Abstract
Cleaning behaviour is considered to be a classical example of mutualism. However, no studies, to our knowledge, have measured the benefits to clients in terms of growth. In the longest experimental study of its kind, over an 8 year period, cleaner fish Labroides dimidiatus were consistently removed from seven patch reefs (61–285 m2) and left undisturbed on nine control reefs, and the growth and parasite load of the damselfish Pomacentrus moluccensis determined. After 8 years, growth was reduced and parasitic copepod abundance was higher on fish from removal reefs compared with controls, but only in larger individuals. Behavioural observations revealed that P. moluccensis cleaned by L. dimidiatus were 27 per cent larger than nearby conspecifics. The selective cleaning by L. dimidiatus probably explains why only larger P. moluccensis individuals benefited from cleaning. This is the first demonstration, to our knowledge, that cleaners affect the growth rate of client individuals; a greater size for a given age should result in increased fecundity at a given time. The effect of the removal of so few small fish on the size of another fish species is unprecedented on coral reefs.
Keywords: symbiosis, cooperation, coral reef ecology, fish behaviour, Labridae, parasitism
1. Introduction
Mutualisms play a significant role in the diversity and stability of ecosystems [1]. Therefore, determining the benefits to participants is essential for understanding the selective processes involved in the evolution of such key interactions. Alas, this information is often not available, particularly in marine systems [2]. One of the most common interactions among coral reef fishes is cleaning behaviour, where cleaners benefit from removing and eating ectoparasites from clients and presumably control client parasite loads [2]. Cleaning behaviour of Labroides dimidiatus is currently used to test general theories about cooperation (e.g. [3]), under the assumption that both partners benefit. However, while the diets of obligate cleaner fishes [4], and hence the benefits to cleaners, are well known, no studies, to our knowledge, have examined the effect of cleaning on client growth.
Previous experimental studies involving the removal of cleaner fish to examine their effect on client parasites show conflicting results. Early studies lasting six months to 2 years [5,6] found no detectable effects; by contrast, for caged, relatively large, Hemigymnus melapterus clients, the absence of L. dimidiatus cleaners increased the abundance of parasitic isopods over 24 h and 12 days [7,8]. Whether cleaners affect the parasite load of small species remains unclear. Clients with natural cleaner access had higher body condition and a lower antibody response than those without, suggesting that cleaner access decreases the need for active immunity, releasing resources allocated to somatic growth [9]. Cleaner absence for 8.5 years reduced fish length in two damselfishes, but fish growth was not measured [10]. These conflicting results raised questions about the proximate and ultimate causes of cleaning behaviour in clients [6].
Therefore, we examined the effects of 8 years of L. dimidiatus absence on the growth and parasites of the small, common, site-attached damselfish Pomacentrus moluccensis, and whether the size of individual P. moluccensis affects their likelihood of being cleaned, as there may be an interaction between the benefits of cleaning and client size. No studies, to our knowledge, have examined the effects of cleaner fish presence on parasite loads over the long term (greater than 2 years). Therefore, this study was conducted on reefs where cleaner presence was experimentally manipulated for 8 years.
2. Material and methods
This study is a continuation of an experiment conducted off Lizard Island, Great Barrier Reef, at two sites, Casuarina Beach and the Lagoon (see Grutter et al. [11] for reef distributions and sample sizes). All L. dimidiatus (one to five adults, zero to three juveniles/reef) were removed from seven, randomly selected, patch reefs in September 2000 and left undisturbed on nine controls; removal reefs were inspected every few months for L. dimidiatus recruits and these were again removed (29% of reef inspections, 81% involving one to two individuals per reef, usually juveniles, electronic supplementary material, table S1).
