Abstract
Nonhuman primate ageing resembles its human counterpart. Moreover, ratings of subjective well-being traits in chimpanzees, orang-utans and rhesus macaques are similar to those of humans: they are intercorrelated, heritable, and phenotypically and genetically related to personality. We examined whether, as in humans, orang-utan subjective well-being was related to longer life. The sample included 184 zoo-housed orang-utans followed up for approximately 7 years. Age, sex, species and number of transfers were available for all subjects and 172 subjects were rated on at least one item of a subjective well-being scale. Of the 31 orang-utans that died, 25 died a mean of 3.4 years after being rated. Even in a model that included, and therefore, statistically adjusted for, sex, age, species and transfers, orang-utans rated as being “happier” lived longer. The risk differential between orang-utans that were one standard deviation above and one standard deviation below baseline in subjective well-being was comparable with approximately 11 years in age. This finding suggests that impressions of the subjective well-being of captive great apes are valid indicators of their welfare and longevity.
Keywords: primate, subjective well-being, welfare, happiness, mortality, senescence
1. Introduction
Lay persons correctly see human happiness or subjective well-being as indicative of positive or negative events in the lives of individuals [1,2]. However, studies of twins and families show that subjective well-being also has a genetic basis [3,4]. Moreover, prospective longitudinal studies have shown that human subjective well-being is related to success in work and relationships [5] and how long individuals live [6]. While it is unlikely that these relationships come about because subjective well-being plays a causal role, these studies do suggest that subjective well-being is a valid indicator of better psychological adjustment and health.
Nonhuman primates show similar ageing profiles to humans [7]. Moreover, behavioural cues, including facial expressions [8], signal happiness in humans and nonhuman primates. Therefore, it is not surprising that independent judges can provide reliable and repeatable ratings of subjective well-being in nonhuman primates [9–11].
For the present study, we used a prospective longitudinal design to determine whether subjective well-being was related to longer life in zoo-housed orang-utans. If so, it would validate measures of nonhuman primate subjective well-being and indicate that these measures can be used to assess the health and welfare of captive orang-utans.
2. Material and methods
(a). Subjects
Subjects were 100 Sumatran (Pongo abelii), 54 Bornean (Pongo pygmaeus) and 30 hybrid orang-utans (mean age = 21.6, s.d. = 12.0) housed in 42 zoological parks (38 in the United States, two in Canada, one in Australia and one in Singapore). Of these subjects, 152 were previously used to examine their personality structure and the relationship between personality and subjective well-being [9].
(b). Raters
Raters were 113 zoo employees who regularly worked with individual orang-utans and thus were highly familiar with their typical behaviour and affect. Orang-utans were assessed by one to six raters (median = 2) and each rater assessed between one and 22 orang-utans (median = 3). Raters were instructed not to discuss their ratings with one another.
(c). Instrument
Of the 184 subjects, 172 were rated on at least one item on a four-item subjective well-being questionnaire that was similar to human subjective well-being scales, though adapted for rating nonhuman primates [9]. The first three items asked raters to indicate on a seven-point scale the subject's frequency of positive versus negative moods, pleasure derived from social interactions and ability to achieve its goals. The fourth item asked raters to indicate on a seven-point scale how happy they would be if they were the orang-utan for a short period of time. This instrument can be obtained from A.W.
A previous study of 152 of these subjects found that the four items formed a single factor with loadings ranging from 0.60–0.89, and that the interrater reliability of this factor, as assessed by intraclass correlations of mean ratings across raters, was acceptable (ICC[3,k] = 0.58) and that this factor was related to personality in a manner similar to that in humans, i.e. with higher subjective well-being being related to lower neuroticism and higher extraversion and higher agreeableness [9]. A subsequent study found that this factor was heritable [12].
