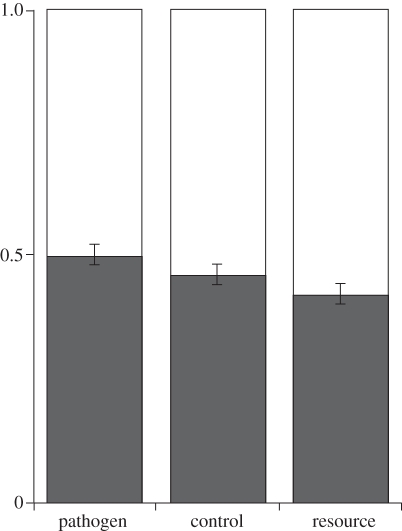
Across all conditions, participants tended to invest more in good-dad traits (mean = 13.53, s.d. = 2.58) than good-genes traits (mean = 11.48, s.d. = 2.58). As shown in figure 1, participants in the pathogen prevalence condition invested more mate dollars in good-genes traits than those in the irrelevant threat condition, who in turn invested more than those in the resource scarcity condition. This pattern was reversed for spending on good-dad traits. These trends are in-line with our predictions.
Figure 1.
Proportion of mate dollars invested in good-genes (dark bar) and good-dad (light bar) traits across the pathogen prevalence, unrelated threat and resource scarcity conditions.
Since an increase in mate dollars invested in good-genes traits resulted in a direct decrease in spending on good-dad traits and vice versa, subsequent statistical testing focused on spending on good-genes traits (with the results applying equally to good-dad traits). A one-way between-subjects ANOVA revealed that preferences for good-genes traits varied significantly among different prime conditions, F2,57 = 3.59, p = 0.034, supporting our prediction that cues of environmental factors would shift women's mate preferences. Since our hypotheses predict that the pathogen prevalence condition would show the highest investment in good-genes traits, the resource condition the lowest investment, and the control condition intermediate, a linear contrast was conducted to test for a linear effect in mate dollars invested in good-genes traits. This was found to be significant, F1,57 = 7.18, p = 0.010. Given the overall significant difference between conditions, and the significant linear pattern of mean differences in accordance with predictions, our results are consistent with the hypothesis that women's mate preferences shift towards good-genes traits when primed with pathogen prevalence and towards good-dad traits when primed with resource scarcity.
4. Discussion
As predicted, women's mate preferences shifted towards good-genes traits when primed with pathogen prevalence and towards good-dad traits when primed with resource scarcity. This indicates that pathogen and resource-related environmental cues can directly influence women's mate preferences.
As previously mentioned, inferences of causality could not be made from earlier correlational studies which revealed that residents of unhealthier countries had greater preference for good-gene traits [12–14], because the association could be caused by other factors that covary with pathogen prevalence, such as income, inequality or violence [15]. The present experimental study suggests that those observed associations were probably owing, at least partly, to a direct relationship between pathogen prevalence and/or resource scarcity and mate preferences. The current study is also consistent with findings from recent experimental studies on the effect of environmental cues on preferences for masculine/feminine facial features [16,17], suggesting that the effect of pathogen prevalence and resource scarcity on mate preferences extends beyond facial features to a much broader range of traits.
Furthermore, along with the two aforementioned experimental studies, the current study provides insight into which of two possible processes underlie the regional variation in preferences found in cross-cultural research. If this variation were solely owing to environmental factors placing selection pressures that change the genetic component of preferences throughout a population, we would not have observed environmental cues causing shifts in mate preferences. Since we did observe these shifts, evolved plasticity must play a role whereby mate preferences are modified in response to perceived local levels of pathogen prevelance and resource scarcity. This mechanism may underlie cultural variations in mate preferences, as different regions are exposed to different environmental conditions.
