Abstract
The oldest annelid fossils are polychaetes from the Cambrian Period. They are representatives of the annelid stem group and thus vital in any discussion of how we polarize the evolution of the crown group. Here, we describe a fossil polychaete from the Early Cambrian Sirius Passet fauna, Pygocirrus butyricampum gen. et sp. nov., with structures identified as pygidial cirri, which are recorded for the first time from Cambrian annelids. The body is slender and has biramous parapodia with chaetae organized in laterally oriented bundles. The presence of pygidial cirri is one of the characters that hitherto has defined the annelid crown group, which diversified during the Cambrian–Ordovician transition. The newly described fossil shows that this character had already developed within the total group by the Early Cambrian.
Keywords: Annelida, Polychaeta, Cambrian, fossil, pygidial cirri
1. Introduction
Polychaetes (Annelida) are common constituents of modern marine habitats. With their distinctive segmented body plan and lateral appendages, called parapodia, which usually have two bundles of chitinous bristles (neuro- and notochaetae), they display several modes of life, for example, as epibenthic predators and scavengers, infaunal burrowers, sessile filter feeders and even pelagic predators and planktotrophs [1]. The clitellates, which include the more familiar earthworms and leeches, evolved within the annelids and have invaded most freshwater and terrestrial habitats.
Fossil annelids are rare, but jaw elements (scolecodonts) from some polychaetes (eunicidans and glycerids) appeared in the fossil record in the Early Ordovician (488 Ma) [2]. Calcified polychaete tubes from mainly serpulids are known since the Jurassic [3,4], whereas complete polychaetes with some soft-tissue preservation are reported from a limited number of localities ranging in age from the Cambrian to the Cretaceous. Some of the most important are described from the Cambrian Burgess Shale [5,6], the Devonian Hunsrück Slate [7], the Carboniferous Mazon Creek fauna [8–10] and the largely undescribed Cretaceous Hakel polychaete fauna [11,12].
The oldest known fossil polychaete is Phragmochaeta canicularis Conway Morris and Peel, 2008 from the Early Cambrian Sirius Passet fauna [13]. In this paper, we describe a new genus and species, Pygocirrus butyricampum gen. et sp. nov., from the same locality and with preserved pygidial cirri, and discuss its implications for our understanding of evolution towards the annelid crown group.
2. Systematic description
crown group Lophotrochozoa Halanych et al. 1995
stem group Annelida Lamarck 1909
Pygocirrus butyricampum gen. et sp. nov.
(a). Etymology
Pygo: for pygidium (Latin), terminal body region—and cirrus: thread, used for a tendril-like appendage in zoology.
This species is named in honour of Dr Nicholas Butterfield (Department of Earth Sciences, Cambridge University, UK) in recognition of his work on Cambrian metazoan palynomorphs. Butyrum: butter and campus: field; butyricampum is a noun in apposition.
(b). Locality and material
Sirius Passet, North Greenland, Lower Cambrian (possibly Atdabanian [14]). Collected from the exposure of a very fissile dark shale unit within the Buen Formation, with abundant fossils preserved as two-dimensional reflective films. Holotype: part and counterpart (figure 1), Geological Museum of Copenhagen, MGUH 29288. Paratype: part and counterpart (electronic supplementary material, figure S1), MGUH 29289.
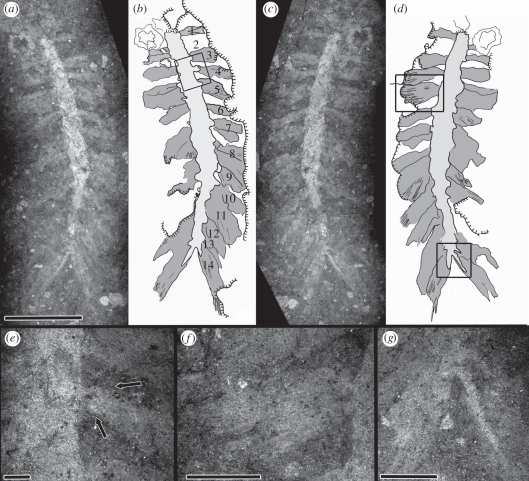
Figure 1.
Pygocirrus butyricampum sp. et gen. nov. Holotype MGUH 29288. From Sirius Passet, North Greenland, Early Cambrian. (a) Part and (b) corresponding interpretive camera lucida drawing; numbering denotes individual parapodia. (c) Counterpart and (d) corresponding interpretive camera lucida drawing. (e) Parapodium, detail of area indicated in (b). (f) Two chaetal bundles emerging from a parapodium, detail of anterior area indicated in (d). (g) The pygidial cirri on the counterpart, detail of posterior area indicated in (d). Scale bars, (a–d) 5 mm; (e–g) 1 mm.
