Abstract
Animal communication through colour signals is a central theme in sexual selection. Structural colours can be just as costly and honest signals as pigment-based colours. Ultraviolet (UV) is a structural colour that can be important both in intrasexual competition and mate choice. However, it is still unknown if a UV signal alone can determine the outcome of male–male fights. European green lizard (Lacerta viridis) males develop a nuptial throat coloration with a strong UV component. Among males differing only in their manipulated UV colour, females prefer males with higher UV. Here, we experimentally decreased the UV coloration of randomly chosen males from otherwise similar male pairs to test the hypothesis that a difference in UV colour alone can affect fight success during male–male competition. Our results fully supported the hypotheses: in almost 90 per cent of the contests the male with reduced UV lost the fight. Our results show that UV can be an important signal, affecting both female mate choice and determining male fight success.
Keywords: communication, Lacerta viridis, male–male competition, sexual selection, signal, ultraviolet
1. Introduction
Conspicuous coloration is a key feature in sexual selection that has been studied in a wide variety of taxa in terms of mate preference, contest competition and species recognition [1–4]. After some controversy in the past, it has been accepted recently that structural colours (e.g. ultraviolet, UV) have development and maintenance costs just like pigment-based colours [5], and thus they can act as honest signals of individual quality (sensu [6,7]). However, the exact information conveyed by UV signals is rarely revealed. Previous studies showed that both male and female mate preference can be based on UV signals in different taxa [8–11]. Fewer studies have investigated the role of UV colours during male–male competition [12–16], even though showing that a UV signal alone can affect the outcome of male aggressive encounters would indicate a direct connection to male quality, and further strengthen the similarities between pigment-based and structural colours. However, experiments where only the strength of the UV signal differs between contestants and where staged aggressive encounters are assessed are still lacking.
The aim of the present study was to examine whether the success of male–male fights is influenced by the UV reflectance of the nuptial throat patch of male European green lizards (Lacerta viridis). Males with high UV reflectance on their throats are preferred by receptive females, based on tests where females chose between males differing only in (manipulated) UV colour [11]. If throat UV was a signal in both inter- and intrasexual selection, the range of possible evolutionary explanations could be narrowed. Here, we studied if male L. viridis, with artificially reduced throat UV reflectance, chosen randomly from morphologically matching pairs, are more likely to lose fights (males fight vigorously during the mating season) than the corresponding control males from a pair.
2. Material and methods
(a). Sampling and measurements
Lacerta viridis is one of the most common Lacertid lizards in Europe. Males develop blue nuptial coloration on their throats, which has strong reflectance in the UV range [11,17]. We captured 40 males in April 2007 near Tápiószentmárton, Hungary; (47°20′25″ N, 19°47′11″ E). They were housed individually, and fed with mealworms (Tenebrio molitor) and crickets (Gryllus bimaculatus) dusted with vitamin powder ad libitum. We measured lizards' body weight (BW) to the nearest 0.01 g and snout-vent length (SVL), head length, head width and head depth to the nearest 0.1 mm. We ran a principal components analysis (PCA) on the head measures, which resulted in one PC with an eigenvalue greater than 1 (eigenvalue = 2.623, variation explained = 87.43%), describing head size (factor loadings less than −0.9). Throat colour was measured using spectrometer-type Ocean Optics 2000 [15], complete with a Mini-D2 deuterium–halogen lamp connected to fibre-optic probe (for details of the measurements, see [11]). We measured spectral reflectance (R) from 320 to 700 nm immediately before and after manipulation (see below). We calculated three variables describing throat colour [11,18]: (i) brightness, the total reflectance (R320–700 nm), (ii) UV chroma (R320–400/R320–700), and (iii) blue chroma (R400–490/R320–700).
(b). Experiment
We created 20 pairs of males with a maximum SVL difference of 2 mm. Males within pairs were assigned randomly to the control or UV-reduced groups. Initial morphological and colour differences between treatment groups were tested with paired t-tests. UV-reduction was carried out as explained in Bajer et al. [11]. Control males' throats were treated with duck preen gland fat, while UV-reduced males received a treatment with UV-reducing agents mixed with the fat. To see whether our UV-reducing treatment was successful, we used paired t-tests to compare colour variables between treatment groups.
