Abstract
Although the cardioprotection of late preconditioning (PC) is known to be mediated by both inducible NO synthase (iNOS) and cyclooxygenase-2 (COX-2), the signaling mechanism responsible for COX-2 upregulation and the interaction between iNOS and COX-2 remain unknown. A total of 122 mice were used to address this issue. In wild-type mice preconditioned with six cycles of 4-min coronary occlusion-4-min reperfusion, ischemic PC resulted in rapid activation of nuclear STAT1/3 through tyrosine phosphorylation (STAT1: 339 ± 48% of control; STAT3: 389 ± 46% of control) and increased STAT1/3-DNA binding activity (687 ± 58% of control) at 30 min after PC, with subsequent upregulation of COX-2 protein (373 ± 60% of control) and activity(increased myocardial levels of PGE2, PGF2α, and 6-keto-PGF1α) at 24 h. However, COX-1 protein was not changed 24 h after ischemic PC. Pretreatment with the Janus tyrosine kinase (JAK) inhibitor AG-490 before the six occlusion-reperfusion cycles blocked both the tyrosine phosphorylation of STAT1/3 and the subsequent upregulation of COX-2 protein, demonstrating a necessary role of the JAK-STAT pathway in the induction of COX-2. Targeted disruption of the iNOS gene (iNOS−/−) did not block the increased expression of COX-2 protein 24 h after ischemic PC but completely blocked the increase in COX-2 activity, whereas targeted disruption of the COX-2 gene (COX-2−/−) did not alter ischemic PC-induced iNOS induction. Immunoprecipitation of preconditioned heart tissues with anti-COX-2 antibodies followed by immunoblotting with anti-iNOS antibodies revealed that the increased iNOS protein co-precipitated with COX-2. We conclude that (i) the upregulation of COX-2 protein expression after ischemic PC is mediated by a JAK1/2-STAT1/3-signaling cascade; (ii) COX-2 activity requires upregulated iNOS and iNOS-derived NO; and (iii) COX-2 forms complexes with iNOS, supporting a direct interaction between these two proteins. To our knowledge, this is the first evidence that myocardial COX-2 is upregulated via a JAK1/2-STAT1/3 pathway.
Keywords: Ischemia, Myocardial infarction, iNOS−/−, COX-2, STAT proteins, prostaglandin
1. Introduction
The late phase of ischemic preconditioning (PC) is a delayed protective adaptation whereby brief episodes of ischemia enhance the heart’s resistance to subsequent ischemia-reperfusion injury 24–72 h later [1–17]. The protection afforded by late PC is sustained and broad, involving both alleviation of myocardial stunning and limitation of infarct size [13]. As a result, considerable attention has focused on the pathophysiology and potential clinical implications of this defensive cardiac adaptation. Previous studies have shown that the initial signals that trigger the development of late PC after the ischemic PC challenge (on day 1) involve the rapid generation of NO via activation of eNOS [4,5,14] and the formation of reactive oxygen species (ROS) [3,13], followed by activation of protein kinase C (PKC) [6,9] and Src protein tyrosine kinases (PTKs) [7,9] which in turn activate nuclear factor-κB (NF-κB) [10] resulting in transcriptional activation of target genes and synthesis of cardioprotective proteins. Both cyclooxygenase-2 (COX-2) [11,12,15] and inducible NO synthase (iNOS) [14,16,17] have been found to be upregulated following a sublethal ischemic insult and to mediate the cardioprotective effects of late PC.
Inhibition of COX-2 activity completely abrogates the infarct-sparing actions of late PC [11,12], indicating a crucial role of this enzyme. Although pharmacological studies in rabbits suggest that ischemic PC upregulates COX-2 through a mechanism involving activation of PKC, Src PTKs, and NF-κB [15], the pathways responsible for transcriptional activation of the COX-2 gene in the heart following a sublethal ischemic stress remain poorly understood. The activation of COX-2 gene transcription is known to be a complex process that requires the coordinated interaction of multiple transcription regulatory elements [18–20]. Since the promoter of the mouse COX-2 gene contains the interferon-γ activation site (GAS) consensus sequence (the site for the binding of signal transducers and activators of transcription (STATs)) [16,21–23] and since our previous studies have shown that ischemic PC activates Janus tyrosine kinases (JAKs) and STATs, which are necessary for the development of the cardioprotective effects of late PC [16], we tested the hypothesis that ischemic PC upregulates COX-2 protein expression by activating the JAK-STAT-signaling cascade.
