Abstract
Background
Central nervous system (CNS) atypical teratoid/rhabdoid tumors (AT/RT) are aggressive tumors usually diagnosed in young children and characterized by SMARCB1 (INI1, hSNF5) gene abnormalities. Despite initial chemo-radiation responsiveness, most children die of progressive disease (PD). Little data regarding familial AT/RT clinical course exist. This study described and compared familial (F) versus sporadic (S) AT/RT and elucidated SMARCB1 mutations and inheritance patterns.
Methods
A retrospective chart review, pedigree, and SMARCB1 analysis were done.
Results
Between January 1989 and June 2009, 20 children with CNS AT/RT were diagnosed, 8-S and 12-F. Median age at diagnosis (months) of S and F patient were: 13 and 4.8, respectively. Median survival (months) was S-21, F4.5, and 8-all. Pedigree analyses showed unaffected parent carriers with multiple affected offspring.
Conclusions
Children with F-AT/RT are younger, have more extensive disease, and are more likely to die from PD than children with S-AT/RT. Surgery, radiation, and chemotherapy were important in achieving long-term survival. Pedigree analysis supports autosomal dominant inheritance pattern with incomplete penetrance. Germline SMARCB1 mutation analysis is important in all patients diagnosed with AT/RT to (1) determine actual incidence of F-AT/RT, (2) determine penetrance of predisposing mutations, (3) provide appropriate genetic counseling, and (4) establish surveillance screening guidelines.
Keywords: atypical teratoid rhabdoid (AT/RT), brain tumor, familial, SMARCB1, treatment
BACKGROUND
Central nervous system (CNS) atypical teratoid/rhabdoid tumors (AT/RT) are clinically aggressive malignancies most frequently diagnosed in young children <4 years of age. They occur more often in males than females and are usually cerebellar in location [1]. Although often initially responsive to chemotherapy, response duration is usually brief. Most children die of progressive disseminated disease, with or without local recurrence, despite aggressive therapy [1–4]. However, long-term survivors have been reported [5–13].
By histological appearance, AT/RT’s are dense, highly cellular sheets of undifferentiated cells, including variable numbers of rhabdoid cells characterized by eccentric nuclei, a prominent nucleolus, eosinophilic cytoplasm, and eosinophilic inclusion body. AT/RT is distinguished from other malignant brain tumors using a panel of immunohistochemical (IHC) markers. Staining is diffusely positive for vimentin, cytokeratin, smooth muscle actin, and epithelial membrane antigen; variable staining for neuron-specific enolase, Leu7, S-100 markers; and negative staining for germ cell tumor markers, desmin and myogenin [1]. The tumors have a variety of chromosome 22 abnormalities that result in loss of function of the SMARCB1 gene in 22q11.2 [14,15]. Deletions and/or mutations of the SMARCB1 gene are diagnostic for AT/RT, even in the absence of rhabdoid histology. IHC staining for the SMARCB1 protein in tumor nuclei is negative due to gene inactivation [16–18].
Recent publications have shown a pre-disposition to AT/RT resulting from germline loss of function mutations in the SMARCB1 gene and subsequent somatic inactivation of the remaining copy of this tumor suppressor gene, resulting in malignant transformation [15,19,20]. Simultaneous presentation of CNS AT/RT was reported in two sisters diagnosed within a few weeks of each other [21]. Coexistence of AT/RT, a SMARCB1 abnormality, and myoepithelioma in a three-generation family [22], and co-occurrence of AT/RT and schwannomatosis in a family with a single predisposing mutation from this institution [23] have also been reported.
Between January 1989 and March 2009, 20 children were diagnosed with CNS AT/RT at our institution, some of whom were siblings also diagnosed with CNSAT/RT. The purposes of this study were to (1) describe and compare clinical features and outcomes of patients diagnosed with familial (F) versus sporadic (S) AT/RT and (2) further elucidate the genetic alterations involved in the familial AT/RT syndrome.
METHODS
Chart Review
After approval by the University of Utah Institutional Review Board, a retrospective chart review of the 20 patients diagnosed with CNS AT/RT at this institution between January 1989 and June 2009 was conducted. Data analyzed included age at diagnosis, gender, primary tumor site, presence of metastases, treatment administered, response to treatment, overall and disease-free survival (DFS), and time to tumor progression and death.