The experiments were carried out in the natural habitat of L. viridis on sunny, low wind and no rain days. Trials were conducted from 15 to 18 May 2007 between 08.00 and 16.00 h, in five glass terraria (40 × 80 × 40 cm; width × length × height, respectively) with a removable opaque divider in the middle. First, we put males of a given pair randomly into the separated compartments and let them acclimatize for 10 min. Then, we raised the middle wall to allow males to interact. Observations were made from a blind during all trials. Between subsequent trials, we washed the terraria with detergent in order to remove any chemical stimuli left by lizards from the previous trial. Every male was used only once. If lizards did not display aggressive behaviour (approaching contestant with the back arched, head lowered and throat inflated, forcing the contestant either to respond aggressively or escape) within 20 min, we considered the trial unsuccessful. Three of the 20 trials were unsuccessful, while there were clear winners/losers in the rest. The trials were terminated when a male first escaped the other. The escaping male was assigned as loser, while the other as winner. We did not aim to analyse fine details of behaviour, but rather focused solely on the functional outcome of the encounter. Fight success was analysed with a χ2-test based on a 2 × 2 contingency table with treatment and success entered. When experiments finished, all males were released unharmed at the site of capture.
3. Results
Neither morphology (SVL: t16 = −0.58, p = 0.57; BW: t16 = −0.823, p = 0.42; head size: t16 = 0.181, p = 0.86) nor colour (UV chroma: t16 = 0.34, p = 0.74; blue chroma: t16 = 1.54, p = 0.14; brightness: t16 = 0.32, p = 0.75) differed between the UV-reduced versus control males prior to manipulation.
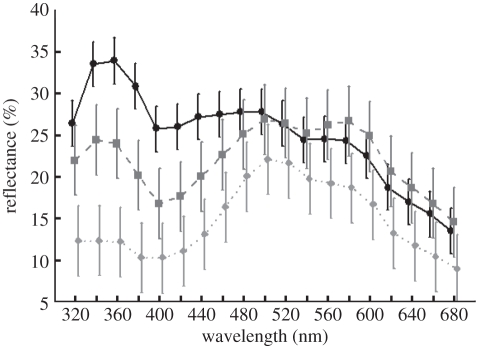
After manipulations, total brightness and UV chroma of UV-reduced males were significantly lower than those of control males (brightness: t16 = −3.711, p = 0.002; UV chroma: t16 = −2.391, p = 0.03), while blue chroma did not differ (t16 = −0.021, p = 0.98). Hence, our treatments were effective in reducing relative UV reflectance (and were strong enough to affect the overall reflectance; figure 1). The manipulation of throat UV reflectance determined fight success (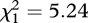 ; p = 0.02). Out of the 17 staged aggressive encounters where the males showed aggressive behaviour, the UV-reduced male retreated 15 times.
; p = 0.02). Out of the 17 staged aggressive encounters where the males showed aggressive behaviour, the UV-reduced male retreated 15 times.
Figure 1.
Mean reflectance (+95% CI) measured per 20 nm in the 320–700 nm range on the throat patch of male Lacerta viridis prior to manipulation (filled circles; n = 40) and after the UV-reducing (filled diamonds; n = 20) and control (filled squares; n = 20) treatments. UV range = 320–400 nm, blue range = 400–490 nm.
4. Discussion
We found that male L. viridis with experimentally reduced throat UV were more likely to lose the fights than control males, even though the contestants were matching in body and head size. Our results are interesting because, owing to our experimental design, the differently treated males within fighting male pairs could not systematically differ in any other correlated trait; it was the manipulated UV alone that determined the success of the fights with 88 per cent probability. Therefore, throat UV is clearly a male quality signal in L. viridis.
When considering random male pairs, body size alone, or body size combined with relative head size are likely to be the real determinants of fight success [19–21]. However, such differences might not be easy and quick to assess for males. Signals advertising fighting ability allow male lizards to assess probability of winning and, therefore, to avoid energetically costly escalated fighting, injuries, and increased predation risk [22,23], and to gain time and energy for other tasks like feeding, mate search or thermoregulation [4]. The role of UV signals in advertising dominance status or aggression has been shown in different taxa [13–16]. In our experiment, aggressive displays were abundant, but they rarely escalated to physical fights. Still, in 17 out of 20 cases, one male gave up and tried to escape from the other who pursued the loser, suggesting that males made their decision without taking the risks involved in actual fights. As everything else was equal (or differed randomly), the manipulated UV signal alone must have been the cue used in males' decisions. A possible explanation is that throat colour may be an amplifier of head size, which usually correlates with bite force [24], and thus males may use throat colour as an indicator of each other's bite force [25].