(d). Mortality
We determined date of death over a period of approximately 7 years using the orang-utan studbook [13]. At each of six time points—2003, 2004, 2005, 2006, 2007 or 1 January 2008–1 August 2009 (2008–2009)—we coded subjects as alive (0), dead (1) or having died in a previous year (missing). Of the 184 subjects, 31 died. Of the 31 deceased subjects, 25 died a mean of 3.4 years after they were rated.
(e). Transfers
Using the orang-utan studbook [13], we counted the number of transfers after birth to other facilities for all 184 subjects. If an orang-utan was wild-caught, this was considered an additional transfer.
(f). Analysis
To determine whether predictor variables were related to mortality risk over time, we conducted a discrete-time survival analysis [14]. Discrete-time survival analysis is a type of generalized linear-mixed model in which, for each subject (i), one models the probability that a discrete event (T = mortality status in the present study) occurs at a specific time point (j = 2002, 2003, 2004, 2005, 2006, 2007 and 2008–2009 in the present study) given that the event has not occurred on a previous time point and given a set of n predictor variables Xn. This conditional probability is known as the hazard, h(tij). Because event occurrence is a binary variable, it is linked to the n linear predictor variables using a logit link function. This link function enables one to perform a regression of hazard onto the predictor variables in a manner similar to how it enables one to relate predictor variables to binary outcomes in logistic regression.
The full description of the model is provided in the electronic supplementary material. The predictor variables in our model were year of observation, sex, age, species, number of transfers and subjective well-being. Year of observation was represented by six dummy-coded variables equal to 1 at time j and 0 otherwise, sex was effects-coded (males = 1; females = −1), age was mean-centred by subtracting mean age (in years) of the sample from each individual's age (in years), and species' effects were tested using two contrast-coded variables. The first contrast-coded variable compared risk between Sumatran and Bornean orang-utans (Sumatran = 1; Bornean = −1; hybrids = 0). The second contrast-coded variable compared risk between purebred and hybrid orang-utans (Sumatran or Bornean = −0.5; hybrid = 1). Number of transfers was treated as a continuous variable. Subjective well-being was a random variable estimated for each subject from their scores on the subjective well-being items. For ease of interpretation, estimated subjective well-being scores were transformed into z-scores (mean = 0; s.d. = 1).
We used Mplus v. 6.1 [15] to estimate model parameters via maximum likelihood. We chose maximum likelihood because it enabled us to model the relationships using information from all 184 orang-utans, including those with no subjective well-being data, and, as a result, it provides more accurate parameter estimates.
3. Results
We expressed the risk associated with each characteristic of the subjects (e.g. sex) via the hazard odds ratio (HOR). For the HOR associated with any predictor, a value of 1.00 indicates that the predictor is not related to mortality risk, a value less than 1.00 indicates that the predictor is related to decreased mortality risk, and a value greater than 1.00 indicates that the predictor is related to increased mortality risk. We obtained HORs by finding the antilog, i.e. raising the base of the natural log (e) to the power of the parameter estimates (β) [14]. Male orang-utans were almost two and a half times more likely to die than females over the follow-up period (HOR = 2.38, 95% confidence interval (CI95%) = (1.18, 4.82), p = 0.042). Older orang-utans were at significantly greater risk; each year was associated with a 10 per cent increase in risk (HOR = 1.10, CI95% = (1.07, 1.14), p < 0.001). There was no significant difference in mortality risk between Sumatran and Bornean orang-utans (HOR = 1.14, CI95% = (0.75, 1.75), p = 0.603), or between purebred and hybrid orang-utans (HOR = 0.88, CI95% = (0.42, 1.82), p = 0.772). Number of transfers were not significantly related to increased risk of death (HOR = 1.20, CI95% = (0.94, 1.53), p = 0.210).
