Abstract
Lactation is the most energetically expensive component of reproduction in mammals. Theory predicts that reproducing females will adjust their behaviour to compensate for increased nutritional demands. However, experimental tests are required, since comparisons of the behaviour of naturally reproducing and non-reproducing females cannot distinguish between true costs of reproduction, individual differences or seasonal variation. We experimentally manipulated reproduction in free-ranging, eastern grey kangaroos (Macropus giganteus), using a fertility control agent. Our novel field experiment revealed that females altered their behaviour in direct response to the energetic demands of reproduction: reproducing females increased bite rates, and thus food intake, when the energetic demands of lactation were highest. Reproducing females did not reduce the time spent on vigilance for predators, but increased their forage intake on faecal-contaminated pasture, thereby increasing the risk of infection by gastrointestinal parasites—a largely unrecognized potential cost of reproduction.
Keywords: cost of reproduction, eastern grey kangaroo, foraging behaviour, lactation, Macropus giganteus
1. Introduction
A fundamental assumption of life-history theory is that reproduction is costly [1]. These costs are typically borne by females, through maternal care of offspring, requiring sufficient resources to meet metabolic demands of both mother and offspring. In mammals, energetic costs are associated with the transfer of resources from a mother to her offspring, especially during lactation. Energetic expenditure during peak lactation varies with the number of offspring [2] and can be up to twice that of non-lactating females [3]. Other maternal behaviours, such as grooming [4] and avoiding predation [5], may impose additional costs upon a mother. These costs may be reduced by compensatory behavioural adjustments, with mothers increasing energy intake rather than draining somatic reserves [3].
Studies of reproductive costs in the wild typically focus on the natural covariation between reproductive status and foraging, and may be confounded by either the inherent quality of reproducing and non-reproducing females, or seasonal variation in environmental variables. Intrinsic differences among individuals, including age, social rank, prior breeding experience, body condition and ability to conceive and genetic quality can affect breeding success [6]. Foraging behaviour may similarly vary with these intrinsic differences [7]. Likewise, feeding rates are often affected by seasonal food availability [8], which can covary with reproductive activity. Consequently, it is unclear whether changes in the behaviour of females during reproduction are due to reproductive state or other underlying factors.
Field experiments using anti-fertility agents to manipulate reproduction in females can control for these confounding factors [9]. In a rare example, MacWhirter [10] treated Columbian ground squirrels, Spermophilus columbianus, with a chemosterilant, and showed that parous females spent more time foraging above-ground than treated females. Marsupials are an ideal model taxon for such experiments, because their brief gestation is followed by an extended period of lactation in the pouch, so that reproductive status and the stage of offspring development can be easily discerned. In marsupials, the energetic demands of lactation are greatest around the time of permanent emergence from the pouch, when growth and development of the young are most rapid [11].
We experimentally examined the impact of reproduction on the foraging behaviour of free-ranging female eastern grey kangaroos (Macropus giganteus), using an anti-fertility agent. This gregarious species forages in open grassy areas, where females carrying large pouch young and young that have recently left the pouch permanently, are particularly vulnerable to predators [12]. Foraging individuals are also at risk of infection by intestinal parasites, if they feed on forage contaminated by infective larvae, and so may reduce this risk by avoiding patches of forage contaminated with the faeces of conspecifics [13]. Any changes in foraging rates may have implications for the acquisition of intestinal parasites [14].
2. Material and methods
Field experiments took place in a 22 ha paddock with a homogeneous, closely cropped sward of pasture, within Serendip Sanctuary, Victoria, Australia. Macropus giganteus has a peak in births over summer. At Serendip, M. giganteus avoids patches of forage contaminated with the faeces of conspecifics to reduce the risk of acquiring gastrointestinal parasite larvae [13]. Potential predators at the site include red foxes, Vulpes vulpes, and domestic dogs, Canis familiaris.
We captured adult female kangaroos using draw-string traps, and immobilized them with an intra-muscular injection of Zoletil 100 (1 : 1 Zolezapam and Tiletamine, dose 5 mg kg−1). We marked individuals with a unique combination of coloured ear tags, took standard morphometric measurements and randomly allocated them to either an experimental (non-reproducing) or control treatment (n = 10 each group). Non-reproducing females had two subcutaneous deslorelin implants (4.7 mg, Suprelorelin, a GnRH agonist, Peptech, Australia) inserted with a trocar needle, while control females were injected twice with an empty trocar. In captive trials, deslorelin implants inhibited reproduction in female eastern grey kangaroos for greater than or equal to 510 days and had no direct effects on feeding behaviour [15]. At the conclusion of the study, we recaptured 14 of the study animals and took body measurements again.
