Abstract
HCV NS3 protein plays a central role in viral polyprotein processing and RNA replication. We demonstrate that the NS3 protease (NS3pro) domain alone can specifically bind to HCV-IRES RNA, predominantly in the SLIV region. The cleavage activity of the NS3 protease domain is reduced upon HCV-RNA binding. More importantly, NS3pro binding to the SLIV hinders the interaction of La protein, a cellular IRES-trans acting factor required for HCV IRES-mediated translation, resulting in inhibition of HCV-IRES activity. Although overexpression of both NS3pro as well as the full length NS3 protein decreased the level of HCV IRES mediated translation, replication of HCV replicon RNA was enhanced significantly. These observations suggest that the NS3pro binding to HCV IRES reduces translation in favor of RNA replication. The competition between the host factor (La) and the viral protein (NS3) for binding to HCV IRES might regulate the molecular switch from translation to replication of HCV.
Various stages of viral life cycles are coordinated by a complex interplay of viral proteins and host (cellular) factors. In positive strand RNA viruses, the switch from translation, the initial obligatory step post-infection, to replication, the essential step for viral proliferation, is thought to be mediated by multiple interactions between viral and cellular proteins. Hepatitis C virus (HCV) non-structural protein 3 (NS3) plays a central role in polyprotein processing and viral RNA replication. The multifunctional enzyme HCV-NS3 has two major domains: the serine protease domain (1–180aa) and helicase domain (181–632aa) 1. NS4A act as the cofactor for its protease activity. The DExH/D-box helicase activity of NS3 is an important component of the replicative complex of the virus. On the other hand, the protease activity has been reported to enhance the RNA binding 2 and duplex unwinding 3 abilities of NS3 helicase, indicating a direct or indirect role of NS3 protease in RNA replication. Interestingly, the protease domain being the most positively charged region of the NS3 protein 2 raises the question whether it can bind to HCV RNA by itself.
HCV proteins are synthesized by the IRES mediated translation of the viral genomic RNA, which is the initial obligatory step after infection. Once viral proteins are synthesized, the viral RNA is replicated. However the mechanism of switch from translation to viral RNA replication is not well understood. Earlier, PCBP2 (an IRES trans acting factor, ITAF) and 3CD (viral protein) have been shown to play role in the switch for poliovirus RNA 4. The study provided evidence of a complex interplay between the viral and cellular protein in binding to viral RNA and influencing the switch from translation to replication. Some canonical initiation factors (eIF2 and eIF3) and non-canonical ITAFs, have been shown to interact with the HCV-IRES and influence its function 5,6,7,8. Earlier, results from our laboratory and others have shown, that human La autoantigen binds to HCV IRES RNA both in vitro and ex vivo and help in ribosome assembly during internal initiation of translation of HCV RNA 5,9,10. The central RNA recognition motif (RRM 112–184) of human La protein was shown to interact at the GCAC near initiator AUG and trigger a conformational alteration, which facilitates 40S binding during internal initiation 11. La protein has also been shown to bind 3′UTR and might help in HCV RNA replication 12.
As the NS3pro is thought to be involved in HCV replication and La is involved in translation, we investigated whether the translation-replication switch in HCV is regulated by interplay between NS3pro and La, and if the regulation is mediated by the ability of these proteins to bind the HCV RNA.
Results
NS3 protease specifically interacts with HCV IRES RNA
To investigate whether the protease domain of the NS3 protein (Fig. 1a) is capable of binding to HCV IRES RNA, direct RNA-protein UV cross linking assay was performed using purified NS3 protease from plasmid pYB43 13. Additionally, the above experiment was repeated to analyze the binding at physiological salt concentration i.e. 140mM KCl (Supplementary Information, Fig. S1a). The results showed that NS3pro interacts efficiently with HCV IRES and the binding increases with increasing protein concentration (Fig. 1b). Interestingly, NS3pro showed no appreciable binding with the HCV 3′UTR (Supplementary information, Fig S1b). Further, to demonstrate that NS3 protein indeed binds to HCV IRES RNA, the ribonucleoprotein (RNP) complex isolated from Huh7 cells harboring HCV monocistronic replicon (generous gift from R. Bartenschlager), was immunoprecipitated using anti-NS3 antibody. Anti-actin antibody was used as negative control. The HCV IRES RNA was detected by RT-PCR using the RNA isolated from the pull-down complex (Fig. 1c, lane 3). Additionally, we have observed considerable binding of full length NS3 protein with the HCV IRES RNA (Supplementary information, Fig. S1c). Results reconfirm the interaction of NS3 protein with HCV IRES RNA.
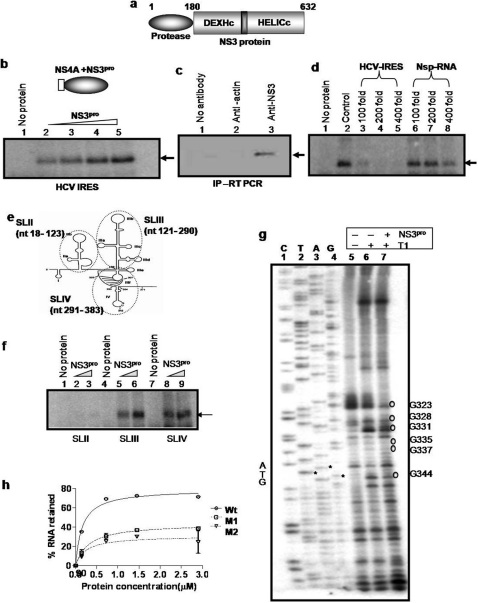
Figure 1. NS3pro specifically binds to HCV IRES RNA.