Immunohistochemistry
IHC staining was performed on formalin-fixed, paraffinembedded tissue sections from AT/RT sections using commercially available monoclonal antibodies directed against selected cell associated antigens according to standard techniques [16–18].
Molecular Analyses
Cytogenetic analysis and fluorescence in situ hybridization (FISH) were performed using SMARCB1 and Ewing’s sarcoma-specific chromosome band 22q12 probes as previously described [15]. High-resolution genomic profiling and comprehensive targeted analysis of SMARCB1 was performed using Illumina single-nucleotide polymorphism-based oligonucleotide arrays, multiplex ligation-dependant probe amplification (MLPA), and coding sequence analysis [15,23–25].
Chemotherapy
Chemotherapy treatment regimens administered included CCG 9921 [26], Head start II protocol [13], CCG 99701 [27], CCG 99703 [28], and ACNS0333 [29], depending on the age of the patient and available therapeutic options at the time of diagnosis.
Radiation Therapy
Radiation therapy doses and field designs varied among patients, depending on the age of the patient at the time of diagnosis, tumor site, specific study, and curative versus palliative intent.
RESULTS
Twenty children with CNS AT/RT were diagnosed between January 1989 and June 2009. Twelve patients from six families had positive family histories and/or proven germline mutations in a parent, while eight appeared to be sporadic. Treatment following diagnosis varied and included supportive care measures only, chemotherapy, radiation therapy, and high-dose chemotherapy with hematopoietic stem cell rescue, depending on the age of the patient when diagnosed, parental decisions, and available therapeutic options when diagnosed (Tables I and II).
TABLE I.
Patient Characteristics
| Pt/family | Gender | Age | Site | Metastases | Surgery | Chemo | XRT | Status | Time F/U | Time PD | TTD |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sporadic | |||||||||||
| 1 | F | 18 | ST | Spine | GTR | Y | Y | DOD | 12 | 6 | 6 |
| 2 | F | 2.5 | PF | None | NTR | Y-BMT | Y | ANED | 30 | ||
| 3 | F | 39 | ST | None | GTR | Y | Y | ANED | 77 | ||
| 4 | M | 42 | ST | None | NTR | Y | Y | ANED | 88 | ||
| 5 | F | 11 | PF | None | GTR | Y | Y | ANED | 142 | ||
| 6 | M | 9 | PF | None | GTR | Y-BMT | Y | DOD | 11 | 6 | 5 |
| 7 | M | 6 | C-spine | BP | PR | Y | N | DOD | 3 | 2.5 | 0.5 |
| 8 | F | 15 | PF | None | GTR | Y-BMT | Y | ANED | 8 | ||
| Familial | |||||||||||
| 9-F1 | M | 42 | PF | None | GTR | Y | Y | ANED | 62 | ||
| 10-F1 | M | 18 | PF | None | GTR | Y-BMT | Y | ANED | 13 | ||
| 11-F1 | F | 18 | ST | None | GTR | Y | N | D-infection | 1 | ||
| 12-F2 | M | 0 | PF | None | BX | N | N | DOD | 0.75 | 0 | 0.75 |
| 13-F2 | F | 6 | PF | Spine, skin | GTR | Y | N | DOD | 7 | 6 | 1 |
| 14-F2 | M | 0.5 | PF | None | GTR | Y | Y | DOD | 8 | 4.5 | 3.5 |
| 15-F3 | F | 18 | Spine | Spine | BX | Y | N | DOD | 2 | 1 | 1 |
| 16-F3 | F | 5 | PF | None | GTR | Y | N | D-infection | 9 | ||
| 17-F4 | M | 0 | CNS | Widespread | BX | N | N | DOD | 0 | 0 | 0 |
| 18-F4 | F | 3 | PF | None | NTR | N | N | DOD | 2 | 0 | 2 |
| 19-F5 | M | 4.5 | PF | None | GTR | Y | Y | ANED | 8 | ||
| 20-F6 | F | 2 | PF | Renal | NTR | N | N | DOD | 2 | 0 | 2 |
Age, time, in months; PF, posterior fossa; BP, brachial plexus; ST, supratentorial; Bx, biopsy; PR, partial resection; NTR, near total resection; GTR, gross total resection; XRT, radiation; F/U, follow-up; D-infection, died of infection; ANED, alive no evidence of disease; PD, progressive disease; DOD, dead of disease; TTD, time to death after PD; gl, germline mutation; no+, mother with same germline mutation.