Signals important in one context (intra- versus intersexual) may not be important in the other [1,25]. In our case, male throat UV is not only an important cue in female mate choice [11], but it also determines fight success, making Fisherian runaway [26] as the process behind the signal's evolution highly unlikely. UV was accepted as conferring comparable costs with pigment-based colours only recently [5]. For instance, higher UV colour is negatively correlated with health state in the lizard Lacerta schreiberi [25]. In L. viridis, we found that high throat UV is negatively correlated with body condition in the field (O. Molnár, K. Bajer, J. Török & G. Herczeg 2011, unpublished data), and its annual development is dependent on the time available for maintaining optimal body temperature (K. Bajer, O. Molnár, J. Török & G. Herczeg 2011, unpublished data), suggesting that it is a costly signal and thus can honestly reveal individual quality. Our present results are in line with this scenario, suggesting that throat UV is a reliable signal of fight ability, allowing male L. viridis to judge each other without engaging in costly physical fights. Because UV in this species is important in both settling aggressive encounters between males and female mate choice, we suggest that UV, or other structural colours, can be more important in sexual selection than previously presumed.
Acknowledgements
Experiments were performed according to the guidelines of the Hungarian Act of Animal Care and Experimentation (1998, XXVIII, section 243/1998), which conforms to the regulation of animal experiments by the European Union. The experiment was carried out under the license of the Middle-Danube-Valley Inspectorate for Environmental Protection, Nature Conservation and Water Management (no. 21765/2007).
References
- 1.Andersson M. 1994. Sexual selection. Princeton, NJ: Princeton University Press [Google Scholar]
- 2.Losey G. S. J., Cronin T. W., Goldsmith T. H., Hyde D., Marshall N. J., McFarland W. N. 1999. The UV visual world of fishes: a review. J. Fish Biol. 54, 921–943 10.1111/j.1095-8649.1999.tb00848.x (doi:10.1111/j.1095-8649.1999.tb00848.x) [DOI] [Google Scholar]
- 3.Hoffman E. A., Boulin M. S. 2000. A review of colour and pattern polymorphisms in anurans. Biol. J. Linnean Soc. 70, 633–665 10.1111/j.1095-8312.2000.tb00221.x (doi:10.1111/j.1095-8312.2000.tb00221.x) [DOI] [Google Scholar]
- 4.Whiting M. J., Nagy K. A., Bateman P. W. 2003. Evolution and maintenance of social status signalling badges: experimental manipulations in lizards. In Lizard social behavior (eds Fox S. F., McCoy J. K., Baird T. A.), pp. 47–82 Baltimore, MD: Johns Hopkins University Press [Google Scholar]
- 5.Prum R. O. 2006. Anatomy, physics, and evolution of structural colors. In Bird coloration, volume 1: mechanisms and measurements (eds Hill G. E., McGraw K. J.), pp. 295–353 Cambridge, MA: Harvard University Press [Google Scholar]
- 6.Grafen A. 1990. Biological signals as handicaps. J. Theor. Biol. 144, 517–546 10.1016/S0022-5193(05)80088-8 (doi:10.1016/S0022-5193(05)80088-8) [DOI] [PubMed] [Google Scholar]
- 7.Guilford T. 1995. Animal signals: all honesty and light? Trends Ecol. Evol. 10, 100–101 10.1016/S0169-5347(00)89001-1 (doi:10.1016/S0169-5347(00)89001-1) [DOI] [PubMed] [Google Scholar]
- 8.Andersson S., Ornborg J., Andersson M. 1998. Ultraviolet sexual dimorphism and assortative mating in blue tits. Proc. R. Soc. Lond. B 265, 445–450 10.1098/rspb.1998.0315 (doi:10.1098/rspb.1998.0315) [DOI] [Google Scholar]
- 9.LeBas N. R., Marshall N. J. 2000. The role of colour in signalling and male choice in the agamid lizard Ctenophorus ornatus. Proc. R. Soc. Lond. B 267, 445–452 10.1098/rspb.2000.1020 (doi:10.1098/rspb.2000.1020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rick I. P., Bakker T. C. M. 2008. UV wavelengths make female threespined sticklebacks (Gasterosteus aculeatus) more attractive for males. Behav. Ecol. Sociobiol. 62, 439–445 10.1007/s00265-007-0471-6 (doi:10.1007/s00265-007-0471-6) [DOI] [Google Scholar]