Panel a: Schematic representation of the domain organization of HCV NS3 protein. Panel b: UV-crosslinking. [α32P]UTP labeled HCV IRES RNA was UV cross linked with increasing concentrations (0.1, 0.2, 0.4 and 0.8 µM) of NS3pro (lane 2 –5). Lane 1 shows only probe control. Schematic representation of the NS3pro protein is shown above the panel. Panel c: Immuno-pulldown assay and RT-PCR. NS3-RNA complex was immunoprecipitated from HCV replicon bearing cells using anti-NS3 antibody. RNA was isolated and RT-PCR was performed for HCV IRES (lane 3). No antibody (lane 1) and anti-actin antibodies (lane 2) were used as negative controls for the pull down. Panel d: Competition UV-crosslinking. [α32P]UTP labeled HCV IRES RNA was UV cross linked with NS3pro in the absence (lane 2) and presence of molar excess of unlabeled HCV IRES RNA (lanes 3–5) or a nonspecific RNA (lanes 6–8). Lane 1 shows only probe control. Panel e: Schematic representation of HCV IRES RNA showing the stem and loop (SL) regions 20. The domains that are used as SLII, III and IV in the study are encircled and indicated. Panel f: UV-crosslinking. [α32P]UTP labeled HCV SLII (lane 2–3); SLIII (lane 5–6) and SLIV (lane 8–9) RNAs were UV cross linked to increasing concentrations (0.2 and 0.4µM) of NS3pro. Lanes 1, 4 and 7 represents only probe controls for SLII, SLIII and SLIV respectively. Panel g: RNase T1 Foot-printing assay. Binding reactions of in vitro transcribed HCV IRES RNA were carried out in absence (lane 6) or presence (lane 7) of NS3 protease. The RNA was then digested with RNase T1. RNA was reverse-transcribed with an end labeled primer. The cDNA was resolved in along with a reference sequencing reaction (lanes 1–4). Lane 5 represents the no T1 control. Panel h: Filter-binding assay. [α32P]UTP labeled HCV IRES RNA or mutant IRES RNA (M1 and M2) was bound to increasing concentrations of NS3pro.
The interaction between 32P IRES RNA and NS3pro was further examined by competition UV cross linking experiment with 100, 200 and 400 fold molar excess of unlabeled self RNA or a non-specific RNA (Nsp), which showed the specificity of the interaction (Fig. 1d). NS3pro did not bind Polio IRES efficiently (Supplementary Information, Fig. S2a). Further, NS3pro showed greater affinity for the IRES RNA derived from positive strand compared to the corresponding negative strand RNA (data not shown).
Also, it was observed that the protease domain binds to both SLIII (nt121- 290) and SL IV (nt291- 383) regions more efficiently compared to SLII (nt18- 123) RNA (Fig. 1e and f). Competition UV cross-linking assay using cold competitor RNA (200 and 400 fold molar excess) corresponding to different stem-loop region reconfirmed the observation and showed significant competition with SLIV RNA (Supplementary Information, Fig. S2b). Results suggest that NS3pro might bind to HCV IRES at different pockets but predominantly at the SLIV region which harbors the iAUG and also contains La binding sites critical for translation initiation.
To characterize the binding sites of the NS3pro within IRES RNA, toe-printing assay was carried out. Prominent toe-prints corresponding to NS3pro were observed at G328, G331, G335, G337, G344, and G346 (Supplementary Information, Fig. S3a). Similarly, RNaseT1 foot-printing assay showed prominent foot prints at G323, G331 and G344 in stem loop IV region (Fig. 1g). Also, minor foot prints were observed at G335 and G337 suggesting that NS3pro makes primary contact between G328-G344 within HCV IRES (Fig. 1g, Supplementary Information, Fig. S3b).
Since human La protein was also shown to bind at similar region, two mutant HCV IRES RNAs (M1 and M2) (Supplementary Information, Fig. S4a) were used that are known to have reduced affinity for binding to La protein 11. Results showed drastic decrease in RNA binding of NS3pro with these mutants, suggesting a significant overlap between binding sites of La and NS3pro in the HCV IRES (Fig 1h), Supplementary Information, Fig. S4b).
Effect of RNA binding on NS3pro cleavage activity
To investigate whether the RNA binding of NS3Pro had an effect on the canonical proteolytic activity of NS3pro, NS3 cleavage assay was performed in the absence or presence of HCV IRES RNA. For cleavage, NS5A/5B substrate was used that harbors the NS3 cleavage site at the NS5A/5B junction. In presence of NS3pro, distinct cleavage product was obtained which showed dose dependent decrease upon addition of HCV IRES RNA but not with non specific RNA (Nsp RNA) (Fig. 2a). Interestingly, addition of excess enzyme (NS3pro), but not BSA, could rescue the inhibition, possibly by titrating out the RNA and rendering NS3pro available to cleave the substrate (Fig. 2b). This was reconfirmed by addition of antisense IRES RNA which could successfully rescue the inhibitions of cleavage activity (Fig. 2c). Furthermore, presence of mutant HCV IRES (M1 and M2) RNA harboring mutations in NS3pro binding region did not show significant reduction of cleavage activity (Fig. 2d). The results suggest binding with HCV IRES RNA reduces the proteolytic activity of NS3pro.
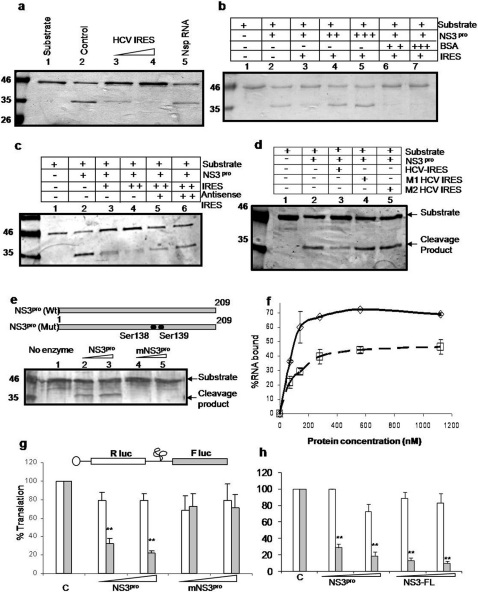
Figure 2. Effect of HCV RNA binding on the catalytic activity of NS3 protease.