We observed the behaviour of marked females through a telescope from a 3 m tower beside the study paddock. We collected observational data during three discrete, 7 day periods that covered three stages of lactation (early, mid and peak), according to the size of pouch young [16]. We used focal animal sampling to investigate fine-scale foraging behaviour, selecting focal individuals using stratified random sampling, and alternating between the reproducing and non-reproducing groups. We observed focal females continuously for 3 min, recording the duration of all behaviours with a digital voice recorder. We calculated two key foraging variables: gross bite rate, a measure of resource intake rate and step rate (number of steps per minute), an indication of selection for higher quality forage. We assumed that bite size was constant because the sward was uniformly low.
We constructed diurnal time–energy budgets for the third period of observations (peak energetic demands) using data collected from 138 scans, including only individuals sampled at least four times. We log-transformed data to meet the assumptions of normality for ANOVA, or else used log–linear models. There was no correlation (r = +0.08, p = 0.22) between group size and foraging time, so group size was not included in the models. We analysed bite rates (calculated with JWatcher v. 1.0 [17]) using restricted maximum-likelihood analyses (REML), with time and treatment as fixed factors, and kangaroo identity as a random factor to account for repeated measures. We used ANCOVA to analyse differences in body mass between the two groups at the end of the study, with initial body mass as the covariate.
3. Results
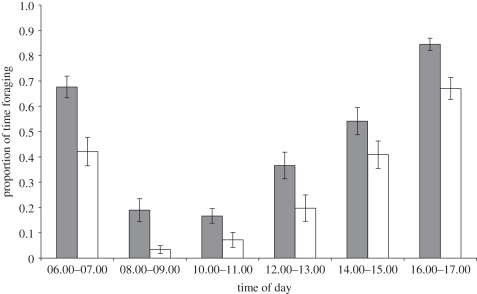
Kangaroos spent most daylight hours feeding (50.7%), resting (33.1%) or vigilant (8.8%). The proportion of the time spent feeding was influenced by the time of day (F5,109 = 52.11, p < 0.001) and reproducing females consistently spent, on average, 16 per cent more time feeding than non-reproducing females in peak lactation (F1,109 = 38.39, p<0.001; interaction: F5,109 = 0.817, p = 0.54; figure 1). The amount of time females spent resting in peak lactation also changed over the time of day (F5,113 = 82.53, p < 0.001), with reproducing females spending around 13 per cent less time resting than non-reproducing females (F1,113 = 26.12, p < 0.001; interaction: F5,113 = 1.08, p = 0.38).
Figure 1.
Proportion of time spent feeding by reproducing and non-reproducing female M. giganteus across all hours of daylight, from scan sampling in peak lactation. Error bars indicate standard error. Grey bars, reproducing; white bars, non-reproducing.
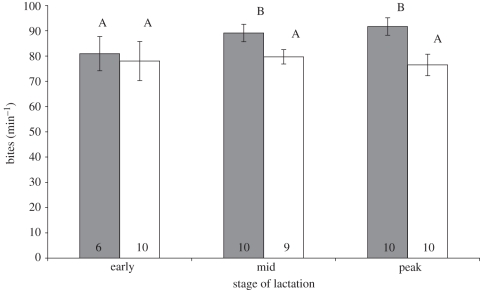
Variation in bite rate across the three reproductive phases was explained by reproductive status (F1,16 = 38.21, p < 0.001; figure 2), reproductive phase (F2,31 = 5.37, p = 0.01), and their interaction (F2,31 = 7.25, p = 0.003). Tukey's HSD revealed that peak and mid-term reproducing females had significantly (α = 0.05) higher bite rates than the other females. In peak lactation, reproducing females took around 20 per cent more bites per minute than did non-reproducing females. In contrast, step rate across the three reproductive phases was not influenced by reproductive status (F1,54 = 0.40, p = 0.53), reproductive phase (F2,54 = 3.03, p = 0.06) or their interaction (F2,54 = 0.32, p = 0.73). Reproducing and non-reproducing females were equivalent in body mass at the end of the study, after controlling for the effect of initial mass (F1,11 = 3.41, p = 0.09, ηp = 0.24).
Figure 2.
