Abstract
Coral reefs are currently experiencing a number of worsening anthropogenic stressors, with nearshore reefs suffering from increasing sedimentation because of growing human populations and development in coastal regions. In habitats where vision and olfaction serve as the primary sources of information, reduced visual input from suspended sediment may lead to significant alterations in prey fish behaviour. Here, we test whether prey compensate for reduced visual information by increasing their antipredator responses to chemically mediated risk cues in turbid conditions. Experiments with the spiny damselfish, Acanthochromis polyacanthus, found that baseline activity levels were reduced by 23 per cent in high turbidity conditions relative to low turbidity conditions. Furthermore, risk cues elicited strong antipredator responses at all turbidity levels; the strongest antipredator responses were observed in high turbidity conditions, with fish reducing their foraging by almost 40 per cent, as compared with 17 per cent for fish in clear conditions. This provides unambiguous evidence of sensory compensation in a predation context for a tropical marine fish, and suggests that prey fish may be able to behaviourally offset some of the fitness reductions resulting from anthropogenic sedimentation of their habitats.
Keywords: sedimentation, chemical alarm cues, sensory compensation, turbidity, antipredator behaviour, coral reef fish
1. Introduction
Sedimentation is arguably the primary land-based anthropogenic stressor affecting coastal coral reef systems and is only expected to worsen as human populations continue to grow [1]. The effects of reduced irradiance and increased particle deposition have been well documented for corals [2], but most of our understanding of sediment effects on fishes are limited to freshwater [3,4] and temperate marine and estuarine systems [5].
In aquatic systems, organisms predominantly rely on chemical and visual cues to learn about their surroundings, using such cues to inform decisions on settlement location, optimal foraging sites, mate selection and predator avoidance [6]. Both visual and chemical cues are necessary for prey species to detect potential threats and to distinguish predators from non-predators [7]. However, these two types of cue differ in their availability and reliability. Visual cues are highly reliable in space and time, but are not available in environments with visual obstructions such as habitat complexity [8] or high turbidity [9]. Conversely, chemical cues are available at all times, but are not as reliable in space or time; for example, chemical predation cues can be detected far from a predator and long after the predator is gone [7]. Reliance on chemical cues is generally predicted to increase under conditions of reduced visibility, such that organisms compensate for reduced visual information by increasing their reliance on chemical cues [3,10]; this ‘sensory compensation’ has been previously shown in fathead minnows [3], three-spined sticklebacks [11] and diving beetles [12].
Within the context of predation in aquatic environments, chemical alarm cues (CACs) are chemicals involuntarily released by organisms from a number of taxa as a result of direct mechanical damage to the skin, and informing nearby conspecifics of an ongoing or recent predation event. CACs have been shown to elicit survival-promoting overt antipredator behaviours in conspecific organisms and closely related heterospecifics upon detection [7].
We expect that under future sedimentation scenarios, prey species may increase their reliance on chemicals such as CACs to compensate for the reduction in visual information. The present study tested this ‘sensory compensation’ hypothesis by observing the response of the damselfish Acanthochromis polyacanthus, a species ubiquitous to the Great Barrier Reef (GBR), to conspecific CACs under different turbidity conditions. Fish were maintained under three turbidity treatments (clear-water, low and high turbidity), and their behavioural responses to three different chemical cues were monitored; stimuli included two non-risk cues (a seawater control and a skin extract from a distantly related fish) and one risk cue (skin extract from a conspecific). As per the sensory compensation hypothesis, we predict that responses to chemical risk cues should increase with decreased availability of visual information (i.e. increased turbidity).
2. Material and methods
Acanthochromis polyacanthus is a tropical planktivorous fish with a broad Indo-Pacific distribution. It is found on all parts of the GBR lagoon, from inner to outer barrier locations. Behavioural assays were conducted in May 2010 on juvenile A. polyacanthus (standard length 28.8 ± 2.1 mm s.d.) reared at the James Cook University aquarium facility. Freshwater swordtails, Xiphophorus helleri, were obtained from commercial breeders to serve as sources of skin extract to control for behavioural changes resulting from exposure to the extract of any injured fish.
Skin extracts were prepared 15 min prior to each trial; chemical cues were collected by making 25 shallow vertical incisions down each flank of the donor using a fresh scalpel blade, rinsing the donor with 15 ml of filtered seawater and filtering the solution to remove particulate matter. A total of 60 A. polyacanthus and 60 female X. helleri were euthanized for cue production.