Besides examining the role of the JAK-STAT system in COX-2 expression, we sought to explore the relationship between iNOS and COX-2 in late PC. Pharmacological and genetic evidence has demonstrated that iNOS plays a necessary role in mediating the cardioprotective effects of late PC [4,14,17]. For example, disruption of the iNOS gene abrogates late PC [17]. In principle, iNOS and COX-2 could act in parallel (i.e. independently) or in series (i.e. in a hierarchical order). In noncardiac tissues, NO has been found to enhance COX-2 activity [24–28], to decrease it [29–33], or to have no effect [34–36]. Using a pharmacologic approach, we have recently found in conscious rabbits that the increase in myocardial levels of PGE2 and 6-keto-PGF1α 24 h after the initial ischemic stress is blocked by the administration of the iNOS inhibitors S -methylisothiourea (SMT) and 1400W [15], suggesting that COX-2 activity is driven by iNOS-derived NO. However, since both SMT and 1400W have nonspecific actions unrelated to NOS inhibition [37–39], a molecular genetic approach would be required to provide conclusive evidence that COX-2 activity in preconditioned myocardium is iNOS dependent.
The two major objectives of the present study were (i) to determine whether recruitment of STATs is an important mechanism responsible for the upregulation of COX-2 that underlies late PC and (ii) to elucidate the interaction between COX-2 and iNOS in preconditioned myocardium. The following specific questions were addressed: (i) does inhibition of the JAK-STAT pathway interfere with the upregulation of COX-2 protein expression and activity after ischemic PC? (ii) does targeted disruption of the iNOS gene alter the levels of COX-2 protein expression or activity 24 h after ischemic PC? (iii) conversely, does targeted disruption of the COX-2 gene alter the levels of iNOS protein expression or activity 24 h after ischemic PC?, and (iv) is there a physical interaction between iNOS and COX-2? To address these issues, mice with targeted disruption of the iNOS gene (iNOS−/−) and the COX-2 gene (COX-2−/−) were used in an established model of late PC in which fundamental physiological variables that impact on infarct size and cardiac response to stress (body temperature, arterial oxygenation, acid-base balance, heart rate, and arterial blood pressure) are carefully controlled and kept within normal limits [17,40].
2. Methods
2.1. Wild-type and gene knockout mice
iNOS−/− mice were purchased from the Jackson Laboratory (Bar Harbor, ME) and acclimated to our facility for >2 weeks before experiments. The iNOS−/− mice were of hybrid genetic background (B6129). Wild-type mice (purchased from the Jackson Laboratory) with a genetic background as close as possible to the iNOS−/− mice (B6129PF2/J) [17] were used as controls. COX-2−/− mice and wild-type controls were on a mixed background of 129 Ola/C57Bl/6 [41]. All mice were maintained in sterile microisolator cages under pathogen-free conditions in a low-stress environment (low-noise and a 12-h light-dark cycle). Food and water were autoclaved, and all handling was done under a laminar flow hood following standard procedures for maintaining pathogen-free transgenic mice. The iNOS−/− and COX-2−/− mice were genotyped by PCR and Southern blot hybridization as previously described [17] using DNA prepared from tissue samples taken at the end of the experiments.