(c). Diagnosis
Annelid with biramous parapodia, each ramus containing seven to 10 laterally oriented capillary chaetae. One pair of pygidial cirri present.
(d). Description
The holotype is a posterior fragment, lacking the head and an unknown, but presumably small, number of anterior chaetigers. The preserved body is 14 mm long excluding pygidial cirri, and 1.2 mm wide without parapodia, 1.7 mm with parapodia and 5.3 mm with parapodia and chaetae. A total of 14 chaetigers are present. The median body region is straight-sided in the first nine chaetigers; from chaetiger 10 the body tapers towards the pygidium. The parapodia are short and those of certain chaetigers appear to be bilobed (figure 1e). The parapodia are biramous; the most well-developed parapodia show two fascicles of seven to 10 capillary chaetae. The two bundles are semi-parallel, but with separate fascicles (figure 1f). Chaetae of the posterior segments are almost 1.5 times longer than on more anterior segments and are directed postero-laterally. Pygidium with two elongate, distally tapering cirri, V-shaped in outline. The left cirrus on the part (figure 1a,g) is more or less completely preserved, whereas the right cirrus is incompletely exposed.
The paratype is a median fragment with 10 chaetigers; it is 11.3 mm long, 1.7 mm wide lacking well-preserved parapodia and 9.5 mm wide with parapodia and chaetae.
Neither specimen shows any sign that dorsal cirri, ventral cirri or aciculae were present. The paratype is more decayed than the holotype: some of the parapodia are partially detached from the body and show no evidence of aciculae.
3. Discussion
The presence of pygidial cirri in P. butyricampum is unique among annelids known from the Cambrian Period. Therefore, although the species description provided here is incomplete (the anterior end is unknown), this form marks the origin of an important character for our understanding of annelid evolution. Pygidial cirri are widely distributed among modern annelids and have been considered one of the key autapomorphies of polychaetes [15,16]. The consistent result that clitellates (which lack pygidial cirri) are derived from within the polychaetes [16–19] indicates that this feature is one of the characteristics of the phylum as a whole.
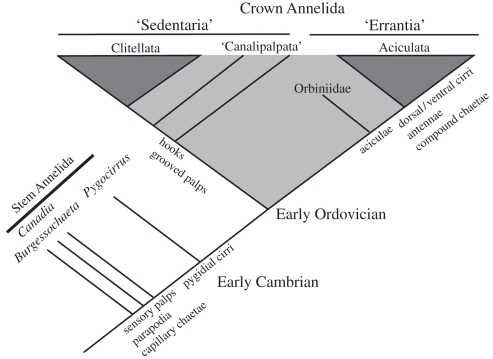
Understanding the phylogenetic relationships among major subclades of annelid polychaetes has been problematic owing to major conflicts between molecular systematics and morphology, e.g. [20], although recent published research displays a high degree of agreement between the two [19]. This contribution supports the recognition of two major groups. The first is Sedentaria (including the Clitellata), which contains groups that have grooved peristomial palps for food collection or are infaunal burrowers lacking large external head appendages (broadly conforming to Canalipalpata + Scolecida, both sensu [21], as well as Clitellata). The second major group is Errantia (as with Sedentaria, originally introduced in De Quatrefages [22]), most members of which have non-grooved, sensory prostomial palps and are often motile surface dwellers; this group consists mainly of the Aciculata, sensu [21], with the addition of Orbiniidae. The exact position of some groups remains problematic in this analysis, such as the ectoparasitic Myzostomidae and the Chaetopteridae, which are placed at the base of the annelids, below the sipunculans. Other studies that relied on less homoplastic characters such as rare genomic changes [23] have indicated that Sipuncula is the sister group of Annelida, as a separate phylum [18,24], which suggests that the position of myzostomids and chaetopterids below sipunculans is erroneous: they exhibit features that suggest an aciculate and canalipalpate affinity, respectively. This would also be in agreement with the fossil evidence, as crown group sipunculans are known from the Early Cambrian Chengjiang fauna of South China [25], which is similar in age to the Sirius Passet fauna. Thus, the primitive morphology of crown annelids can be reconstructed as an animal with anterior non-grooved palps, pygidial cirri, nuchal organs and parapodia that contain two bundles of simple chaetae [15,16].