Mean bite rate of reproducing female M. giganteus across three stages of lactation, and of non-reproducing females at equivalent times, during 3 min focal observations. Error bars indicate standard error and numbers on columns indicate sample size. Columns not labelled with the same letter are significantly different. Grey bars, reproducing; white bars, non-reproducing.
4. Discussion
Female M. giganteus accommodate the energetic cost of reproduction by increasing the time allocated to foraging. Our field experiment augments the interpretations of numerous field studies of natural variation in foraging and reproductive condition (e.g. [18]). The greater proportion of time spent foraging occurs at the expense of time spent resting, suggesting that reproducing female kangaroos must reorganize their time budgets during peak lactation. The energetic costs incurred in these reproductive phases have not been determined in kangaroos, but lactating tammar wallabies, Macropus eugenii, can increase their energy intake to 174 per cent of non-lactating levels [19]. These changes in behaviour are unlikely to result from direct physiological effects of the fertility control agent. Woodward et al. [15] reported no changes in the feeding behaviour of female M. giganteus following the application of deslorelin. Similarly, no behavioural alterations have been reported for GnRH agonists in eutherian mammals [20].
Reproducing female kangaroos increase their forage intake during their most active foraging periods in mid and peak lactation, rather than foraging selectively, as would be evident from a higher step rate. The higher bite rate of reproductive females is unlikely to be affected by sward height, digestive constraints and/or competition with conspecifics, because these females fed on a largely uniform, heavily grazed sward, with only modest seasonal changes in food availability (J.K.C., 2008, unpublished data). Importantly, reproducing and non-reproducing females foraged in the same groups in 78 per cent of observations.
There are several consequences for female kangaroos of increased energetic demands of lactation. Reproducing females in peak lactation spent 16 per cent more time foraging and took 20 per cent more bites than did non-reproducing females, thereby ingesting around 49 per cent more forage in daylight hours (assuming constant bite size). Obviously, this disparity may be even greater if nocturnal activities are taken into account. Surprisingly, there was no obvious increased vigilance for reproducing females, despite the potential for this reproductive class to be more susceptible to predators [12]. Reproducing females instead reorganized their time-budgets to reduce time spent resting. The implications of this are unclear. However, the higher but non-selective forage intake of females in peak lactation may magnify the risk of acquiring gastrointestinal parasite larvae because avoiding patches of faecal contamination [13], while trying to consume more forage, would be challenging. The effect of increased risk of parasitism in female kangaroos during lactation requires investigation, and could highlight a further, largely neglected cost of reproduction.
Our novel field experiment demonstrated that female kangaroos altered their behaviour in direct response to the energetic demands of reproduction. When lactational demands were high, reproducing females increased bite rates, and thus their food intake. They did not reduce the time spent vigilant for predators, instead reducing their resting time.
Acknowledgements
We thank Mike Helman and Mick Smith at Serendip Sanctuary; volunteers who assisted with fieldwork; and Marco Festa-Bianchet, Jenny Martin, Sarah Garnick and two anonymous referees for their perceptive comments on the manuscript. This research was approved by the University of Melbourne's Animal Ethics Committee (no. 06146) and Department of Sustainability and Environment (no. 10004041), and funded by Australian Research Council Linkage Project (LP0560344).