2.2. Coronary occlusion-reperfusion protocol
The murine model of myocardial ischemia and reperfusion has been previously described in detail [17,40]. Briefly, mice were anesthetized with sodium pentobarbital (50 mg/kg i.p.) and ventilated using carefully selected parameters [40]. After administration of antibiotics, the chest was opened through a midline sternotomy, and a nontraumatic balloon occluder was implanted around the mid-left anterior descending coronary artery by using an 8-0 nylon suture. To rule out the possibility that the effects of late PC on the phosphorylation and the DNA-binding activity of STAT1/3 and the expression of COX-2 and iNOS were caused by the surgical procedure, including suture placement, control groups underwent a 1-h open-chest state (sham control) and sutures were placed just as in the PC groups. To prevent hypotension, blood from a donor mouse was given at serial times during surgery [40]. Rectal temperature was carefully monitored and maintained between 36.7 and 37.3°C throughout the experiment.
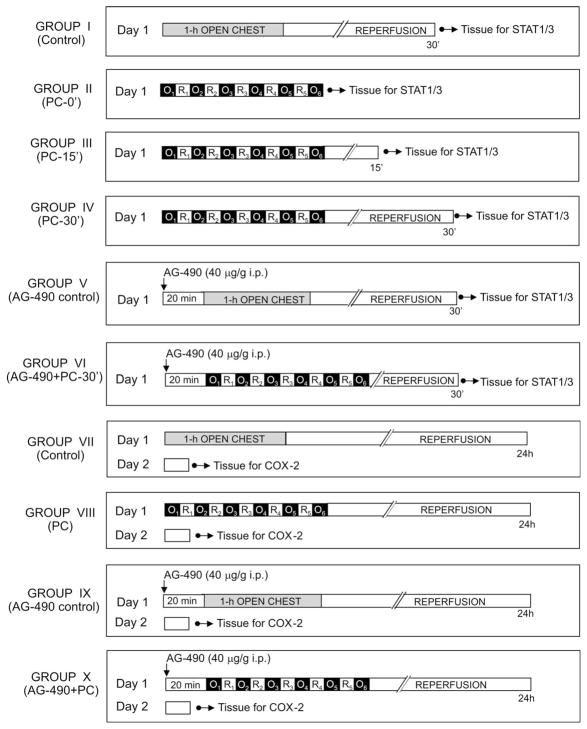
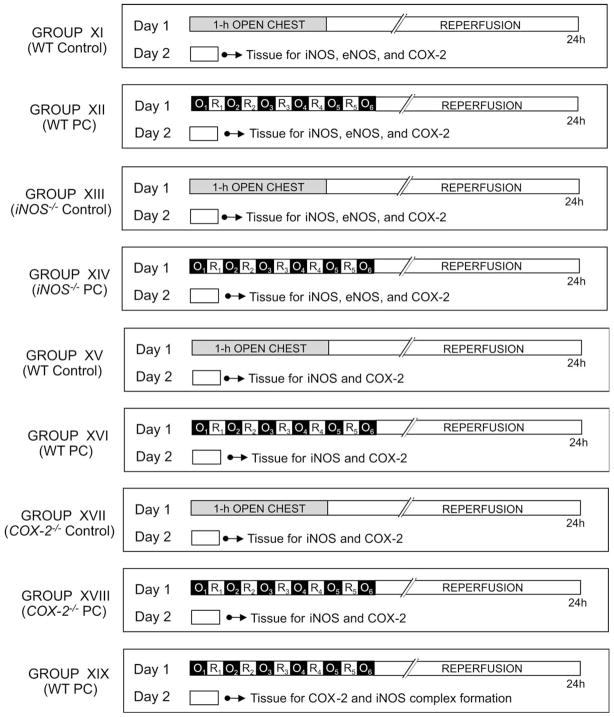
This investigation consisted of two successive phases (I and II) (Figs. 1 and 2). The objective of phase I was to determine whether activation of STAT1 and STAT3 is necessary for upregulation of COX-2. The objective of phase II was to investigate whether iNOS plays a critical role in the regulation of COX-2 protein expression and/or activity.
Fig. 1.
Diagram of the experimental protocol for phase I. O: indicates 4-min coronary occlusion and R: 4-min reperfusion.
Fig. 2.
