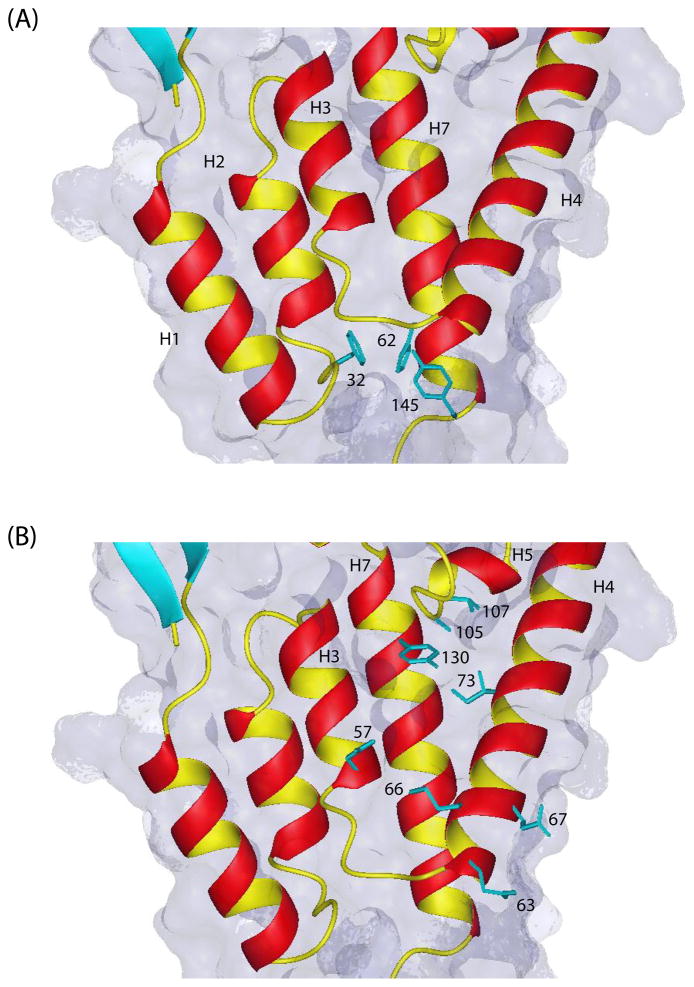
Figure 7.
(a) Close-up view of the hydrophobic binding pocket on the NTD domain between helices 1, 2, 4 and 7 for the assembly inhibitor CAP-1 (13). Though there is some distribution in sidechain orientations among the 20 structures, the aromatic rings of Phe32, His62, and Tyr145 tend to point inwards toward the bottom of the cavity. The binding of the inhibitor is accompanied by a displacement of these aromatic sidechains. (b) The binding pocket for the antiviral compound PF-3450074 formed by helices 3, 4, 5 and 7 on the NTD domain (10). Some of the binding pocket residues involved in interactions with the inhibitor are also shown (from the crystal structure of the inhibitor with a mutant NTD (10)). This inhibitor targets both early and late events in the replication cycle. The cyclophilin-dependent mechanism of antiviral activity of PF-3450074 is different from that of CAP-1.