A possible explanation for why such plasticity in women's mate preferences has evolved could be that it allows women to effectively trade-off genetic and parental quality and choose a mate that maximizes the probability of their own or their offspring's fitness in any given environment, even when the environment changes. This is evolutionarily advantageous over a fixed set of preferences as women would be able to adapt their preferences to rapid changes in the environment, such as a pathogen outbreak or a famine. It should be noted, however, that our findings do not rule out regional genetic variation in mate preferences, which could also play a role in regional variation.
Acknowledgements
This study has been cleared in accordance with the ethical review processes of the University of Queensland and within the guidelines of the National Statement on Ethical Conduct in Human Research.
We thank Bill von Hippel and several anonymous reviewers for very helpful comments, and Madeline Farmer for help with data collection.
References
- 1.Zahavi A. 1975. Mate selection: selection for a handicap. J. Theor. Biol. 53, 205–214 10.1016/0022-5193(75)90111-3 (doi:10.1016/0022-5193(75)90111-3) [DOI] [PubMed] [Google Scholar]
- 2.Miller G. 2000. The mating mind. New York, NY: Doubleday [Google Scholar]
- 3.Haselton M. G., Miller G. F. 2006. Women's fertility across the cycle increases the short-term attractiveness of creative intelligence. Hum. Nat. 17, 50–73 10.1007/s12110-006-1020-0 (doi:10.1007/s12110-006-1020-0) [DOI] [PubMed] [Google Scholar]
- 4.Swaddle J. P., Reierson G. W. 2002. Testosterone increases perceived dominance but not attractiveness in human males. Proc. R. Soc. Lond. B 269, 2285–2289 10.1098/rspb.2002.2165 (doi:10.1098/rspb.2002.2165) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roberts S. C., Little A. C. 2008. Good genes, complementary genes and human mate preferences. Genetica 132, 309–321 10.1007/s10709-007-9174-1 (doi:10.1007/s10709-007-9174-1) [DOI] [PubMed] [Google Scholar]
- 6.Thornhill R., Gangestad S. W. 2006. Facial sexual dimorphism, developmental stability, and susceptibility to disease in men and women. Evol. Hum. Behav. 27, 131–144 10.1016/j.evolhumbehav.2005.06.001 (doi:10.1016/j.evolhumbehav.2005.06.001) [DOI] [Google Scholar]
- 7.Li N. P., Bailey J. M., Kenrick D. T., Linsenmeier J. A. W. 2002. The necessities and luxuries of mate preferences. J. Pers. Soc. Psychol. 82, 947–955 10.1037/0022-3514.82.6.947 (doi:10.1037/0022-3514.82.6.947) [DOI] [PubMed] [Google Scholar]
- 8.Boothroyd L. G., Jones B. C., Burt D. M., Perrett D. I. 2007. Partner characteristics associated with masculinity, health and maturity in male faces. Pers. Indiv. Differ. 43, 1161–1173 10.1016/j.paid.2007.03.008 (doi:10.1016/j.paid.2007.03.008) [DOI] [Google Scholar]
- 9.McIntyre M., Gangestad S. W., Gray P. B., Chapman J. F., Burnham T. C., O'Rourke M. T., Thornhill R. 2006. Romantic involvement often reduces men's testosterone levels—but not always: the moderating role of extrapair sexual interest. Interpers. Relat. Group Process. 91, 642–651 [DOI] [PubMed] [Google Scholar]
- 10.Van Anders S. M., Hamilton L. D., Watson N. V. 2007. Multiple partners are associated with higher testosterone in North American men and women. Horm. Behav. 51, 454–459 10.1016/j.yhbeh.2007.01.002 (doi:10.1016/j.yhbeh.2007.01.002) [DOI] [PubMed] [Google Scholar]