TABLE II.
Chromosome 22 Abnormalities
| Patient/family | Family history | Ch22 abnormality |
|---|---|---|
| Sporadic | Tumor | |
| 1 | No | Not tested |
| 2 | No | c.1143delG, c.1145delC |
| 3 | No | c.601C > T, INI1 CN LOH |
| 4 | No | c.601C > T, htz del 22q |
| 5 | No | Not tested |
| 6 | No | Normal |
| 7 | No | c.548_566dup19 |
| 8 | No | Hmz del E7, htz del INI1 |
| Familial | Germline/tumor | |
| 9-F1 | Yes | c.986 + 1G > C,CN-ILOH |
| 10-F1 | Yes | c.986 + 1G > C, htz del INI1 |
| 11-F1 | Yes | c.986 + 1G > C,CN-LOH |
| 12-F2 | Yes | Not tested |
| 13-F2 | Yes | Dup E4-5 |
| 14-F2 | Yes | Dup E4-5 |
| 15-F3 | Yes | Dup E6, c.578_585dup8 |
| 16-F3 | Yes | Dup E6, Htz del INI1 |
| 17-F4 | Yes | Not tested |
| 18-F4 | Yes | Not tested |
| 19-F5 | No+ | Del E7, htz del INI1 |
| 20-F6 | No++ | c.601C > T |
no+, mother with germline mutation; ++, germline mutation.
Age at Diagnosis
The median age at diagnosis 4.8 months for children with FAT/RT (range 0–46 months), considerably younger than the median age of children diagnosed with sporadic tumors, which was 13 months (range 2.5–42 months). However, considerable overlap between age ranges in these two groups existed. For example, one infant with a sporadic tumor was diagnosed at only 2.5 months of age, while in Family 1, three siblings with familial CNSAT/RT were not diagnosed until 46, 18, and 42 months of age (Table III).
TABLE III.
Outcome of Evaluable Treated Patients With CNS AT/RT
| Pt/family | Year | Age | Surgery | BMT | Chemo | XRT | Intent | Dose | Site | Status |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2008 | 18 | GTR | HS | Y | P | 36 | CS | DOD | |
| 2 | 2007 | 2.5 | NTR | ×1 | 9921a | Y | C | 48 | IF | ANED |
| 3 | 2002 | 39 | GTR | 99701 | Y | C | 36/55.8 | CS/PF | ANED | |
| 4 | 2001 | 42 | NTR | 99701 | Y | C | 36/50.4 | CS/IF | ANED | |
| 5 | 1999 | 11 | GTR | 9921a | Y | C | 46 | PF | ANED | |
| 6 | 2004 | 9 | GTR | ×1 | 9921a | Y | P | 20 | IF | DOD |
| 7 | 2009 | 6.5 | PR | HS | N | DOD | ||||
| 8 | 2009 | 15 | GTR | ×3 | ACNS0333 | Y | C | 50.4 | IF | ANED |
| 9-F1 | 2007 | 18 | GTR | ×1 | HS | Y | C | 50.4 | IF | ANED |
| 10-F1 | 2004 | 42 | GTR | 99701 | Y | C | 36/55.8 | CS/PF | ANED | |
| 13-F2 | 1993 | 5 | GTR | 9921b | N | DOD | ||||
| 14-F2 | 2001 | 0.5 | GTR | 9921a | Y | P | 24 | PF | DOD | |
| 15-F3 | 2006 | 18 | BX | 9921a | N | DOD | ||||
| 19-F5 | 2009 | 4.5 | GTR | ×3 | ACNS0333 | Y | C | 50.4 | PF | ANED |
Year, year diagnosed; age, in months at diagnosis; PR, partial resection; GTR, gross total resection; NTR, near total resection; Bx, biopsy; chemo, chemotherapy protocol; HS, Headstart II; BMT, high-dose chemotherapy autologous stem cell rescue; XRT, radiation; intent: C, curative, P, palliative; IF, involved field; ANED, alive, no evidence of disease; DOD, dead of disease.
Tumor Location at Diagnosis
Children with S-AT/RT usually had localized disease at diagnosis. Seven patients presented with supratentorial tumors, four had posterior fossa (PF) tumors, and one had a spinal primary tumor. In contrast, children with F-AT/RT usually had PF tumors, although one had a supratentorial tumor, and one had a localized spine tumor, but had widespread CNS disease at diagnosis.