The diverse polychaete fauna from the Burgess Shale includes a number of stem annelids [6]. These forms all have elaborate, usually biramous, parapodia with simple chaetae and many forms exhibit anterior (presumably prostomial) palps and thus conform to the expected presence of these characters in the ancestral annelid, except that they all lack pygidial cirri. The fossil Cambrian annelids therefore enable us to polarize the morphology of the ancestral annelid body plan as a surface dwelling errant worm with palps and biramous parapodia, but without compound chaetae or aciculae. None of these forms exhibit unequivocal pygidial cirri like those in P. butyricampum [6]. A cladistic analysis (electronic supplementary material, S2) finds that P. butyricampum resolves at a node above Canadia from the Burgess Shale in a polytomy with the crown group. While nothing is known about the anterior region of this new species, we predict that it also possesses sensory palps and might be located in a more derived position on the annelid stem lineage than the forms hitherto known from the Cambrian (figure 2). While it could be argued that it belongs to the crown group, we hypothesize that it diverged further down the lineage subtending the crown group. It has been argued that the crown group diverged in the Late Cambrian/Early Ordovician [6,26].
Figure 2.
Hypothesized position of Pygocirrus butyricampum on the annelid stem lineage denoting the appearances of important morphological characters. The position of P. butyricampum is hypothesized based on a cladistic analysis (electronic supplementary material, S2) as a stem form subtending the crown group.
Continued studies of annelids from the Cambrian Period will provide more detail to our emerging picture of the appearance of apomorphic morphological characters among forms preceding the origin of the crown group near the Cambrian–Ordovician transition.
Acknowledgements
We would like to thank Geocenter Denmark and POLOG for financial and logistic support, respectively, for our expedition to North Greenland. M. Paul Smith (Birmingham) and Arne T. Nielsen (Copenhagen) assisted in the field. The Invertebrate Palaeontology division of the Peabody Museum supported a visit to Yale University for D. E.-J. Derek E. G. Briggs generously commented on the manuscript. Robert Tunney (Yale) assisted with Latin vocabulary and grammar.
References
- 1.Fauchald K., Rouse G. 1997. Polychaete systematics: past and present. Zool. Scripta 26, 71–138 10.1111/j.1463-6409.1997.tb00411.x (doi:10.1111/j.1463-6409.1997.tb00411.x) [DOI] [Google Scholar]
- 2.Hints O., Eriksson M. E. 2007. Diversification and biogeography of scolecodont-bearing polychaetes in the Ordovician. Palaeogeogr. Palaeoclimatol. Palaeoecol. 245, 95–114 10.1016/j.palaeo.2006.02.029 (doi:10.1016/j.palaeo.2006.02.029) [DOI] [Google Scholar]
- 3.Vinn O., Ten Hove H. A., Mutvei H. 2008. On the tube ultrastructure and origin of calcification in sabellids (Annelida, Polychaeta). Palaeontology 51, 295–301 10.1111/j.1475-4983.2008.00763.x (doi:10.1111/j.1475-4983.2008.00763.x) [DOI] [Google Scholar]
- 4.Vinn O., Jäger M., Kirsimäe K. 2008. Microscopic evidence of serpulid affinities of the problematic fossil tube ‘Serpula’ etalensis from the Lower Jurassic of Germany. Lethaia 41, 417–421 10.1111/j.1502-3931.2008.00093.x (doi:10.1111/j.1502-3931.2008.00093.x) [DOI] [Google Scholar]
- 5.Conway Morris S. 1979. Middle Cambrian polychaetes from the Burgess Shale of British Columbia. Phil. Trans. R. Soc. Lond. B 285, 227–274 10.1098/rstb.1979.0006 (doi:10.1098/rstb.1979.0006) [DOI] [Google Scholar]
- 6.Eibye-Jacobsen D. 2004. A reevaluation of Wiwaxia and the polychaetes of the Burgess Shale. Lethaia 37, 317–335 10.1080/00241160410002027 (doi:10.1080/00241160410002027) [DOI] [Google Scholar]
- 7.Briggs D. E. G., Bartels C. 2010. Annelids from the Lower Devonian Hunsrück Slate (Lower Emsian, Rhenish Massif, Germany). Palaeontology 53, 215–232 10.1111/j.1475-4983.2009.00927.x (doi:10.1111/j.1475-4983.2009.00927.x). [DOI] [Google Scholar]
- 8.Thompson I. 1979. Errant polychaetes (Annelida) from the Pennsylvanian Essex fauna of northern Illinois. Palaeontographica A163, 169–199 [Google Scholar]