Panel a: Recombinant NS3 protease was allowed to form complex with increasing amount of unlabeled HCV IRES RNA (lanes 3–4). Lane 2 shows the control without HCV IRES RNA. NS5A/5B protein was used as the substrate for NS3pro. Lane 1 shows substrate alone. Non specific RNA has been used (lane 5) to test for the specificity of the reaction. Panel b: Cleavage assay was carried out in the presence of HCV IRES RNA. The cleavage inhibition (lane 3) caused by the RNA was rescued by addition of excess amount of NS3pro (lanes 4–5). BSA was used as a negative control (lanes 6–7). Panel c: Cleavage assay was carried out in the presence of HCV IRES RNA. The cleavage inhibition (lane 3–4) caused by the RNA was rescued by addition of increasing concentrations of antisense RNA (lanes 5–6). Panel d: Cleavage assay in presence of the two mutant HCV IRES RNAs (M1 and M2). Panel e: Cleavage assay using increasing concentrations of wild type NS3pro (lanes 2 and 3) and mutant NS3pro (lanes 4 and 5). Panel f: Filter-binding assay. [α32P]UTP labeled HCV IRES RNA was bound to increasing concentrations of NS3pro and mutant NS3pro. Panel g: In vitro transcribed capped bicistronic RNA (shown above panel) was in vitro translated in the absence or presence of increasing concentrations (0.2 and 0.4µM) of either NS3pro or mutant NS3pro. Luciferase assay was performed. Percent luciferase activities corresponding to Rluc (white bar) and Fluc (grey bar) values were plotted against the protein concentrations. Values which significantly differ from control (P value<0.001) are indicated as asterisks. Panel h: In vitro transcribed capped bicistronic RNA was in vitro translated in the absence or presence of increasing concentrations of either NS3pro (0.2 and 0.4µM) or full length NS3 (0.2 and 0.4µM). Luciferase assay was performed. Percent luciferase activities corresponding to Rluc (white bar) and Fluc (grey bar) values were plotted against the protein concentrations.
The catalytic triad of NS3 protease is composed of three amino acid residues: His-57, Asp-81, and Ser-139. Since Ser139 has been shown to be critical for the catalytic activity of NS3 protease and lies in the C terminal half of NS3 protease domain (which has been shown to bind HCV IRES), we generated a mutant NS3pro which harbors substitution mutations (serine to alanine) at ser138 and ser139 (Supplementary Information, Fig. S5a). The purified recombinant mutant NS3pro (Supplementary Information, Fig. S5b) failed to show the cleavage activity (Fig 2e) and also showed a drastic reduction in HCV IRES RNA binding in the filter binding assay (Fig 2f) and UV cross linking assay (Supplementary Information, Fig. S5c).
Effect of NS3pro binding on HCV IRES function
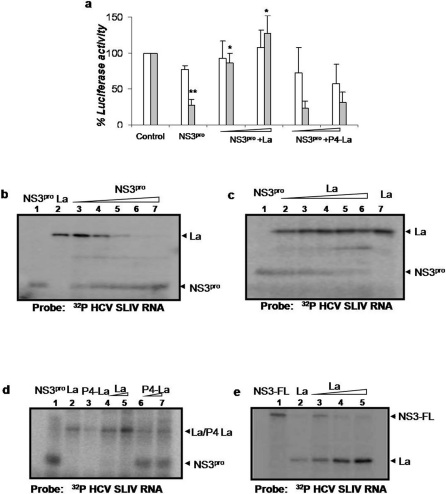
Since the NS3pro binds to SLIV region near initiator AUG, which plays a crucial role in HCV IRES-mediated translation, we investigated the possible effect on the IRES function. in vitro translations using capped HCV bicistronic RNA (cap-Rluc-HCV IRES-Fluc) in presence of NS3pro showed significant inhibition of HCV IRES mediated translation of Fluc (Fig.2g). However, the mutant NS3pro failed to inhibit HCV IRES function (Fig 2g). Additionally, when the full length NS3 protein was used in the assay, a similar degree of inhibition was observed suggesting that NS3 protease domain binds to HCV IRES and inhibits translation in the context of full length NS3 protein (Fig. 2h). Interestingly, addition of increasing concentration of La protein could successfully rescue the NS3pro mediated inhibition of HCV IRES function whereas a mutant La protein (P4-La), which lacks the binding ability near SLIV 14 failed to rescue the inhibition (Fig 3a). Additionally, transient co-transfection of NS3pro encoding plasmid along with the HCV bicistronic construct in Huh7 cells showed dose dependent inhibition of HCV IRES mediated translation (Supplementary Information, Fig. S6a). Also, when tested in similar reaction conditions, Poliovirus IRES mediated translation did not show reduction due to HCV NS3 protease (Supplementary Information, Fig. S6b). A possible mechanism of this inhibition is that NS3pro binding to SLIV region competes with the binding of important transacting factors and consequently inhibits the IRES function.
Figure 3. Panel a: In vitro transcribed capped bicistronic RNA was in vitro translated in the absence or presence of NS3pro.
Luciferase assay was performed. Percent luciferase activities corresponding to Rluc (white bar) and Fluc (grey bar) values were plotted against the protein concentrations. The translation inhibition was rescued by adding equimolar and also 2 fold molar excess of wild type La protein. P4-La mutant was used as a negative control. Values which significantly differ from control are indicated as asterisk. ** indicates P value<0.001 and * indicates P value<0.01. Panel b: UV-crosslinking assay: [α32P] UTP labeled HCV SLIV RNA was UV crosslinked with La protein (0.2 µM) in the presence of equimolar, 2 fold, 4 fold, 8 fold and 10 fold molar excess of purified HCV NS3pro (lanes 3–7). The position of NS3pro and La protein is indicated. Lane 1 and 2 represents binding due to NS3pro and La protein alone respectively. Panel c: UV-crosslinking assay: [α32P]UTP labeled HCV SLIV RNA was UV cross linked to NS3pro (0.2 µM) in the presence of equimolar, 2 fold, 4 fold, 8 fold and 10 fold molar excess La protein (lanes 2–6). Lane 1 and 7 represents binding due to NS3pro and La protein alone respectively. Panel d: UV-crosslinking assay: [α32P]UTP labeled HCV SLIV RNA was UV cross linked to NS3pro (0.2µM) in the presence of equimolar or 2 fold molar excess of either wild type La protein (lanes 4 and 5) or P4-La (lanes 6 and 7). Lanes 1, 2 and 3 represent binding due to NS3pro and wild type La protein and P4-La alone respectively. Panel e: UV-crosslinking assay: [α32P] UTP labeled HCV SLIV RNA was UV crosslinked with the purified recombinant full-length NS3 protein (0.3µM) in the presence of equimolar, 2 fold, and 4 fold molar excess of the La protein (lanes 3–5). The position of NS3 and La protein is indicated. Lane 1 and 2 represents binding due to NS3-FL (0.3µM) and La protein (0.3µM) alone respectively.