Treatment
Sixteen of the 20 patients diagnosed with AT/RT were treated with systemic chemotherapy and 11 were also treated with radiation. Chemotherapy and radiation therapy were associated with prolongation of overall survival (OS) and disease-free survival (DFS). Four patients, eventually determined to have F-AT/RT, were treated with supportive care measures only, and all expired within 2 months following presentation. Two additional patients in the familial cohort expired of infectious complications during chemotherapy, one during the first month from overwhelming sepsis associated with neutropenia (7-F1), and one from pneumonia 4 months into chemotherapy (14-F3). These two patients are thus not evaluable for tumor response. Data describing the treatment and outcome for the 14 treated and evaluable patients are summarized in Table III.
Survival
Death from tumor progression occurred very rapidly in children who were not treated at the time of diagnosis. Once progression occurred in patients being treated, death occurred rapidly. However, there were three noteworthy exceptions. Patient 1 experienced PD following five courses of Headstart II, prior to planned HDC-SCR and radiation. She was treated with craniospinal radiation and temozolomide during radiation, followed by temozolomide, irinotecan, and bevacuzimab and had disease stabilization for 6 months, before her death from tumor cell dissemination within the cerebrospinal fluid. Patient 12-F2 survived 3.5 months following palliative involved field radiation administered after PD. Finally, Patient 6 experienced PD after chemotherapy, HDC-SCR, and radiation. He was treated with temozolomide for 4 months prior to PD again, and then with oxaliplatin for 3 months but expired 5 months after initial PD.
Genetic Analyses
In the sporadic tumor cohort, tumors from two patients were not tested. One was negative by FISH, MLPA, and PCR-based coding sequence analysis. However, there was no additional material remaining for subsequent SNP array analysis. One tumor showed two common single base deletions in exon 9; and three cases had mutations in exon 5. The final tumor had an exon 7 deletion in one allele and loss of the second allele.
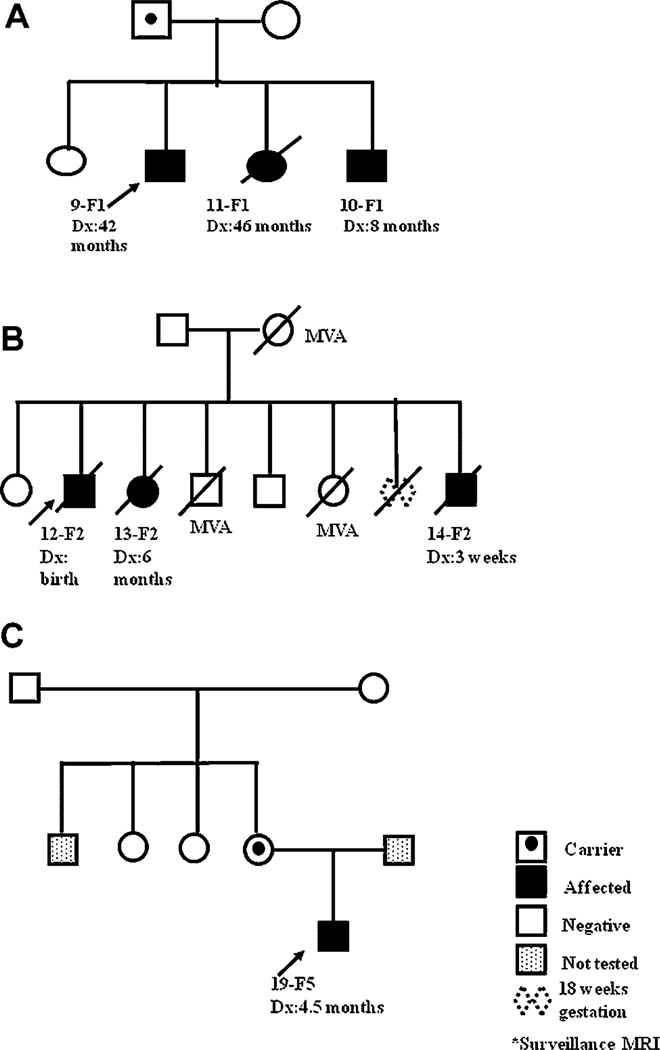
In the familial cohort, five of the six families had material available for genetic analysis. A germline splice site mutation in intron 7 was present in the blood samples from the affected children and the father in Family 1. There was an exon 4,5 duplication in the three siblings from Family 2. Family 3 demonstrated a germline duplication of exon 6 [23]. Material from the two children in Family 4 was not available for testing. In Family 5, exon 7 was deleted in the patient and his biologic mother. Patient 20-F6 had a germline mutation in exon 5. The pedigrees from three families available for more complete evaluation are shown in Figure 1A–C, and the analyses descriptions are presented below. The pedigree from family F3 has previously been published [23].