Diagram of the experimental protocol for phase II. O: indicates 4-min coronary occlusion and R: 4-min reperfusion.
2.2.1. Phase I: effect of ischemic PC on STAT1/3 and on COX-2
Mice were assigned to 10 groups (Fig. 1). Mice in group I (control) underwent 1 h of open-chest state without coronary occlusion. Mice in groups II (PC-0′), III (PC-15′), IV (PC-30′), and VI (AG-490 + PC-30′) underwent a sequence of six cycles of 4-min coronary occlusion-4-min reperfusion (a protocol that induces late PC) [17,40] and were euthanized at 0, 15, or 30 min after the last occlusion. Mice in group V (AG-490 control) received the JAK inhibitor AG-490 (Cal-Biochem; 40 μg/g i.p. dissolved in 45% DMSO) 20 min before 1 h of open-chest state, whereas group VI (AG-490 + PC-30′) received AG-490 20 min before the first occlusion. This dose of AG-490 inhibits the development of late PC and the concomitant upregulation of iNOS in this murine model [16]. Mice in group VII (PC control) underwent 1 h of open-chest state without coronary occlusion, whereas mice in group VIII (PC-24 h) underwent a sequence of six cycles of 4-min coronary occlusion-4-min reperfusion. Mice in group IX (AG-490 control) received AG-490 20 min before 1 h of open-chest state, whereas group X (AG-490 + PC-24 h) received AG-490 20 min before the first occlusion. Mice in groups VII-X were euthanized 24 h after the last occlusion. Myocardial samples were rapidly removed from the ischemic-reperfused region (whose boundaries had been marked with 10-0 nylon sutures at the time of coronary occlusion) and frozen in liquid nitrogen until used.
2.2.2. Phase II: Effect of targeted disruption of the iNOS and COX-2 genes
Mice were assigned to nine groups (Fig. 2). Mice in groups XI (wild-type control), XIII (iNOS−/− control), XV (wild-type control), and XVII (COX-2−/− control) underwent 1 h of open-chest state without coronary occlusion. Mice in groups XII (wild-type PC-24 h), XIV (iNOS−/− PC-24 h), XVI (wild-type PC-24 h), XVIII (COX-2−/− PC-24 h), and XIX (wild-type PC-24 h for COX-2-iNOS complex formation) underwent a sequence of six cycles of 4-min coronary occlusion-4-min reperfusion. All mice in groups XI-XIX were euthanized 24 h after the last occlusion. Myocardial samples were removed from the ischemic-reperfused region and frozen in liquid nitrogen until used.
2.3. Preparation of cytosolic, membranous, and nuclear fractions
Cytosolic, membranous, and nuclear fractions were prepared from heart samples as previously described in detail [10,16,17].
2.4. Western immunoblotting
Western immunoblotting analysis was performed using standard SDS-PAGE western immunoblotting techniques [10,16]. Tyrosine-phosphorylated (activated) forms of STAT1 and STAT3 were determined using specific anti-pTyr(701)-STAT1 (Upstate Biotechnology) and anti-pTyr(705)-STAT3 antibodies (Cell Signaling Technology), respectively, as previously described [16]. Specific polyclonal anti-COX-2 antibodies (Cayman Chemical), monoclonal anti-COX-1 antibodies (Santa Cruz), polyclonal anti-iNOS antibodies (Upstate Biotechnology), and monoclonal anti-eNOS antibodies (Transduction Laboratories) were used to detect COX-2, COX-1, iNOS, and eNOS. Despite a careful attempt to achieve equal protein loading in all lanes of the gel, the total amounts of proteins transferred from each lane to the nitrocellulose membranes during blotting are rarely identical. Therefore, given the critical importance of quantitating signals as accurately as possible, each specific signal pertaining to the tyrosine phophorylation of STAT1 and STAT3 and to the expression of COX-2 and iNOS was normalized to the corresponding Ponceau stain signal determined by densitometric analysis of the Ponceau stain record, as previously described [10,16,17].