The interplay between NS3pro and La protein for binding to SLIV within HCV IRES
Since La is known to bind to HCV IRES near the initiator AUG, with an overlapping binding site with NS3pro, we have explored whether the binding of the two proteins was antagonistic. Competition UV crosslinking assay using increasing concentrations of NS3pro, showed increased binding of NS3pro with HCV SLIV and corresponded with reduction in La binding (Fig. 3b). Conversely, addition of increasing concentrations of La protein resulted in gradual decrease in NS3pro binding (Fig. 3c) confirming the antagonistic binding between these two proteins. Mutant La protein (P4-La) did not show appreciable competition with NS3pro for binding to HCV SIV (Fig 3d) confirming that both NS3pro and the wild type La protein bind to a similar region in SLIV. More importantly, La protein could successfully compete with the full length NS3 protein for binding to the HCV IRES (Fig 3e). These observations suggest that the mutually antagonistic binding of NS3 and La protein with the SLIV of HCV IRES may constitute a molecular switch from translation to replication of viral RNA.
HCV translation versus replication: role of NS3pro
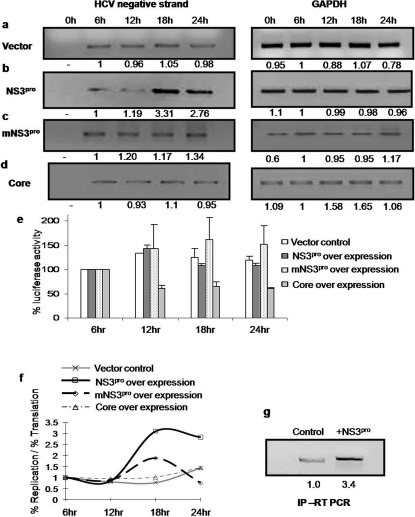
To investigate the possible role of NS3pro in replication of HCV RNA, pSGR JFH1 luc replicon RNA 15 was transiently cotransfected with either NS3pro encoding construct or vector DNA. At different time points (0, 6, 12, 18 and 24 hour) HCV negative strand RNA was quantified by semi quantitative RT PCR (Fig. 4a–d). GAPDH was used as an internal control. Over expression of NS3pro (Fig. 4b), but not of mutant NS3pro (Fig. 4c) or vector control (Fig. 4a, Supplementary information, Fig S7) showed increased level of HCV negative strand at 18th hour. Over expression of Core protein did not show appreciable changes in the levels of negative strand RNA (Fig 4d).
Figure 4. Effect of NS3pro over expression on HCV RNA replication versus translation.
Huh7 cells were cotransfected with of pSGR JFH1/Luc RNA and construct expressing NS3pro, or vector or mutant NS3pro or core. RNA was isolated at different time points and HCV RNA was quantitated using semi quantitative RT PCR (left side of Panel a, b, c and d). GAPDH was used as internal control (right hand side of Panel a, b, c and d ). Panel e: For the experiments performed in fig. 4a–d, luciferase assay was carried out to detect translation levels. The percentage luciferase activities were plotted for each reaction at different time intervals taking 6 hour time point as 100 (control). Panel f: The ratio of percentage of replication to translation levels in vector control cells was compared with cells overexpressing NS3pro plasmid or the other controls and graphically represented by plotting ratio of percentage replication versus translation on Y axis and time on X axis. Panel g: RNA protein complex was pulled down at 18th hour time point from cells cotransfected with pSGR JFH1/Luc RNA and construct expressing NS3pro using anti-NS5B antibody followed by RT-PCR for HCV IRES.
In parallel to assess the rate of IRES mediated translation at different time points, starting 6 hr post transfection, luciferase assay was performed (Fig. 4e). Ratios of HCV RNA amplified (representing replication) and luciferase synthesis (representing translation) at each time point showed that HCV negative strand RNA level at 18hr post transfection was around 3.5 fold higher than vector control (Fig 4f). However, under same conditions, luciferase translation from the same replicon showed no concomitant increase, suggesting an adverse effect on IRES function. Interestingly, overexpression of HCV-core protein (known to inhibit HCV IRES function) also showed no significant change in the level of replication to translation ratio.
Although, NS3pro binding to HCV IRES was found to dislodge the La protein binding with SLIV region and inhibit translation, overexpression of La protein in Huh7 cells didn't show appreciable change in replication profile of the negative strand (Supplementary information, Fig. S8). Transient transfection of increasing concentration of pCDLa showed overexpression of La protein but didn't show corresponding change in the negative strand RNA synthesis. However, in a similar experiment overexpression of NS3pro showed considerable increase in the negative strand synthesis (Supplementary information, Fig S9).
Finally, to reconfirm our observation the replication complex was immunoprecipitated by using antiNS5B antibody and the RNA isolated from the complex was measured for negative strand by RT-PCR analysis. Results clearly showed considerable increase of negative strand synthesis in the immunoprecipitate from the cells transfected with NS3pro overexpressing plasmid construct (Fig 4h).
We have also assayed the negative strand synthesis more quantitatively using real time RT-PCR. Results showed approximately 3.5 fold increase in negative strand synthesis (between 6–18hrs post transfection) upon overexpression of NS3pro (Supplementary information, Fig. S9).
Additionally, to rule out DNA contamination in RNA samples and verify the specificity of the negative strand primers used in the RT-PCR analysis several control experiments were performed (Supplementary information, Fig S10).