Turbidity regimes were produced by pre-mixing Eckalite kaolin clay (Imerys Minerals, Pittong, VIC, Australia) with seawater and stirring it into trial tanks, generating three turbidity treatments: clear (0 mg l−1 of suspended clay), low turbidity (9 mg l−1) and high turbidity (41 mg l−1), equivalent to 4.5, 8.8 and 24 NTU, respectively. The low turbidity treatment is comparable to current sedimentation rates on reefs unaffected by human activities, while the high turbidity level falls within the range of reported sedimentation rates for human-impacted reefs (10–100 mg l−1, [13]), and represents the highest concentration of sediment at which fish could be reliably monitored. These concentrations of kaolin clay did not significantly alter water pH or hardness, which may affect transmission of chemical information such as CACs [14]. Following turbidity preparation, individual A. polyacanthus were moved into the trial tanks and left to acclimate for 2 h.
Behavioural observations took place in 9 l tanks (30 × 20 × 15 cm) filled with 7.2 l seawater and equipped with an airstone and a 5 cm diameter terracotta pot used as shelter. An 1 m injection tube was attached to the airstone to introduce the stimuli without disturbance. A 6 × 4 grid placed under the tank provided a frame for measuring activity levels during trials. Tanks were surrounded by black plastic sheeting to visually isolate fish. Observations were conducted via an angled mirror placed above the tanks.
Fish were exposed to one of three stimuli (seawater control, X. helleri skin extract control, A. polyacanthus alarm cue) under one of three turbidity regimes (clear, low and high) for a total of nine treatments (n = 20 per treatment). Following injection of 3 ml of an Artemia solution containing approximately 250 nauplii per millilitre, pre- and post-stimulus behaviours were assessed by recording three measures of activity for a period of 5 min: (i) line crosses: the number of gridlines crossed during each observation period, (ii) time active: the total time (s) spent actively swimming, and (iii) horizontal area use: the number of grid cells visited. Decreased activity and area use are common antipredator responses in prey fishes [7]. Post-stimulus observations began following injection of another 3 ml of Artemia and 15 ml of previously prepared stimulus (seawater or skin extract).
Trials in which abnormally low baseline activity levels indicated improper fish acclimation were excluded from all analyses (n = 30). The effect of turbidity on pre-stimulus baseline behaviours was assessed across all treatments using a single-factor MANOVA. Data were then converted into a per cent behavioural change between pre- and post-stimulus observations ((post–pre)/pre) and used as our raw data to test for the effects of turbidity (none, low or high) and cue (seawater, heterospecific or conspecific skin extract) on the fish antipredator responses using a two-factor MANOVA. Significant effects were further investigated using ANOVA followed by Tukey's HSD means comparisons. Raw data met parametric test assumptions. See the electronic supplementary materials for further details on fish maintenance, stimulus preparation, experimental set-up, behavioural assay procedures and statistical analyses.
3. Results
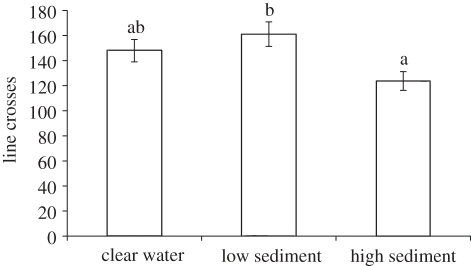
Pre-stimulus behaviours were significantly different among turbidity treatments (Pillai's Trace: F6,292 = 4.4, p < 0.001, n = 150), driven by a significant difference in line crosses (F2,147 = 4.7, p = 0.010; figure 1). The baseline number of line crosses for the low turbidity treatment was higher than that for the high turbidity treatment, but neither was significantly different from the clear-water treatment.
Figure 1.
Baseline number of line crosses (per 5 min) for juvenile Acanthochromis polyacanthus in each turbidity condition. Letters indicate groupings from Tukey's HSD means comparisons.