- 9.Hay A. A. 2002. Flabelligerida from the Francis Creek Shale of Illinois. J. Paleontol. 76, 764–766 (doi:10.1666/0022-3360(2002)076<0764:FFTFCS>2.0.CO;2) [DOI] [Google Scholar]
- 10.Fitzhugh K., Sroka S., Kruty M. D., Henderson A. A. 1997. Polychaete worms. In Richardson's guide to the fossil fauna of Mazon creek (eds Shabica C. W., Hay A. A.), pp. 64–83 Chicago, IL: Northeastern Illinois University [Google Scholar]
- 11.Bracchi G., Alessandrello A. 2005. Paleodiversity of the free-living polychaetes (Annelida, Polychaeta) and description of new taxa from the Upper Cretaceous Lagerstätten of Haqel, Hadjula and Al-Namoura (Lebanon). Memorie della Società Italiana de Scienze Naturali e del Museo Civico di Storia Naturale di Milano 32, 1–64 [Google Scholar]
- 12.Alessandrello A., Teruzzi G. 1986. Eunicites phoenicius n. sp., a new fossil polychaete annelid of the Cenomanian of Hakel, Lebanon. Atti della Società Italiana di Scienze Naturali e del Museo Civico di Storia Naturale de Milano 127, 321–325 [Google Scholar]
- 13.Conway Morris S., Peel J. S. 2008. The earliest Annelids. Acta Palaeontol. Polonica 53, 137–148 10.4202/app.2008.0110 (doi:10.4202/app.2008.0110) [DOI] [Google Scholar]
- 14.Babcock L., Peel J. S. 2007. Palaeobiology, Taphonomy and Stratigraphic Significance of the Trilobite Buenellus from the Sirius Passet Biota, Cambrian of North Greenland. Mem. Assoc. Australas. Palaeontol. 34, 401–418 [Google Scholar]
- 15.Westheide W. 1997. The direction of evolution within the Polychaeta. J. Nat. Hist. 31, 1–15 10.1080/00222939700770011 (doi:10.1080/00222939700770011) [DOI] [Google Scholar]
- 16.Purschke G. N. 2002. On the ground pattern of Annelida. Organ. Divers. Evol. 2, 181–196 10.1078/1439-6092-00042 (doi:10.1078/1439-6092-00042) [DOI] [Google Scholar]
- 17.Dunn C. W., et al. 2008. Broad phylogenomic sampling improves resolution of the animal tree of life. Nature 452, 745–749 10.1038/nature06614 (doi:10.1038/nature06614) [DOI] [PubMed] [Google Scholar]
- 18.Sperling E. A., Vinther J., Moy V. N., Wheeler B. M., Sémon M., Briggs D. E. G., Peterson K. J. 2009. MicroRNAs resolve an apparent conflict between annelid systematics and their fossil record. Proc. R. Soc. B 276, 4315–4322 10.1098/rspb.2009.1340 (doi:10.1098/rspb.2009.1340) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Struck T. H., et al. 2011. Phylogenomic analyses unravel annelid evolution. Nature 471, 95–98 10.1038/nature09864 (doi:10.1038/nature09864) [DOI] [PubMed] [Google Scholar]
- 20.Rousset V., Pleijel F., Rouse G. W., Erseus C., Siddall M. E. 2007. A molecular phylogeny of annelids. Cladistics 23, 41–63 10.1111/j.1096-0031.2006.00128.x (doi:10.1111/j.1096-0031.2006.00128.x) [DOI] [PubMed] [Google Scholar]
- 21.Rouse G. W., Fauchald K. 1997. Cladistics and polychaetes. Zool. Scripta 26, 139–204 10.1111/j.1463-6409.1997.tb00412.x (doi:10.1111/j.1463-6409.1997.tb00412.x) [DOI] [Google Scholar]
- 22.De Quatrefages A. M. 1866. Histoire naturelle des Annelés marins et d'eau douce: Annélides et Géphyriens. Paris, France: Libraire Encyclopédique de Roret [Google Scholar]
- 23.Rokas A., Holland P. W. H. 2000. Rare genomic changes as a tool for phylogenetics. Trends Ecol. Evol. 15, 454–459 10.1016/S0169-5347(00)01967-4 (doi:10.1016/S0169-5347(00)01967-4) [DOI] [PubMed] [Google Scholar]
- 24.Mwinyi A., Meyer A., Bleidorn C., Lieb B., Bartolomaeus T., Podsiadlowski L. 2009. Mitochondrial genome sequence and gene order of Sipunculus nudus give additional support for an inclusion of Sipuncula into Annelida. BMC Genomics 10, 27, 16 pp. (doi:10.1186/1471-2164-10-27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang D.-Y., Chen J.-Y., Vannier J., Saiz Salinas J. I. 2004. Early Cambrian sipunculan worms from southwest China. Proc. R. Soc. Lond. B 271, 1671–1676 10.1098/rspb.2004.2774 (doi:10.1098/rspb.2004.2774) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Budd G. E., Jensen S. 2000. A critical reappraisal of the fossil record of the bilaterian phyla. Biol. Rev. 75, 253–295 10.1017/S000632310000548X (doi:10.1017/S000632310000548X) [DOI] [PubMed] [Google Scholar]