NS3-FL also supports the switch from translation to replication
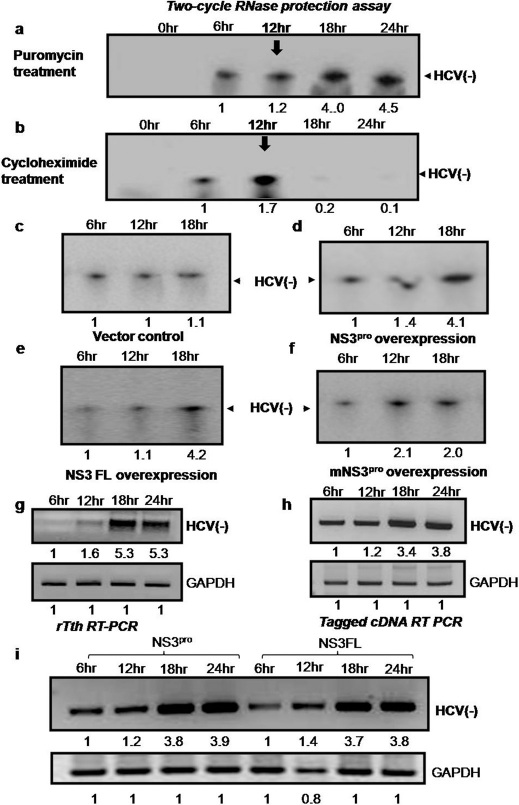
To further investigate the switch from translation to replication puromycin and cycloheximide were used that induce the dissociation and freezing of polysomes respectively and have the opposite effects on negative strand RNA synthesis. For this purpose, Huh7 cells were transfected with HCV replicon and treated with the respective drugs followed by detection of negative strand synthesis at different time points.
Considering the fact that the large molar excess of HCV positive strands is expected to interfere with hybridization-based methods of quantification of minus strand (semiquantitative RT-PCR), to specifically detect the negative strands ‘two-cycle RNase protection' assays were performed in parallel 16. Results clearly showed that treatment with puromycin resulted in considerable increase in negative strand synthesis at 18th hour post transfection (Fig 5a). However, treatment with cycloheximide showed gradual decrease in negative strand synthesis (Fig 5b). These results are consistent with the mechanism of action of the two compounds.
Figure 5. Panel a: Huh 7 cells were transfected with pSGR JFH1 luc RNA.
12hours after transfection, cells were treated with puromycin. At different time points total cellular RNA was isolated and was subjected to two cycle RNase protection assay. Negative strand RNA was probed using radiolabelled positive strand HCV IRES RNA followed by RNAse digestion. Protected fragment was detected by running on 4% polyacrylamide-8M urea gel. The arrow (below 12hr) indicates the time of addition of puromycin. Panel b: Huh 7 cells were transfected with pSGR JFH1 luc RNA. 12hours after transfection, cells were treated with cycloheximide. At different time points total cellular RNA was isolated and was subjected to two cycle RNase protection assay. Negative strand RNA was probed using radiolabelled positive strand HCV IRES RNA followed by RNAse digestion. Protected fragment was detected by running on 4% polyacrylamide-8M urea gel. The arrow (below 12hr) indicates the time of addition of cycloheximide. Panel c–f: Two cycle RNase protection assay was performed under similar experimental set up as in fig.4 for vector control, NS3pro, NS3 full length and mutant. The total RNA was self hybridized followed by RNase treatment. Negative strand RNA was probed using radiolabelled positive strand HCV IRES RNA followed by RNAse digestion. Protected fragment was detected by running on 4% polyacrylamide-8M urea gel. Panel g: Under similar experimental set up as in fig. 4b, instead of semiquantitative RT-PCR, negative strand was detected using thermostable RT-PCR. Panel h–i: Under similar experimental set up as in fig. 4b, negative strand was detected using a tagged cDNA. Here, cDNA was made using a HCV primer that was tagged with a long non HCV nucleotide tag. This was followed by first round of PCR using tag only primer and HCV reverse primer. A second cycle nested PCR was performed using 50 times diluted first PCR product by a second set of internal primers. Similarly, the above assay was performed under over expression of NS3pro as well as NS3 full length protein as indicated (panel i).
Similarly, the effect of over expression of NS3 protease on HCV negative strand synthesis was further investigated using ‘two cycle RNase protection assay'. The results showed significant increase of negative strand synthesis at 18 hour time point upon over expressions of NS3 protease (Fig 5d). More importantly, similar observation was made with the over expressions of full length NS3 (Fig 5e). However, mutant NS3 protease failed to enhance the negative strand synthesis to the same extent (Fig 5f).
To further validate above observations, we repeated the experiment using the rTth RT-PCR method which rules out the self priming and miss priming events 17 (Fig. 5g). Again, the results showed increase in negative strand RNA synthesis to a similar extent after the overexpression of NS3pro.
Additionally, to discriminate between negative strands versus a vast excess of positive-strands, RT-PCR with a tagged cDNA synthesis primer was used to selectively measure the level of negative strand synthesis 18. Results (Fig. 5h) reconfirmed the earlier observations. Interestingly, over expression of the full length NS3 protein also showed considerable increase in the negative strand synthesis as observed after NS3pro over expressions (Fig. 5i). Results suggest that the full length NS3 protein also supports the switch.
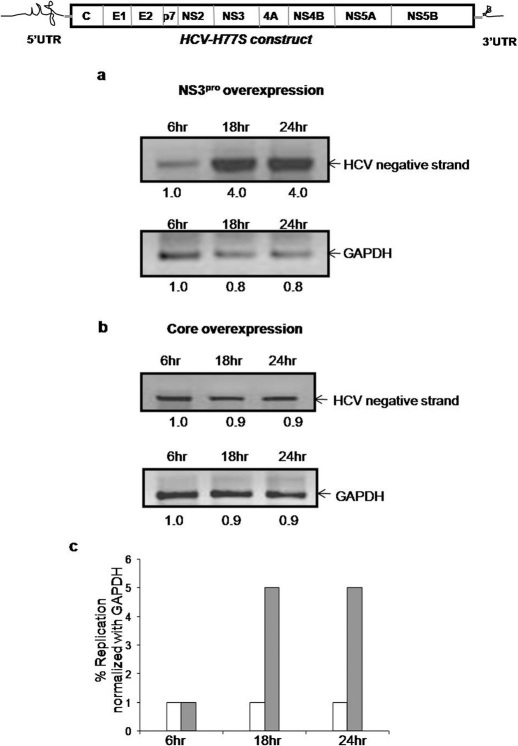
Finally, over expression of NS3pro in the context of all the viral proteins, was investigated with the HCV-H77S RNA (a generous gift from Stanley Lemon, University of Texas, Galveston) 19 in HCV cell culture assay which showed that NS3pro overexpression advances the replication of H77S RNA (Fig 6a) whereas overexpression of core protein did not show similar effect (Fig. 6b and 6c).