Pomacentrus moluccensis live up to 6 years at Lizard Island (S. L. Bray 2009, personal communication); therefore, most individuals probably experienced experimental conditions for their entire lives. Pomacentrus moluccensis (n = 10 per reef) were collected from 9 October until 26 October 2008 and the parasites were quantified (see the electronic supplementary material). Fish growth was measured using fish standard length (SL) and by estimating fish age, using otolith annual rings [12] or daily rings [13] for fish less than 1 year. To determine how client size affects how often they are cleaned, cleaning interactions between P. moluccensis and L. dimidiatus were observed by SCUBA divers between 29 August and 4 September 2009 on control reefs, except Reef 1. A L. dimidiatus was haphazardly selected, its size was estimated (40–86 mm total length), and behaviour was observed until it had engaged in one cleaning interaction (any physical contact between cleaner and client, or greater than 1 s inspection by the cleaner) with P. moluccensis. This location on the reef was marked with a small, labelled weight. The SL of the cleaned P. moluccensis, and conspecifics within a 1 m radius of the marker, were estimated. For client size estimation and statistical analyses, see the electronic supplementary material.
3. Results
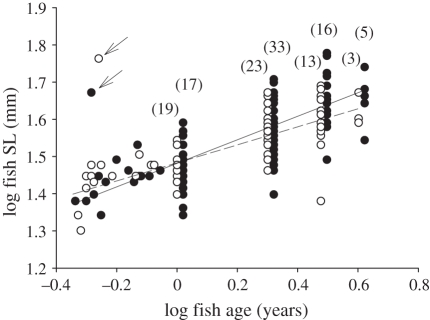
There was a significant interaction between cleaner presence and fish age (linear mixed-effects model, F1,135 = 4.15, p = 0.0435), indicating that the slope of the relationship between response log fish SL and covariate log age differed with cleaner presence (figure 1). Examination of figure 1 suggested that the difference in size between the cleaned and uncleaned fish were greater for older than younger fish. To test this, fish were separated into less than 1 year and greater than or equal to 1 year and data pooled across reefs and analysed as above; this revealed that the interaction between cleaner presence and fish age was not significant for fish of less than 1 year (F1,20 = 0.2626, p = 0.6139) but was significant for fish greater than or equal to 1 year (F1,121 = 5.2578, p = 0.0236) supporting the above interpretation. Thus, for example, for 3 or 4 year old fish, the estimated average size (SL, 95% CI) of fish on reefs with and without cleaners was 42.7, 40.6–44.9 and 40.0, 38.1–42.0 or 46.8, 43.9–49.8 and 42.9, 40.4–45.6 mm, an average decrease of 7 per cent and 9 per cent, respectively. All other interactions were not significant (p ≥ 0.1332). There was a significant effect of site (F1,12 = 16.82, p = 0.0015), owing to fish being smaller at Casuarina Beach (figure 1).
Figure 1.
Fish growth of P. moluccensis, derived from the regression of log fish standard length (SL) and log age, from reefs with (black circles, solid line) and without (white circles, dashed line) cleaner fish L. dimidiatus. Annual ages were jittered to reveal underlying data. Arrows indicate outliers omitted. Sample sizes in brackets.
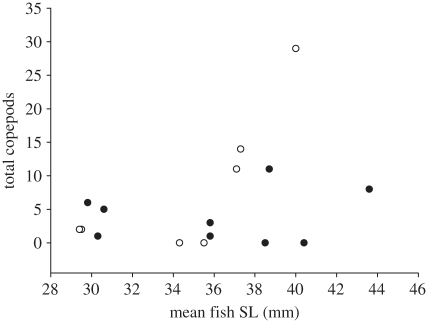
Pomacentrus moluccensis were mainly infected with parasitic copepods (electronic supplementary material, figure S1), identified as juveniles (G. Boxshall 2010, personal communication). There was an interaction between cleaner presence and mean fish SL per reef on the abundance of juvenile copepods (generalized linear model with a quasi-Poisson distribution: F1,12 = 5.5189, p = 0.0368; figure 2); examination of this interaction revealed that on reefs without cleaners, copepod numbers increased with fish size (Spearman rank correlation: rs = 0.82, n = 7, p = 0.025), while on control reefs, this relationship was not significant (rs = 0.08, n = 9, p = 0.84; figure 2). Copepod length (median, 25–75th quantiles) did not vary between reefs with (0.86, 0.8–0.9 mm) and without (0.77, 0.6–1.0 mm) cleaners (Wilcoxan test Z = 1.19, p = 0.234). Other parasites were a turbellarian worm, Transversotrema sp. (Digenea) worm, and female Hatschekia crenulata copepod; all on separate fish from control reefs.