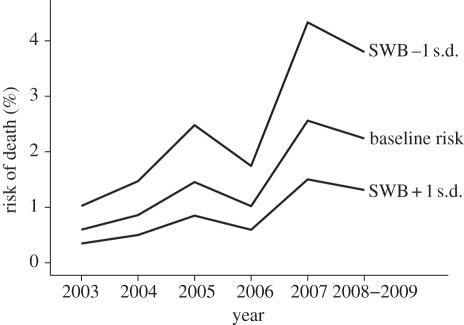
Orang-utans rated as higher in subjective well-being were less likely to die over the follow-up period: each standard deviation was associated with a 42 per cent reduction in risk (HOR = 0.58, CI95% = (0.41, 0.83), p = 0.012; figure 1). On the linear scale, the estimated regression coefficients for the effect of being 1 s.d. higher in subjective well-being and 1 year older were −0.54 and 0.096, respectively. Thus, in terms of risk reduction, the effect of 1 s.d. in subjective well-being was equivalent to a −0.54/0.096 = −5.67 year difference in age. In other words, the difference in risk between individuals that were 1 s.d. above and 1 s.d. below the mean in subjective well-being was equivalent to being 2 × 5.67 = 11.34 years younger.
Figure 1.
The predicted probability (risk) of dying at each time point associated with subjective well-being (SWB) that is one standard deviation below (−1 s.d.) and above (+1 s.d.) baseline risk (0 s.d.). Figure by the authors, licensed under a Creative Commons Attribution 3.0 Un-ported License and published under the terms of this license.
4. Discussion
Indicators of positive affect or happiness are related to longer life in orang-utans. While the mechanisms underlying this relationship cannot be addressed with the present data, there are several possible explanations for this relationship in orang-utans, and, for that matter, in humans and possibly other animals. One possibility is that behavioural indicators of low subjective well-being in orang-utans reflect behavioural reactions to the subsyndromal stages of disease or other health problems. A second possibility is that lower subjective well-being in orang-utans reflects the presence of stressors that lead to chronic hypothalamic–pituitary–adrenal (HPA) axis activation, which, in turn, generates greater allostatic load and poorer health [16]. A third possibility, which is consistent with the heritability of subjective well-being [12], is that subjective well-being is a marker of genetic quality that evolved via sexual selection [17]. While the present study could not test these possibilities, they suggest that future studies of captive and wild populations of orang-utans should examine the environmental causes of orang-utan subjective well-being and mortality, the relationship of subjective well-being to markers of HPA-axis activation, and whether orang-utan subjective well-being and health share a common genetic basis.
The present findings highlight one more characteristic shared between positive affective states or traits in orang-utans and humans. Naturally, only studies of happiness on a wide range of species and eventual comparative studies can determine whether these similarities are analogies or homologies. This finding also has important practical implications. Specifically, it suggests that impressions of the subjective well-being of captive great apes are valid indicators of their welfare. Thus, a quick, easily administered four-item questionnaire could provide important diagnostic information useful for making decisions about health monitoring, assessing the efficacy of enrichment, and otherwise ensuring that orang-utans, too, live ‘happily ever after’.
Acknowledgements
We thank Lori Perkins, the head of the Orangutan SSP Program, who has continued to lend us her generous support and expertise. We also thank Megan Elder, the International Studbook Keeper, for making the orang-utan studbook available to us. Finally, we would like to thank the individual institutions and personnel responsible for the ratings. Without their enthusiastic cooperation, these projects would not be possible.