Figure 6. Panel a: Huh7 cells were cotransfected with H77S RNA (schematic representation shown above panel) and the construct encoding NS3pro.
RNA was isolated at different time points and HCV RNA was quantitated using semi quantitative RT PCR. GAPDH was used as internal control. Panel b: Huh7 cells were cotransfected with H77S RNA and construct encoding HCV core. RNA was isolated at different time points and HCV RNA was quantitated using semi quantitative RT PCR. GAPDH was used as internal control. Panel c: Graph plotted taking percentage replication of HCV RNA normalized with that of GAPDH amplified on Y axis and time on X axis. White bar indicates overexpression of HCV core and grey bar indicates that of NS3pro
Discussion
The two domains of NS3 protein (protease and helicase) are functionally coupled. It has been shown that helicase domain binds to HCV RNA more effectively if the protease domain is included. In this study we demonstrate that NS3pro independently binds HCV genomic RNA which in turn affects its protease activity. The binding of NS3pro to HCV IRES RNA has been found primarily due to the C terminal half of the NS3 protease domain. The recombinant NS3pro used in the study also bears the NS4A core peptide (since NS4A is required for catalytic activity of the protease). However, the C terminal half of NS3pro that is devoid of NS4A showed significant binding with HCV IRES (Supplementary Information, Fig. S11), comparable to NS3pro full-length, ruling out the possibility of any contribution of NS4A core peptide to RNA binding.
Under normal conditions, transfection of HCV replicon RNA showed accumulation of HCV RNA only after 24hr. Results from this study clearly demonstrated that over expression of NS3pro leads to increased replication at 18hr post transfection while no concomitant increase in luciferase translation was observed from the transfected HCV replicon RNA. Considering the half life of luciferase between 6–8 hours and presence of abundant template RNAs, this reflects significant decrease in HCV-IRES function at this time point.
It is possible that overexpression of NS3pro in HCV replicon and HCV cell culture systems could stimulate HCV RNA replication indirectly by promoting HCV polyprotein cleavage and increasing the abundance of NS5B and other “replication” proteins. However, western blot analysis using anti-NS5B antibody did not show considerable increase in NS5B protein expressions upon over expressions of NS3pro (Supplementary Information, Fig. S12). In fact considerable decrease in NS5B was noticed which could be due to inhibition of translation upon NS3pro overexpression.
IRES mediated translation of the input RNA is the first step in HCV life cycle inside the host cell after entry and release of genomic RNA. To restrict the cytotoxicity of accumulated viral proteins, it is essential to halt IRES function once the optimum level of viral protein synthesis is achieved and switch to viral RNA replication. In fact, Zhang et al reported that HCV core protein binds to HCV IRES (between nt 441–473) to inhibit translation and the authors speculated that the down regulation in translation could be required to favor initiation of transcription. However, the molecular mechanism was not clear 20.
Interestingly, when Huh7 S10 extract was UV cross linked to HCV SLIV RNA, several cellular proteins were found to interact including p50, p54, p56 etc. Additions of purified NS3 pro protein apparently competed out the binding of two proteins of approximate molecular mass, p50 and p56. One of the proteins (p50) was immuno-precipitated (Supplememtary Information, Fig. S13 a and b) with anti-La antibody suggesting that NS3pro competes out La protein binding to HCV IRES in the context of Huh7 S10 containing other cellular proteins.
Human La protein has been shown to be required for HCV IRES mediated translation as well as replication. It is tempting to speculate that at the initial stage, La protein is engaged in translation of viral RNA, while NS3pro performs viral polyprotein processing activity. At this stage, the concentration of NS3 may not be sufficient to compete out La protein binding to the IRES. As NS3 concentration rises, La binding to the IRES is antagonized, and La and NS3 finally reach an equilibrium state where the viral life cycle switches to replication (Supplementary Information, Fig. S14).
Taken together, these observations provide insights into a novel regulatory process involving the interplay between a viral and a host protein which determines the essential switch from translation to replication in the viral life cycle.
Methods
Over expression and purification of recombinant proteins
NS3 protease and EGFP-NS5A/5B-CBD proteins were over expressed in E. coli using the pYB-43 and pYB-44 plasmids (obtained as a kind gift from Itai Benhar) and purified as described earlier 13. NS3 pro is a fusion protein of NS4A-NS3pro, which lacks the helicase domain. Similarly all other recombinant full-length or mutant proteins were overexpressed in E coli and purified as described before 14. For replication studies pSGR JFH1 luc construct was used 15.
in vitro transcription and translation
mRNAs were transcribed from different plasmid constructs by run-off transcription reactions using T7 RNA polymerase (Promega). Similarly radiolabeled mRNAs were transcribed in vitro using T7 RNA polymerase and [α32P]UTP.
In vitro translation of capped bicistronic RNA containing HCV IRES (18–383nt) was carried out in a 15µl reaction containing 9µl nuclease treated rabbit reticulocyte lysate (Promega), amino acid mix and RNase inhibitor (as per manufacturer's protocol). Renilla and firefly luciferase activity was measured according to Promega protocol using dual luciferase assay system as described earlier 14
UV-crosslinking assay
The [α32P]UTP labeled RNA of interest was incubated with the protein of interest at 30°C for 15 minutes in RNA binding buffer (containing 5mM HEPES pH 7.6, 25mM KCl, 2mM MgCl2, 3.8% Glycerol, 2mM DTT and 0.1mM EDTA). The reaction mixtures were then UV-cross-linked using a UV lamp for 15 minutes. The reaction mixtures were then treated with RNase A followed by SDS-PAGE analysis and phosphor imaging. For non specific RNA control pGEMT-Easy vector was religated and digested with Sac1 in the polylinker. This template was in vitro transcribed to obtain RNA against the polylinker region which was used as a nonspecific RNA control.