Figure 2.
Parasitic copepod juveniles on P. moluccensis, summed across 10 fish sampled per reef, compared with mean fish SL per reef on reefs with (black circles) and without (white circles) cleaner fish L. dimidiatus.
Pomacentrus moluccensis that were cleaned were significantly larger than the average conspecific within a 1 m radius of the cleaning interaction (F1,99 = 149.09, p < 0.0001). The overall mean SL ± s.e. of the mean SL per reef of cleaned P. moluccensis and individuals within a 1 m radius were 32.4 ± 0.9 mm and 25.7 ± 0.8 mm, respectively. On average, this difference per reef was 27% ± 2; the covariate, cleaner size, was not significant (F1,91 = 0.18, p = 0.670).
4. Discussion
The removal of one to five L. dimidiatus cleaners per reef decreased the growth and increased the parasitic copepod load of damselfish P. moluccensis; however, only larger individuals were affected. In support of this finding, L. dimidiatus appeared to selectively clean larger P. moluccensis compared with conspecifics in the vicinity. This study shows that individuals of a small relatively infrequently cleaned client species [14] benefit from cleaning, and provides, to our knowledge, the first confirmation that cleaner fish affect the growth of client reef fish.
On reefs with cleaner fish, older fish were larger for a given age compared with reefs without cleaner fish. This difference in growth probably affects the fecundity of clients at a given time as female size and fecundity are highly correlated in many fishes [15], and suggests an increased reproductive output of P. moluccensis individuals on reefs with cleaner fish.
This is, to our knowledge, the first study to show an effect of cleaner fish on parasitic copepod abundance. The abundance of parasitic copepod larvae was positively correlated with P. moluccensis size on reefs without cleaner fish, while no such relationship was observed on reefs with cleaners present. This pattern suggests that cleaners may remove parasites more often from larger P. moluccensis than smaller individuals, a conclusion supported by our behavioural observations. Parasites can affect damselfish in many ways, including causing higher metabolism and slower growth [16–18]. Our result differs from a previous six month study [5] at the same location, which found no effect of L. dimidiatus presence on copepod abundance on P. moluccensis. Labroides dimidiatus mostly eats gnathiid isopods and few parasitic copepods [19]. Despite this low feeding rate on copepods, this parasite was still affected by cleaner fish presence. The patterns are unlikely owing to fish movement patterns as P. moluccensis are sedentary; of 700 tagged individuals, recaptured two to four times per year for 6 years, only two moved greater than 10 m [20].
Pomacentrus moluccensis individuals cleaned by L. dimidiatus tended to be 27 per cent larger than the conspecifics present within a 1 m radius of the fish being cleaned. Both cleaners and clients can initiate cleaning behaviour [21]; however, L. dimidiatus prefer large over small model clients [22]. This may explain why parasite loads of larger, but not smaller, individual clients were affected by the presence of cleaner fish. Parasite load is also known to affect the tendency of a client to seek L. dimidiatus [23]. Thus, the higher parasite load of larger fishes suggests that parasite removal may be a proximate cause of cleaning and could also explain why such fish ultimately benefited from cleaning. These findings agree with theoretical predictions that net outcomes of mutualistic interactions can be related to partner size [24].