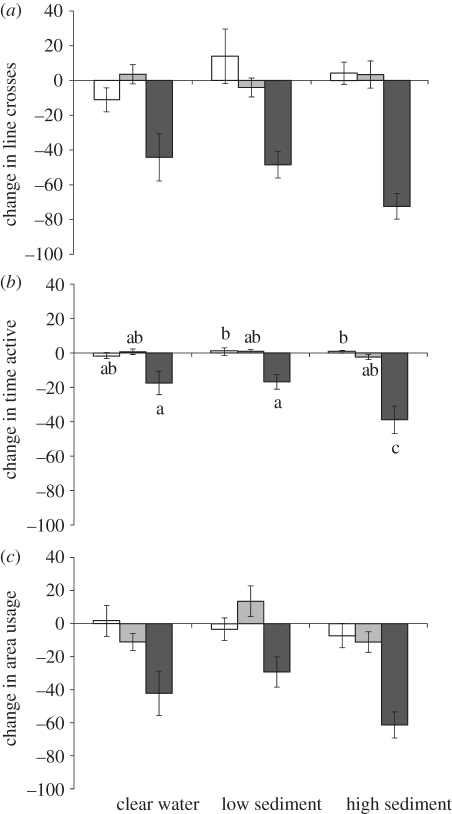
The two-factor MANOVA revealed a significant interaction between turbidity and cue (Pillai's Trace: F12,423 = 2.3, p = 0.008, n = 150; figure 2) on the antipredator responses of A. polyacanthus. This multivariate effect was driven mainly by differences in time active (F4,141 = 3.1, p = 0.0178). The interaction resulted from turbidity influencing the behaviour of fish exposed to CAC (F2,48 = 3.8, p = 0.030), but not that of fish exposed to the two control stimuli (F2,49 = 0.6, p = 0.570 for seawater; F2,44 = 1.8, p = 0.182 for X. helleri skin extract). Fish in high turbidity conditions displayed antipredator responses twice as strong as that of fish maintained in clear (Tukey's HSD, p < 0.006) and low turbidity water (Tukey's HSD, p < 0.003; figure 2b).
Figure 2.
Per cent change (±s.e.) in (a) line crosses, (b) time active and (c) horizontal area use from the pre-stimulus baseline for fish maintained in one of three turbidity levels and exposed to seawater control (white bars), heterospecific skin extracts (light-grey bars) or conspecific chemical alarm cues (dark-grey bars). Letters in chart (b) indicate groupings from Tukey's HSD means comparisons.
4. Discussion
The amplified antipredator behaviours exhibited by fish exposed to conspecific CACs in the high turbidity condition support the ‘sensory compensation’ hypothesis. Antipredator responses were significantly greater in the high turbidity treatment relative to the clear-water treatments for time active, while both area use and line crosses showed similar, albeit non-significant, trends. These results indicate that in situations where visual signals are compromised, prey will increase their response to chemical information, as has been found in mate-selection scenarios [11]. The significant effects of turbidity on the antipredator behaviour of a marine fish, identified here, support previous studies on freshwater fish at similar turbidities [3], and when visual information was reduced via habitat complexity [15] rather than turbidity.
Although alarm cues may be temporally and spatially unreliable, the lack of visual cues informing on the predator's identity or location led to cautious decision-making, i.e. increased antipredator behaviours [15]. As per the ‘sensory compensation’ hypothesis, A. polyacanthus increased its reliance on chemical input to assess its risk of predation in situations where visual information was limited. The finer details of risk assessment at intermediate turbidities have yet to be elucidated, and we encourage further work into the possibility of graded responses or behavioural thresholds in this system.
The differences in baseline behaviours suggest that in the long term, the fitness of prey fish may suffer from high turbidity conditions [4,5], with reductions in activity levels likely corresponding to decreased feeding rates [7], and therefore lower energy allocation to growth and reproduction [5]. Sediment effects may prove particularly detrimental to fish fitness if seasonal peaks in turbidity, and consequent reductions in foraging activity, coincide with vulnerable ontogenetic stages of reef fish (as per Cushing [16]).
Although future sedimentation scenarios may lead to significant reductions in the activity levels of this marine fish, and to reduced organism fitness through reduced energy reserves [5], A. polyacanthus may increase its reliance on chemical cues enough to compensate for the reduction in visual acuity, thereby improving the prey fish's chance of survival in encounters with predators, as predator-recognition ability through visual identification has been shown to decline with increasing turbidity levels [17]. Sensory compensation may therefore enhance organism survival, potentially counterbalancing some losses in fitness caused by globally changing environmental conditions.
Acknowledgements
Animals were cared for and euthanized as per JCU animal care protocol A1512. Funding was provided by the Dorothy M. and Maurice C. Shapiro Traveling Fellowship to S.M.L., the ARC Centre of Excellence for Coral Reef Studies to M.I.M. and NSERC and the University of Saskatchewan to M.C.O.F.