References
- 1.Stearns S. C. 1989. Trade-offs in life-history evolution. Funct. Ecol. 3, 259–268 10.2307/2389364 (doi:10.2307/2389364) [DOI] [Google Scholar]
- 2.Millar J. S. 1978. Energetics of reproduction in Peromyscus leucopus: the cost of lactation. Ecology 59, 1055–1061 10.2307/1938558 (doi:10.2307/1938558) [DOI] [Google Scholar]
- 3.Speakman J. R. 2008. The physiological costs of reproduction in small mammals. Phil. Trans. R. Soc. B 363, 375–398 10.1098/rstb.2007.2145 (doi:10.1098/rstb.2007.2145) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLean J. A., Speakman J. R. 1997. Non-nutritional maternal support in the brown long-eared bat. Anim. Behav. 54, 1193–1204 10.1006/anbe.1997.0498 (doi:10.1006/anbe.1997.0498) [DOI] [PubMed] [Google Scholar]
- 5.Magnhagen C. 1991. Predation risk as a cost of reproduction. Trends. Ecol. Evol. 6, 183–186 10.1016/0169-5347(91)90210-O (doi:10.1016/0169-5347(91)90210-O) [DOI] [PubMed] [Google Scholar]
- 6.Hamel S., Côté S. D., Gaillard J. M., Festa-Bianchet M. 2009. Individual variation in reproductive costs of reproduction: high-quality females always do better. J. Anim. Ecol. 78, 143–151 10.1111/j.1365-2656.2008.01459.x (doi:10.1111/j.1365-2656.2008.01459.x) [DOI] [PubMed] [Google Scholar]
- 7.Ruckstuhl K. E., Fiesta-Bianchet M., Jorgenson J. T. 2003. Bite rates in Rocky Mountain bighorn sheep (Ovis canadensis): effects of season, age, sex and reproductive status. Behav. Ecol. Sociobiol. 54, 167–173 10.1007/s00265-003-0615-2 (doi:10.1007/s00265-003-0615-2) [DOI] [Google Scholar]
- 8.Renecker L. A., Hudson R. J. 1986. Seasonal foraging rates of free-ranging moose. J. Wildl. Manage. 50, 143–147 10.2307/3801504 (doi:10.2307/3801504) [DOI] [Google Scholar]
- 9.Gray M. E., Cameron E. Z. 2010. Does contraceptive treatment in wildlife result in side effects? A review of quantitative and anecdotal evidence. Reproduction 139, 45. 10.1530/REP-08-0456 (doi:10.1530/REP-08-0456) [DOI] [PubMed] [Google Scholar]
- 10.MacWhirter R. B. 1991. Effects of reproduction on activity and foraging behaviour of adult female Columbian ground squirrels. Can. J. Zool. 69, 2209–2216 10.1139/z91-308 (doi:10.1139/z91-308) [DOI] [Google Scholar]
- 11.Green B., Merchant J. 1988. The composition of marsupials milk. In The developing marsupial. Models for biomedical research (eds Tyndale-Biscoe C. H., Janssens P. A.). Berlin, Germany: Springer [Google Scholar]
- 12.Jarman P., Coulson G. 1989. Dynamics and adaptiveness of grouping in macropods. In Kangaroos, wallabies and rat-kangaroos (eds Grigg G., Jarman P., Hume I.), pp. 527–547 New South Wales, Australia: Surrey Beatty & Sons [Google Scholar]
- 13.Garnick S. W., Elgar M. A., Beveridge I., Coulson G. 2010. Foraging efficiency and parasite risk in eastern grey kangaroos (Macropus giganteus). Behav. Ecol. 21, 129–137 10.1093/beheco/arp162 (doi:10.1093/beheco/arp162) [DOI] [Google Scholar]
- 14.Moore J. 2002. Parasites and the behaviour of animals. New York, NY: Oxford University Press [Google Scholar]
- 15.Woodward R., Herberstein M. E., Herbert C. A. 2006. Fertility control in female eastern grey kangaroos using the GnRH agonist deslorelin. II. Effects on behaviour. Wildl. Res. 33, 47–55 10.1071/WR04114 (doi:10.1071/WR04114) [DOI] [Google Scholar]
- 16.Jaremovic R. V., Croft D. B. 1991. Social organization of the eastern grey kangaroo (Macropodidae, Marsupialia) in southeastern New South Wales. I. Groups and group home ranges. Mammalia 55, 169–185 10.1515/mamm.1991.55.2.169 (doi:10.1515/mamm.1991.55.2.169) [DOI] [Google Scholar]
- 17.Blumstein D. T., Daniel J. C., Evans C. S. 2000. JWatcher v 1.0 An introductory user's guide. See http://galliform.psy.mq.edu.au/jwatcher/; http://www.jwatcher.ucla.edu
- 18.Lamoot I., Vandenberghe C., Bauwens D., Hoffmann M. 2005. Grazing behaviour of free-ranging donkeys and Shetland ponies in different reproductive states. J. Ethol. 23, 19–27 10.1007/s10164-004-0123-5 (doi:10.1007/s10164-004-0123-5) [DOI] [Google Scholar]
- 19.Cork S. J. 1991. Meeting the energy requirements for lactation in a macropodid marsupial: current nutrition versus stored body reserves. J. Zool. Lond. 225, 567–576 (doi:10.1111/j.1469-7998.1991.tb04325.x) [Google Scholar]
- 20.Baker D. L., et al. 2002. Effects of GnRH agonist (leuprolide) on reproduction and behaviour in female wapiti (Cervus elaphus nelsoni). Reproduction 60, 155–167 [PubMed] [Google Scholar]