2.5. Co-immunoprecipitation
Myocardial homogenates (600 μg) were incubated with anti-COX-2 or anti-COX-1 antibodies for 4 h followed by incubation with Protein-A-Agarose (Santa Cruz) or Protein G PLUS-Agarose-S (Santa Cruz), respectively, overnight at 4°C. After washing, the anti-COX-2 precipitates were subjected to immunoblotting with anti-COX-2 or anti-iNOS antibodies, whereas the anti-COX-1 precipitates were subjected to immunoblotting with anti-COX-1 or anti-iNOS antibodies.
2.6. Electrophoretic mobility-shift assays
DNA-binding activity of STAT1/3 was measured using electrophoretic mobility shift assays (EMSAs) as previously described [16]. A synthetic double-stranded probe with the sequence 5′-GATCAGCTTCAATTTCCCGTAAATCCCTA-3′ (Gibco) was end-labeled using [γ-32P]ATP (3000 Ci/mmol, Amersham) and T4 polynucleotide kinase, and purified with a G-25 Sephadex column (Pharmacia) [10]. This oligonucleotide has the consensus sequence for GAS elements, as indicated by underlines [16,42,43].
2.7. Measurement of myocardial PGs
PGs were extracted from cardiac samples using ODS-silica reverse-phase columns [11]. Using [3H]-PGE2 as an internal standard, the percent recovery of this extraction was 90 ± 4% (n = 6). The myocardial content of PGE2, PGF2α, and 6-keto-PGF1α were determined by using EIA kits (PGE2 and PGF2α EIA kits from Cayman Chemical; 6-keto-PGF1α kit from Amersham Life Science) and expressed as pg/mg protein [11].
2.8. Statistical analysis
Data are reported as mean ± S.E.M. Measurements were analyzed by ANOVA followed by unpaired Student’s t-tests with the Bonferroni correction. In all western analyses, the content of the specific protein of interest was expressed as a percentage of the corresponding protein in the anterior left ventricular wall of control mice.
3. Results
A total of 122 mice were used in this study.
3.1. Phase I: effect of ischemic PC on STAT1/3 and on COX-2
3.1.1. Effect of ischemic PC on tyrosine phosphorylation and DNA binding activity of STAT1/3
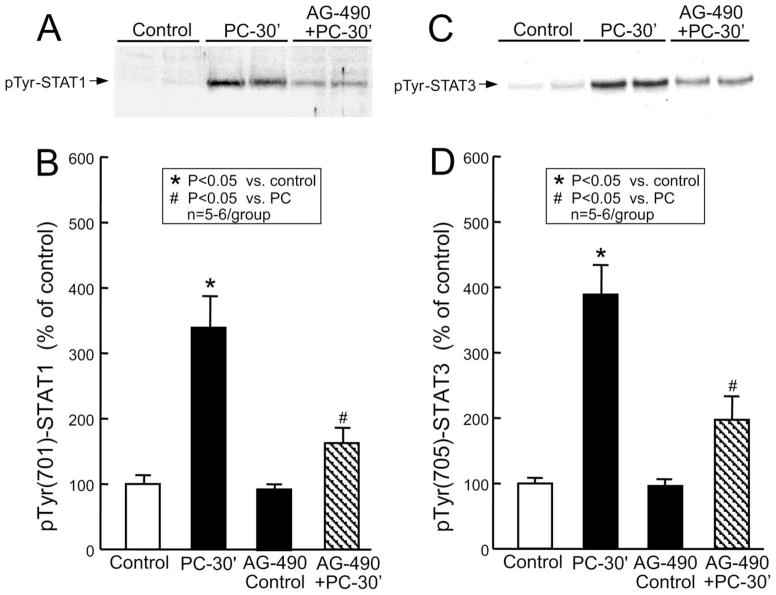
The sequence of six 4-min coronary occlusion-4-min reperfusion cycles resulted in a marked increase in pTyr(701)-STAT1 (339 ± 48% of control, n = 6, P < 0.05 (Fig. 3A,B)) and pTyr(705)-STAT3 (389 ± 46% of control, n = 6, P < 0.05 (Fig. 3C,D)) in nuclear extracts 30 min after the sixth reperfusion, indicating that ischemic PC induced activation of these two transcription factors. Pretreatment with the JAK inhibitor AG-490 decreased the ischemic PC-induced increase in tyrosine phosphorylation of STAT1 by 73% (n = 5, P < 0.05 vs. PC; Fig. 3A,B) and STAT3 by 66% (n = 5, P < 0.05 vs. PC; Fig. 3C,D). In the absence of PC, AG-490 had no effect on tyrosine-phosphorylated STAT1 (93 ± 7% of control, n = 5 (Fig. 3B)) and STAT3 (97 ± 7% of control, n = 5 (Fig. 3D)). These results are consistent with our previous observations [16].