- 11.Gangestad S. W., Simpson J. A. 2000. The evolution of human mating: trade-offs and strategic pluralism. Behav. Brain Sci. 23, 573–644 10.1017/S0140525X0000337X (doi:10.1017/S0140525X0000337X) [DOI] [PubMed] [Google Scholar]
- 12.Gangestad S. W., Haselton M. G., Buss D. M. 2006. Evolutionary foundations of cultural variation: evoked culture and mate preferences. Psychol. Inq. 17, 75–95 10.1207/s15327965pli1702_1 (doi:10.1207/s15327965pli1702_1) [DOI] [Google Scholar]
- 13.Penton-Voak I. S., Jacobson A., Trivers R. 2004. Populational differences in attractiveness judgements of male and female faces: comparing British and Jamaican samples. Evol. Hum. Behav. 25, 355–370 10.1016/j.evolhumbehav.2004.06.002 (doi:10.1016/j.evolhumbehav.2004.06.002) [DOI] [Google Scholar]
- 14.DeBruine L. M., Jones B. C., Crawford J. R., Welling L. L. M., Little A. C. 2010. The health of a nation predicts their mate preferences: cross-cultural variation in women's preferences for masculinized male faces. Proc. R. Soc. B 277, 2405–2410 10.1098/rspb.2009.2184 (doi:10.1098/rspb.2009.2184) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brooks R., Scott I. M., Maklakov A. A., Kasumovic M. M., Clark A. P., Penton-Voak I. S. 2011. National income inequality predicts women's preferences for masculinized faces better than health does. Proc. R. Soc. B 278, 810–812 10.1098/rspb.2010.0964 (doi:10.1098/rspb.2010.0964) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Little A. C., DeBruine L. M., Jones B. C. 2011. Exposure to visual cues of pathogen contagion changes preferences for masculinity and symmetry in opposite-sex faces. Proc. R. Soc. B 278, 2032–2039 10.1098/rspb.2010.1925 (doi:10.1098/rspb.2010.1925) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Little A. C., Cohen D. L., Jones B. C., Belsky J. 2007. Human preferences for facial masculinity change with relationship type and environmental harshness. Behav. Ecol. Sociobiol. 61, 967–973 10.1007/s00265-006-0325-7 (doi:10.1007/s00265-006-0325-7) [DOI] [Google Scholar]
- 18.Bargh J. A., Chartrand T. L. 2000. Studying the mind in the middle: a practical guide to priming and automaticity research. In Handbook of research methods in social psychology (eds Reis H., Judd C.), pp. 253–285 New York, NY: Cambridge University Press [Google Scholar]
- 19.Duncan L. A., Schaller M., Park J. H. 2009. Perceived vulnerability to disease: development and validation of a 15-item self-report instrument. Pers. Indiv. Differ. 47, 441–546 10.1016/j.paid.2009.05.001 (doi:10.1016/j.paid.2009.05.001) [DOI] [Google Scholar]
- 20.Jones W. H., Russell D. W., Nickel T. W. 1977. Belief in the paranormal scale: an instrument to measure beliefs in magical phenomena and causes. JSAS Catalogue Sel. Doc. Psychol. 7, 100 [Google Scholar]
- 21.Lassek W. D., Gaulin S. J. C. 2009. Costs and benefits of fat-free muscle mass in men: relationship to mating success, dietary requirements, and native immunity. Evol. Hum. Behav. 30, 322–328 10.1016/j.evolhumbehav.2009.04.002 (doi:10.1016/j.evolhumbehav.2009.04.002) [DOI] [Google Scholar]
- 22.Simpson J. A., Gangestad S. W. 1992. Sociosexuality and romantic partner choice. J. Pers. 60, 31–51 10.1111/j.1467-6494.1992.tb00264.x (doi:10.1111/j.1467-6494.1992.tb00264.x) [DOI] [Google Scholar]
- 23.Kruger D. J. 2006. Male facial masculinity influences attributions of personality and reproductive strategy. Pers. Relationships 13, 451–463 10.1111/j.1475-6811.2006.00129.x (doi:10.1111/j.1475-6811.2006.00129.x) [DOI] [Google Scholar]