Pomacentrus moluccensis are cleaned relatively infrequently (1.6 s per inspection, 23 inspections per day) [14], yet benefited from cleaning. Since larger species are cleaned more frequently (e.g. H. melapterus, 6.8 s per inspection, 113 inspections per day), they may benefit even more. Thus, it is relatively safe to conclude that larger client species probably also obtain such benefits. This study reveals the impacts of parasites and cleaners on individual fish growth. A small amount of cleaning of small organisms (parasitic copepods) can have a serious and significant consequent effect on individual body size.
Acknowledgements
This research was approved by the University of Queensland Animal Ethics Committee.
We thank Lizard Island Research Station, many field volunteers, G. Boxshall for parasite identification, L. Dong for otolith analyses, S. Blomberg for statistical advice and the Australian Research Council and The University of Queensland for funding.
References
- 1.Stachowicz J. J. 2001. Mutualism, facilitation, and the structure of ecological communities. BioScience 51, 235–246 10.1641/0006-3568(2001)051[0235:MFATSO]2.0.CO;2 (doi:10.1641/0006-3568(2001)051[0235:MFATSO]2.0.CO;2) [DOI] [Google Scholar]
- 2.Grutter A. S., Irving A. D. 2007. Positive interactions in marine communities. In Marine ecology (eds Connell S. D., Gillanders B. M.), pp. 110–137 Oxford, UK: Oxford University Press [Google Scholar]
- 3.Bshary R., Grutter A. S. 2006. Image scoring and cooperation in a cleaner fish mutualism. Nature 441, 975–978 10.1038/nature04755 (doi:10.1038/nature04755) [DOI] [PubMed] [Google Scholar]
- 4.Grutter A. S. 1997. Spatiotemporal variation and feeding selectivity in the diet of the cleaner fish Labroides dimidiatus. Copeia 1997, 346–355 10.2307/1447754 (doi:10.2307/1447754) [DOI] [Google Scholar]
- 5.Grutter A. S. 1996. Experimental demonstration of no effect by the cleaner wrasse Labroides dimidiatus (Cuvier and Valenciennes) on the host fish Pomacentrus moluccensis (Bleeker). J. Exp. Mar. Biol. Ecol. 196, 285–298 10.1016/0022-0981(95)00135-2 (doi:10.1016/0022-0981(95)00135-2) [DOI] [Google Scholar]
- 6.Gorlick D. L., Atkins P. D., Losey G. S. 1987. Effect of cleaning by Labroides dimidiatus (Labridae) on an ectoparasite population infecting Pomacentrus vaiuli (Pomacentridae) at Enewetak Atoll. Copeia 1987, 41–45 10.2307/1446035 (doi:10.2307/1446035) [DOI] [Google Scholar]
- 7.Grutter A. S. 1999. Cleaner fish really do clean. Nature 398, 672–673 10.1038/19443 (doi:10.1038/19443) [DOI] [Google Scholar]
- 8.Grutter A. S., Lester R. J. G. 2002. Cleaner fish Labroides dimidiatus reduce ‘temporary’ parasitic corallanid isopods on the coral reef fish Hemigymnus melapterus. Mar. Ecol. Prog. Ser. 234, 247–255 10.3354/meps234247 (doi:10.3354/meps234247) [DOI] [Google Scholar]
- 9.Ros A. F. H., Lusa J., Meyer M., Soares M., Oliveira R. F., Brossard M., Bshary R. 2011. Does access to the bluestreak cleaner wrasse Labroides dimidiatus affect indicators of stress and health in resident reef fishes in the Red Sea? Horm. Behav. 59, 151–158 10.1016/j.yhbeh.2010.11.006 (doi:10.1016/j.yhbeh.2010.11.006) [DOI] [PubMed] [Google Scholar]