Fig. 1.
Pedigrees for Families F1, F2, and F5.
Family 1
Three siblings, all of whom developed AT/RT, and their father showed a splice site mutation, c.986 + 1G > C, in intron 7 in their blood which was not found in their mother or unaffected sib. RNA from tumor in the proband, Patient 9-F1 showed a deletion of exon 7 as a result of the mutation. The tumor had a loss of most of the long arm of chromosome 22 as a result of a copy number neutral loss of heterozygosity event, as determined by Illumina 610K SNP array analysis. Cytogenetic analysis of blood and tumor samples in his brother, Patient 10-F1, showed a large deletion of chromosome 22 in 65% of tumor cells (46,XY,del(22)(q11.2q12)[13]/46,XY[7]). FISH using the BCR/ABL and EWSR1 probes showed loss of BCR in 54.5% of cells (22q11.2(BCRx1)[109/200]), but retention of EWSR1, indicating an interstitial deletion involving chromosome 22q11.2. This was also confirmed by MLPA in his tumor sample. Patient 11-F1 was found to harbor the same germline mutation and had a supratentorial mass on surveillance brain MRI. She underwent gross total resection of the tumor, which demonstrated copy number neutral loss of heterozygosity of the wild-type allele by SNP array. She died from Clostridium septicum sepsis associated with fever and neutropenia following her initial course of chemotherapy. On physical examination, the carrier-father was found to have multiple, mobile, soft nodules on the trunk and extremities. Biopsy of one of these lesions was characterized as a “dermatofibroma.” IHC staining for S100 was negative. IHC for SMARCB1 was not done (Fig. 1A).
Family 2
All three siblings presented with symptoms: Patient 12-F2, the proband, at birth; Patient 13-F2 at 5 months of age; and Patient 14-F2 at 0.5 months of age. Exons 1–9 of parental blood, and the tumors from the two patients tested exhibited wild-type SMARCB1 sequence and interphase FISH studies were normal. However, both harbored the same exon 4,5 duplication when analyzed with MLPA. Results were confirmed using a quantitative TaqMan assay. The mutation was not detected in either of the parents, suggesting gonadal mosaicism. Parental paternity was confirmed for the two affected children (Fig. 1B).
Family 5
The proband, Patient 19-F5, presented with symptoms at age 4.5 months. Tumor analysis showed no coding sequence mutations detectable by PCR analysis. By MLPA, the tumor tissue showed a heterozygous deletion of exons 1–6 and 8–9 in SMARCB1, and a homozygous deletion of exon 7. The peripheral blood of the patient and his mother showed a heterozygous deletion of exon 7 of SMARCB1 by MLPA. Maternal grandmother and grandfather, and two maternal aunts tested negative. The paternal uncle refused testing. The mutation in the patient’s mother is believed to have arisen de novo although germline mosaicism is a possibility (Fig. 1C).
DISCUSSION
This study comprises the largest single institution experience combining both sporadic and familial AT/RT patients reported to date and yields important comparative clinical and genetic information. Children with F-AT/RT are much younger when diagnosed, and more likely to die from tumor progression than children with SAT/RT, although these data were highly impacted by the fact that 4 of 12 patients in the familial cohort were treated with supportive care only, and were diagnosed early on in the study period. Importantly, long-term survival was achievable in children with familial or sporadic tumors following chemotherapy and radiation therapy, with or without HDC-SCR, consistent with previously published case and small series results but unique to the rare reports of children with F-AT/RT [11,13,30,31]. The institutional experience reported here underscores prior observations that while AT/RT is usually initially responsive to chemotherapy and radiation, maintaining that response remains the major obstacle to overcome. In comparing patients treated with supportive care measures only and patients treated with chemotherapy, chemotherapy did appear to prolong survival, in some cases permitting the younger patients to become older prior to administration of radiation. Radiation therapy appeared to be an essential component of successful therapy. All seven patients who have survived thus far were treated with radiation in addition to chemotherapy. Radiation therapy also prolonged survival in patients with progressive disease following chemotherapy who had not yet been treated with radiation. Recent technical advances that allow conformal radiation may permit more aggressive treatment with acceptable toxicities and late effects in very young patients with AT/RT.