Filter binding assay
The [α32P]UTP labeled RNA was incubated with the protein of interest at 30°C for 15 minutes in RNA binding buffer (containing 5mM HEPES pH 7.6, 25mM KCl, 2mM MgCl2, 3.8% Glycerol, 2mM DTT and 0.1mM EDTA) followed by filter binding assay as described earlier 14.
Ribonuclease T1 footprinting
RNA binding reactions were carried out using in vitro transcribed HCV IRES (18–383nt) RNA (4 μg) in absence or presence of NS3pro protein at 30°C for 15 min. The RNA was then digested with 0.05 unit of RNase T1 (Sigma) for 10 min at 37°C. After incubation the reaction mixture was treated with proteinase K followed by phenol: chloroform extraction and alcohol precipitation. The T1 digestions were carried out using nuclease buffer containing 10mM Tris–Cl (pH 7.5), 10mM MgCl2, and 100mM KCl. The digested RNAs were precipitated with 0.3 M sodium acetate and 2.5 volumes of absolute ethanol. The precipitated RNA was reverse-transcribed with an end labeled primer annealing at 3′ end of the RNA. The cDNAs were precipitated with 0.3M sodium acetate and absolute ethanol and then resolved in 8M urea–8% acrylamide gel along with a reference sequencing reaction carried out using the same end labeled primer.
NS3 protease cleavage assay
To investigate the effect of HCV IRES RNA binding on the NS3 protease cleavage activity, the recombinant NS3 protease was allowed to form complex with increasing amount of cold HCV IRES RNA in binding buffer (5mM HEPES [pH 7.6], 25mM KCl, 2mM MgCl2, 3.8% glycerol, 2mM dithiothreitol, 0.1mM EDTA) at 30°C for 30 min. One of the reaction tubes had no HCV IRES RNA. After incubation at 30°C, NS5A/5B substrate (containing NS3 protease cleavage site) was added to each of the tubes and incubated at 37°C for 45 minutes. The reactions were then loaded on a 15% SDS-PAGE, resolved and subjected for silver-staining.
Transient transfection
pSGR JFH1 luc RNA (5µg) was transiently co-transfected into monolayer (80% confluent) of Huh7 cells in 35mm dishes with either NS3pro, mNS3pro, core, or La encoding plasmid construct using Lipofectamine 2000 reagent (Invitrogen) in antibiotic free medium. Cells were harvested at different time points using Tri reagent (Sigma) for RNA preparation and RT-PCR. To investigate translation levels, cell lysates were prepared using passive lysis buffer (Promega). Luciferase activities were measured using Luciferase assay reagent (Promega).
Semi quantitative RT-PCR
RNA was isolated from Huh 7 cells co-transfected with either HCV dicistronic replicon 15 or H77S RNA 19 (along with either NS3pro over expressing plasmid or the vector control. RNAs were reverse transcribed with HCV 5′ primer and GAPDH 3′ primer using MMLV RT (Promega) for amplification of negative strand of HCV RNA. Resulting cDNA was used for PCR amplification corresponding to HCV IRES. The PCR products were run on 1% agarose gel and quantitated by densitometry. GAPDH was used as an internal control.
Immunoprecipitation
pSGR JFH1 luc RNA (5µg) was transiently co-transfected into monolayer (80% confluent) of Huh7 cells in 35mm dishes with either NS3pro, mNS3pro, core, or La expressing plasmid construct using Lipofectamine 2000 reagent (Invitrogen) in antibiotic free medium. After 18hrs, the cells were lysed with buffer containing 100 mM KCl, 5 mM MgCl2, 10 mM HEPES (pH 7.0), 0.05% NP-40, 1 mM DTT, 100 U/ml RNasin, 25 μl/ml. Supernatants were incubated with anti NS5B antibody overnight. RNP complexes were precipitated by protein-G sepharose beads followed by proteinase-K and DNase treatments. Finally phenol-chloroform extracted and alcohol precipitated RNA was used for RT-PCR for detecting the HCV IRES. Same protocol was followed for pulling down NS3 - RNA complex from HCV monocistronic replicon pFKi383hygubiNS3-3_5.1 cell line 21 using anti NS3 antibody. Anti actin antibody was used as negative control.
Two-cycle RNase protection assay
Total RNA was self hybridized overnight at 60°C in the absence of radiolabelled probe. This was followed by RNase treatment at 7°C for 1hour in a digestion mix containing 500mM NaCl, 10mM Tris HCl (pH 7.5), 5mM EDTA, 4.5µg RNaseA per ml and 350U RNase T1 per ml. Reaction mix was subjected to proteinase K treatment followed by phenol chloroform extraction and ethanol precipitation with 5 µg tRNA. RNA pellet was then suspended in hybridization buffer containing 75fmol of radiolabelled probe and the samples were put through the standard RNase protection assay procedure 16.
Strand specific RT-PCR using tagged HCV primer
Total RNAs were reverse transcribed with tagged HCV 5′ primer using AMV-RT (Promega). Resulting cDNA was amplified by nested PCR. Both rounds were performed in a reaction mix containing 0.2µM of each primer, 1×PCR reaction buffer, 0.1mM dNTP mix and 1U of hot start Taq DNA polymerase (Genei). First round of PCR was performed using tag only primer and HCV IRES reverse primer. The resulting PCR product was diluted 50 times and was used for the second round of PCR using a set of internal primers. GAPDH was amplified as an internal control. Resulting PCR products were analyzed by 2% agarose gel electrophoresis.
rTth reverse transcriptase PCR
In this method reverse transcription was carried out at 60°C for 30 minutes. Reverse transcription activity of the polymerase was inactivated by chelating Mn2+ by addition of chelating buffer containing 1.5mM EGTA and heating the reaction mix at 98 °C for 30 minutes. This was followed by PCR amplification as described earlier 17.
Statistical analysis
Each result represents an average of three independent experiments. All graphs represent mean ± SD. Student's t test was performed and P values of less than 0.01 were considered to be statistically significant (* represent P <0.01; ** represent P <0.001).
Author Contributions
UR designed and performed experiment, analyzed results, prepared figures and written the manuscript. SD designed experiment, analyzed results, prepared figures and written the manuscript.