- 10.Waldie P. A., Blomberg S. P., Cheney K. L., Goldizen A. W., Grutter A. S. In press Long-term effects of the cleaner fish Labroides dimidiatus on coral reef fish communities. PLoS ONE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grutter A. S., Murphy J. M., Choat J. H. 2003. Cleaner fish drives local fish diversity on coral reefs. Curr. Biol. 13, 64–67 10.1016/S0960-9822(02)01393-3 (doi:10.1016/S0960-9822(02)01393-3) [DOI] [PubMed] [Google Scholar]
- 12.Gauldie R. W., Nelson D. G. A. 1990. Otolith growth in fishes. Comp. Biochem. Physiol. A 97, 119–135 10.1016/0300-9629(90)90159-P (doi:10.1016/0300-9629(90)90159-P) [DOI] [Google Scholar]
- 13.Wilson D. T., McCormick M. I. 1997. Spatial and temporal validation of settlement-marks in the otoliths of tropical reef fishes. Mar. Ecol. Prog. Ser. 153, 259–271 10.3354/meps153259 (doi:10.3354/meps153259) [DOI] [Google Scholar]
- 14.Grutter A. S. 1995. Relationship between cleaning rates and ectoparasite loads in coral reef fishes. Mar. Ecol. Prog. Ser. 118, 51–58 10.3354/meps118051 (doi:10.3354/meps118051) [DOI] [Google Scholar]
- 15.Birkeland C., Dayton P. 2005. The importance in fishery management of leaving the big ones. Trends Ecol. Evol. 20, 356–358 [DOI] [PubMed] [Google Scholar]
- 16.Adlard R. D., Lester R. J. G. 1994. Dynamics of the interaction between the parasitic isopod, Anilocra pomacentri, and the coral reef fish, Chromis nitida. Parasitology 109, 311–324 10.1017/S0031182000078343 (doi:10.1017/S0031182000078343) [DOI] [PubMed] [Google Scholar]
- 17.Jones C. M., Grutter A. S. 2008. Reef-based micropredators reduce the growth of post-settlement damselfish in captivity. Coral Reefs 27, 677–684 10.1007/s00338-008-0383-6 (doi:10.1007/s00338-008-0383-6) [DOI] [Google Scholar]
- 18.Grutter A. S., Crean A. J., Curtis L. M., Kuris A. M., Warner R. R., McCormick M. I. 2011. Indirect effects of an ectoparasite reduce successful establishment of a damselfish at settlement. Funct. Ecol. 25, 586–594 10.1111/j.1365-2435.2010.01798.x (doi:10.1111/j.1365-2435.2010.01798.x) [DOI] [Google Scholar]
- 19.Grutter A. S. 1996. Parasite removal rates by the cleaner wrasse Labroides dimidiatus. Mar. Ecol. Prog. Ser. 130, 61–70 10.3354/meps130061 (doi:10.3354/meps130061) [DOI] [Google Scholar]
- 20.Mapstone B. D. 1988. Patterns in the abundance of Pomacentrus moluccensis Bleeker. PhD thesis, pp. 240, University of Sydney, Australia [Google Scholar]
- 21.Côté I. M., Arnal C., Reynolds J. D. 1998. Variation in posing behaviour among fish species visiting cleaning stations. J. Fish Biol. 53, 256–266 10.1111/j.1095-8649.1998.tb01031.x (doi:10.1111/j.1095-8649.1998.tb01031.x) [DOI] [Google Scholar]
- 22.Grutter A. S., Glover S., Bshary R. 2005. Does client size affect cleaner fish choice of client? An empirical test using client fish models. J. Fish Biol. 66, 1748–1752 10.1111/j.0022-1112.2005.00709.x (doi:10.1111/j.0022-1112.2005.00709.x) [DOI] [Google Scholar]
- 23.Grutter A. S. 2001. Parasite infection rather than tactile stimulation is the proximate cause of cleaning behaviour in reef fish. Proc. R. Soc. Lond. B 268, 1361–1365 10.1098/rspb.2001.1658 (doi:10.1098/rspb.2001.1658) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnstone R. A., Bshary R. 2002. From parasitism to mutualism: partner control in asymmetric interactions. Ecol. Lett. 5, 634–639 10.1046/j.1461-0248.2002.00358.x (doi:10.1046/j.1461-0248.2002.00358.x) [DOI] [Google Scholar]