References
- 1.Wilkinson C. R. 1999. Global and local threats to coral reef functioning and existence: review and predictions. Mar. Freshwater Res. 50, 867–878 10.1071/MF99121 (doi:10.1071/MF99121) [DOI] [Google Scholar]
- 2.Fabricius K. E. 2005. Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar. Pollut. Bull. 50, 125–146 10.1016/j.marpolbul.2004.11.028 (doi:10.1016/j.marpolbul.2004.11.028) [DOI] [PubMed] [Google Scholar]
- 3.Hartman E. J., Abrahams M. V. 2000. Sensory compensation and the detection of predators: the interaction between chemical and visual information. Proc. R. Soc. Lond. B 267, 571–575 10.1098/rspb.2000.1039 (doi:10.1098/rspb.2000.1039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lehtiniemi M., Engstrom-Ost J., Viitasalo M. 2005. Turbidity decreases anti-predator behaviours in pike larvae, Esox lucius. Environ. Biol. Fishes 73, 1–9 10.1007/s10641-004-5568-4 (doi:10.1007/s10641-004-5568-4) [DOI] [Google Scholar]
- 5.Sigler J. W., Bjornn T. C., Everest F. H. 1984. Effects of chronic turbidity on density and growth of steelheads and coho salmon. Trans. Am. Fish. Soc. 113, 142–150 [Google Scholar]
- 6.Hay M. E. 2009. Marine chemical ecology: chemical signals and cues structure marine populations, communities, and ecosystems. Ann. Rev. Mar. Sci. 1, 193–212 10.1146/annurev.marine.010908.163708 (doi:10.1146/annurev.marine.010908.163708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrari M. C. O., Wisenden B. D., Chivers D. P. 2010. Chemical ecology of predator-prey interactions in aquatic ecosystems: a review and prospectus. Can. J. Zool. 88, 698–724 10.1139/Z10-029 (doi:10.1139/Z10-029) [DOI] [Google Scholar]
- 8.Rilov G., Figueira W. F., Lyman S. J., Crowder L. B. 2007. Complex habitats may not always benefit prey: linking visual field with reef fish behavior and distribution. Mar. Ecol. Prog. Ser. 329, 225–238 10.3354/meps329225 (doi:10.3354/meps329225) [DOI] [Google Scholar]
- 9.Abrahams M., Kattenfeld M. 1997. The role of turbidity as a constraint on predator–prey interactions in aquatic environments. Behav. Ecol. Sociobiol. 40, 169–174 10.1007/s002650050330 (doi:10.1007/s002650050330) [DOI] [Google Scholar]
- 10.Lima S. L., Steury T. D. 2005. Perception of predation risk: the foundation of non-lethal predator–prey interactions. In Ecology of predator–prey interactions (eds Barbosa P., Castellanos I.), pp. 166–188 Oxford, UK: Oxford University Press [Google Scholar]
- 11.Heuschele J., Mannerla M., Gienapp P., Candolin U. 2009. Environment-dependent use of mate choice cues in sticklebacks. Behav. Ecol. 20, 1223–1227 10.1093/beheco/arp123 (doi:10.1093/beheco/arp123) [DOI] [Google Scholar]
- 12.Abjornsson K., Wagner B. M. A., Axelsson A., Bjerselius R., Olsen K. H. 1997. Responses of Acilius sulcatus (Coleoptera: Dystiscidae) to chemical cues from perch (Perca fluviatilis). Oecologia 111, 166–171 10.1046/j.1365-2427.2002.00883.x (doi:10.1046/j.1365-2427.2002.00883.x) [DOI] [PubMed] [Google Scholar]
- 13.Rogers C. S. 1990. Responses of coral reefs and reef organisms to sedimentation. Mar. Ecol. Prog. Ser. 62, 185–202 10.3354/meps062185 (doi:10.3354/meps062185) [DOI] [Google Scholar]
- 14.Brown G. E., James C., Adrian J., Lewis M. G., Tower J. M. 2002. The effects of reduced pH on chemical alarm signalling in ostariophysan fishes. Can. J. Fish. Aquat. Sci. 59, 1331–1338 10.1139/f02-104 (doi:10.1139/f02-104) [DOI] [Google Scholar]
- 15.Golub J. L., Vermette V., Brown G. E. 2005. Response to conspecific and heterospecific alarm cues by pumpkinseeds in simple and complex habitats: field verification of an ontogenetic shift. J. Fish Biol. 66, 1073–1081 10.1111/j.1095-8649.2005.00658.x (doi:10.1111/j.1095-8649.2005.00658.x) [DOI] [Google Scholar]
- 16.Cushing D. H. 1990. Plankton production and year-class strength in fish populations: an update of the match–mismatch hypothesis. Adv. Mar. Biol. 26, 249–293 10.1016/S0065-2881(08)60202-3 (doi:10.1016/S0065-2881(08)60202-3) [DOI] [Google Scholar]
- 17.Ferrari M. C. O., Lysak K. R., Chivers D. P. 2010. Turbidity as an ecological constraint on learned predator recognition and generalization in a prey fish. Anim. Behav. 79, 515–519 10.1016/j.anbehav.2009.12.006 (doi:10.1016/j.anbehav.2009.12.006) [DOI] [Google Scholar]