Many crucial unanswered questions remain. What is the role for studying all pediatric CNS embryonal tumors for SMARCB1 alterations? In one study, 9 of 26 archival paraffin-embedded pediatric embryonal tumors that showed no INI1 staining lacked the histologic features characteristic of AT/RT but were associated with very aggressive clinical courses and were resistant to conventional therapy [32]. What is the true incidence of germline SMARCB1 mutations in patients diagnosed with AT/RT? The identification of asymptomatic germline mutation carrier parents underscores the importance of testing all newly diagnosed AT/RT patients for germline mutations. Such information would be extremely helpful to neuro-oncologists during their discussions with families of newly diagnoses AT/RT patients. What is the true penetrance of germline SMARCB1 gene mutations, and risk of developing malignant AT/RT in mutation carriers? In data presented here, phenotypically normal parents harboring germline mutations of affected infants or young children are reported. While AT/RT is primarily a pediatric neoplasm, adults with AT/RT have been reported, some of whom have had molecular confirmation of the tumor, including detection of 22q11.2 deletions, and supportive IHC staining although germline SMARCB1 mutation status was not discussed [33–35]. What is the risk of therapy-related second malignancies in children treated for AT/RT who harbor germline mutations? Finally, what is the current role for genetic screening? Given the absence of clinical or radiographic findings that distinguish familial from sporadic AT/RT, it appears prudent to screen all individuals diagnosed with AT/RT for germline mutations. The optimal strategy for following known carriers for subsequent tumor development has yet to be formulated. Current ongoing basic science and cooperative group biologic and therapeutic trials addressing these questions promise to identify new therapeutic strategies to improve the outcome for children who develop this most challenging malignancy.
ACKNOWLEDGMENTS
This work was supported in part by a grant from the National Institutes of Health (CA46274 to J.A.B.). The authors would like to thank Luanne Wainright, Laura Tooke, and Katherine Eaton for technical assistance.
Grant sponsor: National Institutes of Health; Grant number: CA46274.
Footnotes
Conflict of interest: Nothing to declare.
REFERENCES
- 1.Rorke LB, Packer RJ, Biegel JA. Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood: Definition of an entity. J Neurosurg. 1996;85:56–65. doi: 10.3171/jns.1996.85.1.0056. [DOI] [PubMed] [Google Scholar]
- 2.Biegel JA, Rorke LB, Emanuel BS. Monosomy 22 in rhabdoid or atypical teratoid tumors of the brain. N Engl J Med. 1989;321:906. doi: 10.1056/nejm198909283211317. [DOI] [PubMed] [Google Scholar]
- 3.Rorke LB, Packer R, Biegel J. Central nervous system atypical teratoid/rhabdoid tumors of infancy and childhood. J Neurooncol. 1995;24:21–28. doi: 10.1007/BF01052653. [DOI] [PubMed] [Google Scholar]
- 4.Packer RJ, Biegel JA, Blaney S, et al. Atypical teratoid/rhabdoid tumor of the central nervous system: Report on workshop. J Pediatr Hematol Oncol. 2002;24:337–342. doi: 10.1097/00043426-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 5.Olson TA, Bayar E, Kosnik E, et al. Successful treatment of disseminated central nervous system malignant rhabdoid tumor. J Pediatr Hematol Oncol. 1995;17:71–75. doi: 10.1097/00043426-199502000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Weiss E, Behring B, Behnke J, et al. Treatment of primary malignant rhabdoid tumor of the brain: Report of three cases and review of the literature. Int J Radiat Oncol Biol Phys. 1998;41:1013–1019. doi: 10.1016/s0360-3016(98)00106-0. [DOI] [PubMed] [Google Scholar]