Supplementary Material
Supplementary Information
Acknowledgments
We thank Dr. Akio Nomoto, Dr. Itai Benhar, Dr. Ralf Bartenschlager, Dr. Takaji Wakita and Dr. Stanley Lemon for plasmid constructs. We gratefully acknowledge the valuable suggestions and comments on the manuscript from Dr. Charles M Rice and Dr. Peter Sarnow. We thank Dr. Partho Sarothi Ray, Dr. Robin Fahraeus and Dr. Eric Gowans for critical reading of the manuscript and our lab members for their helpful discussion. This work was supported by grant from the Department of Biotechnology; Government of India to SD. UR was supported with pre-doctoral fellowship from CSIR, India.
References
- Barbato G. et al. The solution structure of the N-terminal proteinase domain of the hepatitis C virus (HCV) NS3 protein provides new insights into its activation and catalytic mechanism. J. Mol. Biol. 289, 371–384 (1999). [DOI] [PubMed] [Google Scholar]
- Beran R. K. F., Serebrov V. & Pyle A. M. The serine protease domain of hepatitis C viral NS3 activates RNA helicase activity by promoting the binding of RNA substrate. J. Biol. Chem. 282, 34913–34920 (2007). [DOI] [PubMed] [Google Scholar]
- Frick D. N., Rypma R. S., Lam A. M. I. & Gu B. The nonstructural protein 3 protease/helicase requires an intact protease domain to unwind duplex RNA efficiently. J. Biol. Chem. 279, 1269–1280 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold J. & Andino R. Poliovirus RNA replication requires genome circularization through a protein–protein bridge. Mol. Cell 7, 581–591 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali N., Pruijn G. J. M., Kenan D. J., Keene J. D. & Siddiqqui A. Human La antigen is required for the Hepatitis C virus internal ribosome entry site mediated translation. J. Biol. Chem. 275, 27531–27540 (2000). [DOI] [PubMed] [Google Scholar]
- http://nar.oxfordjournals.org/cgi/content/abstract/35/5/1465 - _blank Fukushi S., Okada M., Kageyama T., Hoshino F. B., Nagai K. & Katayama K. Interaction of poly (rC)-binding protein 2 with the 5′-terminal stem loop of the hepatitis C virus genome. Virus Res. 73, 67–79 (2001). [DOI] [PubMed] [Google Scholar]
- Hellen C. U. T. & Sarnow P. Internal ribosome entry sites in eukaryotic mRNA molecules. Genes Dev. 15, 1593–1612 (2001). [DOI] [PubMed] [Google Scholar]
- Spangberg K. & Schwartz S. Poly (C) -binding protein interacts with the hepatitis C virus 5′ untranslated region. J. Gen. Virol. 81, 1371–1376 (1999). [DOI] [PubMed] [Google Scholar]
- Costa-Mattioli M., Svitkin Y. & Sonenberg N. La autoantigen is necessary for optimal function of the poliovirus and hepatitis C virus internal ribosome entry site in vivo and in vitro. Mol. Cell. Biol. 24, 6861–6870 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudi R., Abhiman S., Srinivasan N. & Das S. Hepatitis C virus internal ribosome entry site-mediated translation is stimulated by specific interaction of independent regions of human La autoantigen. J. Biol. Chem. 278, 12231–12240 (2003). [DOI] [PubMed] [Google Scholar]
- Pudi R., Srinivasan P. & Das S. La protein binding at the GCAC site near the initiator AUG facilitates the ribosomal assembly on the hepatitis C virus RNA to influence internal ribosome entry site mediated translation. J. Biol. Chem. 279, 29879–29888 (2004). [DOI] [PubMed] [Google Scholar]
- Domitrovich A. M., Diebel K. W., Ali N., Sarker S. & Siddiqui A. Role of La autoantigen and polypyrimidine tract-binding protein in HCV replication. Virology 335, 72–76 (2005). [DOI] [PubMed] [Google Scholar]
- Berdichevsky Y. et al. A novel high throughput screening assay for HCV NS3 serine protease inhibitors. J. Viro. Met. 107, 245–255 (2003). [DOI] [PubMed] [Google Scholar]
- Mondal T. & Ray U. et al. Structural determinant of human La protein critical for internal initiation of translation of hepatitis C virus RNA. J. Virol. 82, 11927–11938 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T. et al. Detection of anti-hepatitis C virus effects of interferon and ribavirin by a sensitive replicon system. J. Clin. Microbiol. 43, 5679–5684 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak J. E. & Kirkegaard K. Improved method for detecting poliovirus negative strands used to demonstrate specificity of positive-strand encapsidation and the ratio of positive to negative strands in infected cells. J. Virol. 65, 3384–7 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanford R. E., Chavez D., Chisari F. V. & Sureau C. Lack of detection of negative-strand hepatitis C virus RNA in peripheral blood mononuclear cells and other extrahepatic tissues by the highly strand-specific rTth reverse transcriptase PCR. J. Virol. 69, 8079–83 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craggs J. K., Ball J. K., Thomson B. J., Irving W. L. & Grabowska A. M. Development of a strand-specific RT-PCR based assay to detect the replicative form of hepatitis C virus RNA. J. Virol. Methods. 94, 111–20 (2001). [DOI] [PubMed] [Google Scholar]
- Yi M., Ma Y., Yates J., & Lemon S. M. . Compensatory mutations in E1, p7, NS2 and NS3 enhance yields of cell culture-infectious inter-genotypic chimeric hepatitis C virus. J. Virol. 81, 629–638 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown E. A., Zhang H., Ping L. H. & Lemon S. M. Secondary structure of the 5′ nontranslated regions of hepatitis C virus and pestivirus genomic RNAs. Nucleic Acids Res. 20, 5041–5045 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frese M. K., Barth A., Kaul V., Lohmann V. Schwärzle & R. Bartenschlager. Hepatitis C virus RNA replication is resistant to tumor necrosis factor-α. J. Gen. Virol. 84, 1253–1259 (2003). [DOI] [PubMed] [Google Scholar]
- Zhang J., Yamada O., Yoshida H., Iwai T. & Araki H. Autogenous translational inhibition of core protein: implication for switch from translation to RNA replication in hepatitis C virus. Virology. 293, 141–150 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information