- 11.Bajer K., Molnár O. R., Török J., Herczeg G. 2010. Female European green lizards (Lacerta viridis) prefer males with high ultraviolet throat reflectance. Behav. Ecol. Sociobiol. 64, 2007–2014 10.1007/s00265-010-1012-2 (doi:10.1007/s00265-010-1012-2) [DOI] [Google Scholar]
- 12.Alonso-Alvarez C., Doutrelant C., Sorci G. 2004. Ultraviolet reflectance affects male–male interactions in the blue tit (Parus caeruleus ultramarinus). Behav. Ecol. 15, 805–809 10.1093/beheco/arh083 (doi:10.1093/beheco/arh083) [DOI] [Google Scholar]
- 13.Siebeck U. E. 2004. Communication in coral reef fish: the role of ultraviolet colour patterns in damselfish territorial behaviour. Anim. Behav. 68, 273–282 10.1016/j.anbehav.2003.11.010 (doi:10.1016/j.anbehav.2003.11.010) [DOI] [Google Scholar]
- 14.Siefferman L., Hill G. E. 2005. Evidence for sexual selection on structural plumage coloration in female eastern bluebirds (Sialia sialis). Evolution 59, 1819–1828 [PubMed] [Google Scholar]
- 15.Stapley J., Whiting M. J. 2006. Ultraviolet signals fighting ability in a lizard. Biol. Lett. 22, 169–172 10.1098/rsbl.2005.0419 (doi:10.1098/rsbl.2005.0419) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whiting M. J., Stuart-Fox D. M., O'Connor D., Firth D., Bennett N. C., Blomberg S. P. 2006. Ultraviolet signals ultra-aggression in a lizard. Anim. Behav. 72, 353–363 10.1016/j.anbehav.2005.10.018 (doi:10.1016/j.anbehav.2005.10.018) [DOI] [Google Scholar]
- 17.Václav R., Prokop P., Fekiač V. 2007. Expression of breeding coloration in European green lizards (Lacerta viridis): variation with morphology and tick infestation. Can. J. Zool. 85, 1199–1206 10.1139/Z07-102 (doi:10.1139/Z07-102) [DOI] [Google Scholar]
- 18.Hill G. E., McGraw K. J. 2006. Bird coloration, vol. 1. Mechanisms and measurements. Cambridge, MA: Harvard University Press [Google Scholar]
- 19.Cooper W. E., Jr, Vitt L. J. 1993. Female mate choice of large male broad-headed skinks. Anim. Behav. 45, 683–693 10.1006/anbe.1993.1083 (doi:10.1006/anbe.1993.1083) [DOI] [Google Scholar]
- 20.Censky E. J. 1995. Mating strategy and reproductive success in the teiid lizard, Ameiva plei. Behaviour 132, 529–557 10.1163/156853995X00199 (doi:10.1163/156853995X00199) [DOI] [Google Scholar]
- 21.Salvador A., Veiga J. P. 2001. Male traits and pairing success in the lizard Psammodromus algirus. Herpetologica 57, 77–86 [Google Scholar]
- 22.Zuk M., Kolluru G. R. 1998. Exploitation of sexual signals by predators and parasitoids. Q. Rev. Biol. 73, 415–438 10.1086/420412 (doi:10.1086/420412) [DOI] [Google Scholar]
- 23.López P., Martín J., Cuadrado M. 2004. The role of lateral blue spots in intrasexual relationships between male Iberian rock-lizards, Lacerta monticola. Ethology 110, 543–561 10.1111/j.1439-0310.2004.00996.x (doi:10.1111/j.1439-0310.2004.00996.x) [DOI] [Google Scholar]
- 24.Lappin A. K., Hamilton P. S., Sullivan B. K. 2006. Bite-force performance and head shape in a sexually dimorphic crevice-dwelling lizard, the common chuckwalla [Sauromalus ater (= obesus)]. Biol. J. Linnean Soc. 88, 215–222 10.1111/j.1095-8312.2006.00615.x (doi:10.1111/j.1095-8312.2006.00615.x) [DOI] [Google Scholar]
- 25.Martín J., López P. 2009. Multiple color signals may reveal multiple messages in male Schreiber's green lizards, Lacerta schreiberi. Behav. Ecol. Sociobiol. 63, 1743–1755 10.1007/s00265-009-0794-6 (doi:10.1007/s00265-009-0794-6) [DOI] [Google Scholar]
- 26.Fisher R. A. 1915. The evolution of sexual preference. Eugenics Rev. 7, 115–123 [PMC free article] [PubMed] [Google Scholar]