- 7.Hirth A, Pedersen PH, Wester K, et al. Cerebral atypical teratoid/rhabdoid tumor of infancy: Long-term survival after multimodal treatment, also including triple intrathecal chemotherapy and gamma knife radiosurgery—Case report. Pediatr Hematol Oncol. 2003;20:327–332. [PubMed] [Google Scholar]
- 8.Gardner SL, Asgharzadeh S, Green A, et al. Intensive induction chemotherapy followed by high dose chemotherapy with autologous hematopoietic progenitor cell rescue in young children newly diagnosed with central nervous system atypical teratoid rhabdoid tumors. Pediatr Blood Cancer. 2008;51:235–240. doi: 10.1002/pbc.21578. [DOI] [PubMed] [Google Scholar]
- 9.Zimmerman MA, Goumnerova LC, Proctor M, et al. Continuous remission of newly diagnosed and relapsed central nervous system atypical teratoid/rhabdoid tumor. J Neurooncol. 2005;72:77–84. doi: 10.1007/s11060-004-3115-y. [DOI] [PubMed] [Google Scholar]
- 10.Hilden JM, Meerbaum S, Burger P, et al. Central nervous system atypical teratoid/rhabdoid tumor: Results of therapy in children enrolled in a registry. J Clin Oncol. 2004;22:2877–2884. doi: 10.1200/JCO.2004.07.073. [DOI] [PubMed] [Google Scholar]
- 11.Chen ML, McComb JG, Krieger MD. Atypical teratoid/rhabdoid tumors of the central nervous system: Management and outcomes. Neurosurg Focus. 2005;18:E8. [PubMed] [Google Scholar]
- 12.Hilden JM, Watterson J, Longee DC, et al. Central nervous system atypical teratoid tumor/rhabdoid tumor: Response to intensive therapy and review of the literature. J Neurooncol. 1998;40:265–275. doi: 10.1023/a:1006125120866. [DOI] [PubMed] [Google Scholar]
- 13.Chi SN, Gardner SL, Levy AS, et al. Feasibility and response to induction chemotherapy intensified with high-dose methotrexate for young children with newly diagnosed high-risk disseminated medulloblastoma. J Clin Oncol. 2004;22:4881–4887. doi: 10.1200/JCO.2004.12.126. [DOI] [PubMed] [Google Scholar]
- 14.Versteege I, Sevenet N, Lange J, et al. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature. 1998;394:203–206. doi: 10.1038/28212. [DOI] [PubMed] [Google Scholar]
- 15.Biegel JA, Zhou JY, Rorke LB, et al. Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Res. 1999;59:74–79. [PubMed] [Google Scholar]
- 16.Judkins AR, Mauger J, Ht A, et al. Immunohistochemical analysis of hSNF5/INI1 in pediatric CNS neoplasms. Am J Surg Pathol. 2004;28:644–650. doi: 10.1097/00000478-200405000-00013. [DOI] [PubMed] [Google Scholar]
- 17.Sigauke E, Rakheja D, Maddox DL, et al. Absence of expression of SMARCB1/INI1 in malignant rhabdoid tumors of the central nervous system, kidneys and soft tissue: An immunohistochemical study with implications for diagnosis. Mod Pathol. 2006;19:717–725. doi: 10.1038/modpathol.3800581. [DOI] [PubMed] [Google Scholar]
- 18.Hoot AC, Russo P, Judkins AR, et al. Immunohistochemical analysis of hSNF5/INI1 distinguishes renal and extra-renal malignant rhabdoid tumors from other pediatric soft tissue tumors. Am J Surg Pathol. 2004;28:1485–1491. doi: 10.1097/01.pas.0000141390.14548.34. [DOI] [PubMed] [Google Scholar]
- 19.Janson K, Nedzi LA, David O, et al. Predisposition to atypical teratoid/rhabdoid tumor due to an inherited INI1 mutation. Pediatr Blood Cancer. 2006;47:279–284. doi: 10.1002/pbc.20622. [DOI] [PubMed] [Google Scholar]
- 20.Sevenet N, Lellouch-Tubiana A, Schofield D, et al. Spectrum of hSNF5/INI1 somatic mutations in human cancer and genotype–phenotype correlations. Hum Mol Genet. 1999;8:2359–2368. doi: 10.1093/hmg/8.13.2359. [DOI] [PubMed] [Google Scholar]
- 21.Proust F, Laquerriere A, Constantin B, et al. Simultaneous presentation of atypical teratoid/rhabdoid tumor in siblings. J Neurooncol. 1999;43:63–70. doi: 10.1023/a:1006114732613. [DOI] [PubMed] [Google Scholar]