References
- 1.Lucas R. E., Clark A. E., Georgellis Y., Diener E. 2003. Reexamining adaptation and the set point model of happiness: reactions to changes in marital status. J. Pers. Soc. Psychol. 84, 527–539 10.1037/0022-3514.84.3.527 (doi:10.1037/0022-3514.84.3.527) [DOI] [PubMed] [Google Scholar]
- 2.Lucas R. E., Clark A. E., Georgellis Y., Diener E. 2004. Unemployment alters the set point for life satisfaction. Psychol. Sci. 15, 8–13 10.1111/j.0963-7214.2004.01501002.x (doi:10.1111/j.0963-7214.2004.01501002.x) [DOI] [PubMed] [Google Scholar]
- 3.Nes R. B., Czajkowski N., Tambs K. 2010. Family matters: happiness in nuclear families and twins. Behav. Genet. 40, 577–590 10.1007/s10519-010-9365-x (doi:10.1007/s10519-010-9365-x) [DOI] [PubMed] [Google Scholar]
- 4.Bartels M., Boomsma D. I. 2009. Born to be happy? The etiology of subjective well-being. Behav. Genet. 39, 605–615 10.1007/s10519-009-9294-8 (doi:10.1007/s10519-009-9294-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lyubomirsky S., King L., Diener E. 2005. The benefits of frequent positive affect: does happiness lead to success? Psychol. Bull. 131, 803–855 10.1037/0033-2909.131.6.803 (doi:10.1037/0033-2909.131.6.803) [DOI] [PubMed] [Google Scholar]
- 6.Diener E., Chan M. Y. 2011. Happy people live longer: subjective well-being contributes to health and longevity. Appl. Psychol. Health Well-Being 3, 1–43 10.1111/j.1758-0854.2010.01045.x (doi:10.1111/j.1758-0854.2010.01045.x) [DOI] [Google Scholar]
- 7.Bronikowski A. M., et al. 2011. Aging in the natural world: comparative data reveal similar mortality patterns across primates. Science 331, 1325–1328 10.1126/science.1201571 (doi:10.1126/science.1201571) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parr L. A., Waller B. M. 2006. Understanding chimpanzee facial expression: insights into the evolution of communication. Soc. Cogn. Affect. Neurosci. 1, 221–228 10.1093/scan/nsl031 (doi:10.1093/scan/nsl031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss A., King J. E., Perkins L. 2006. Personality and subjective well-being in orangutans (Pongo pygmaeus and Pongo abelii). J. Pers. Soc. Psychol. 90, 501–511 10.1037/0022-3514.90.3.501 (doi:10.1037/0022-3514.90.3.501) [DOI] [PubMed] [Google Scholar]
- 10.King J. E., Landau V. I. 2003. Can chimpanzee (Pan troglodytes) happiness be estimated by human raters? J. Res. Pers. 37, 1–15 10.1016/S0092-6566(02)00527-5 (doi:10.1016/S0092-6566(02)00527-5) [DOI] [Google Scholar]
- 11.Weiss A., Adams M. J., Widdig A., Gerald M. S. 2011. Rhesus macaques (Macaca mulatta) as living fossils of hominoid personality and subjective well-being. J. Comp. Psychol. 125, 72–83 10.1037/a0021187 (doi:10.1037/a0021187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adams M. J., King J. E., Weiss A. Submitted Happy personalities are ancestral: the genetic structure of personality and subjective well-being in orangutans. Behav. Genet. [Google Scholar]
- 13.Elder M. 2009. 2008 International studbook of the orangutan (Pongo pygmaeus, Pongo abelii). St. Paul, MN: Como Zoo and Conservatory [Google Scholar]
- 14.Singer J. D., Willett J. B. 1993. It's about time: using discrete-time survival analysis to study duration and the timing of events. J. Educ. Stat. 18, 155–195 10.2307/1165085 (doi:10.2307/1165085) [DOI] [Google Scholar]
- 15.Muthén L. K., Muthén B. O. 1998–2010. Mplus user's guide, 6th edn. Los Angeles, CA: Muthén and Muthén [Google Scholar]
- 16.McEwen B. S. 2000. Allostasis and allostatic load: implications for neuropsychopharmacology. Neuropsychopharmacology 22, 108–124 10.1016/S0893-133X(99)00129-3 (doi:10.1016/S0893-133X(99)00129-3) [DOI] [PubMed] [Google Scholar]
- 17.Hunt J., Bussière L. F., Jennions M. D., Brooks R. 2004. What is genetic quality? Trends Ecol. Evol. 19, 329–333 10.1016/j.tree.2004.03.035 (doi:10.1016/j.tree.2004.03.035) [DOI] [PubMed] [Google Scholar]