- 22.Ammerlaan AC, Ararou A, Houben MP, et al. Long-term survival and transmission of INI1-mutation via nonpenetrant males in a family with rhabdoid tumour predisposition syndrome. Br J Cancer. 2008;98:474–479. doi: 10.1038/sj.bjc.6604156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swensen JJ, Keyser J, Coffin CM, et al. Familial occurrence of Schwannomas and malignant rhabdoid tumour associated with a duplication in SMARCB1. J Med Genet. 2009;46:68–72. doi: 10.1136/jmg.2008.060152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jackson EM, Sievert AJ, Gai X, et al. Genomic analysis using high-density single nucleotide polymorphism-based oligonucleotide arrays and multiplex ligation-dependent probe amplification provides a comprehensive analysis of INI1/SMARCB1 in malignant rhabdoid tumors. Clin Cancer Res. 2009;15:1923–1930. doi: 10.1158/1078-0432.CCR-08-2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalpana GV, Marmon S, Wang W, et al. Binding and stimulation of HIV-1 integrase by a human homolog of yeast transcription factor SNF5. Science. 1994;266:2002–2006. doi: 10.1126/science.7801128. [DOI] [PubMed] [Google Scholar]
- 26.Geyer JR, Sposto R, Rorke LB, et al. Multiagent chemotherapy and deferred radiotherapy in infants with malignant brain tumors: A report from the Children’s Cancer Group. J Clin Oncol. 2005;23:7621–7631. doi: 10.1200/JCO.2005.09.095. [DOI] [PubMed] [Google Scholar]
- 27.Jakacki R, Burger P, Zhou T, et al. Outcome for metastatic medulloblastoma treated with carboplatin during craniospinal radiotherapy followed by cyclophosphamide and vincristine: Preliminary results of COG 99701. Journal of Clinical Oncology; ASCO Annual Meeting Proceedings Part I; 2007. Jun 2007, p. 2017. [Google Scholar]
- 28.99703: A pilot study of intensive chemotherapy with peripheral stem cell support for infants with malignant brain tumors. https://members.childrensoncologygroup.org. [Google Scholar]
- 29.Teratoid/rhabdoid tumor if the central nervous system with surgery, intensive chemotherapy and 3D conformal radiation. https://members.childrensoncologygroup.org/.TreatmentofAtypical. [Google Scholar]
- 30.Ronghe MD, Moss TH, Lowis SP. Treatment of CNS malignant rhabdoid tumors. Pediatr Blood Cancer. 2004;42:254–260. doi: 10.1002/pbc.10419. [DOI] [PubMed] [Google Scholar]
- 31.Chen YW, Wong TT, Ho DM, et al. Impact of radiotherapy for pediatric CNS atypical teratoid/rhabdoid tumor (single institute experience) Int J Radiat Oncol Biol Phys. 2006;64:1038–1043. doi: 10.1016/j.ijrobp.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Hablerler C, Laggner U, Slavc I, et al. Immunohistochemical analysis of INI1 protein in malignant pediatric CNS tumors: Lack of INI1 in atypical teratoid/rhabdoid tumors in a fraction of primitive neuroectodermal tumors without rhabdoid phenotype. Am J Surg Pathol. 2006;30:1462–1468. doi: 10.1097/01.pas.0000213329.71745.ef. [DOI] [PubMed] [Google Scholar]
- 33.Makuria AT, Rushing EJ, McGrail KM, et al. Atypical teratoid rhabdoid tumor (AT/RT) in adults: Review of 4 cases. J Neuroooncol. 2008;88:321–330. doi: 10.1007/s11060-008-9571-z. [DOI] [PubMed] [Google Scholar]
- 34.Samaras V, Stamatelli A, Samaras E, et al. Atypical teratoid/rhabdoid tumor of the central nervous system in an 18-year-old patient. Clin Neuropathol. 2009;28:1–10. doi: 10.5414/npp28001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raisanen J, Biegel JA, Hatanpaa KJ, et al. Chromosome 22q deletions in atypical teratoid/rhabdoid tumors in adults. Brain Pathol. 2005;15:23–28. doi: 10.1111/j.1750-3639.2005.tb00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]