Fig. 3.
Effect of ischemic PC on tyrosine phosphorylation of nuclear STAT1 and STAT3. Nuclear extracts of myocardial samples were prepared from mice that underwent a sham operation (1 h of open-chest state without coronary occlusion-reperfusion) without treatment (group I, control) or after receiving AG-490 (group V, AG-490 control) or from the ischemic-reperfused region of preconditioned mice that received either no treatment (group IV, PC-30′) or AG-490 20 min before ischemic PC (group VI, AG-490 + PC-30′). All mice were euthanized 30 min after the sham operation or 30 min after ischemic PC (i.e. after the sixth occlusion), and nuclear extracts of myocardial samples were prepared as described in Section 2. Tyrosine-phosphorylated forms of STAT1 and STAT3 in the nuclear extracts were analyzed by immunoblotting using specific anti-pTyr(701)-STAT1 or anti-pTyr(705)-STAT3 antibodies. (A,C) The immunoreactivity of tyrosine-phosphorylated STAT1 and STAT3 in the nuclear fraction increased markedly 30 min after ischemic PC, and this increase was inhibited by AG-490. (B,D) Densitometric analysis of tyrosine-phosphorylated STAT1 and STAT3. Data are mean ± S.E.M.
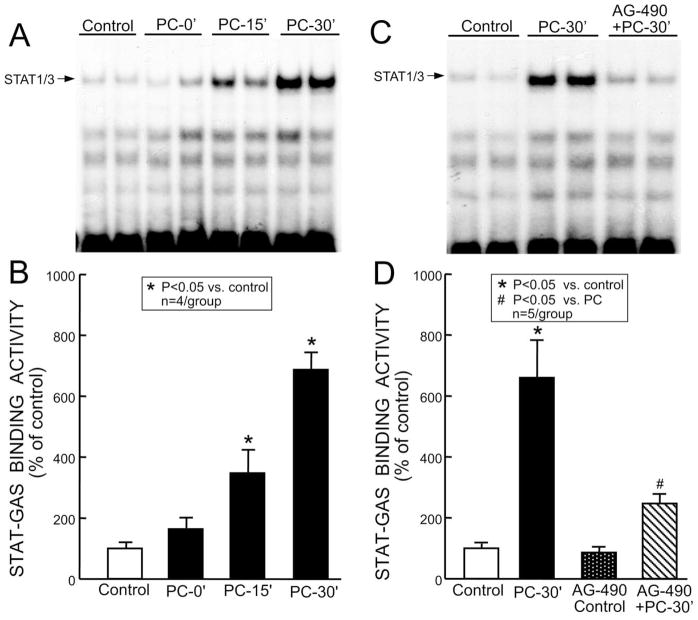
Next, we determined whether the tyrosine-phosphorylated STAT1 and STAT3 bind to GAS motifs. The nuclear samples were analyzed by EMSA using a 32P-labeled GAS oligonucleotide probe. As illustrated in Fig. 4A,B, the six coronary occlusion-reperfusion cycles induced a time-dependent increase in the STAT-DNA complex. The STAT-DNA binding activity detected in the nuclear extracts increased 247 ± 78% above control (n = 4, P < 0.05) at 15 min and 587 ± 58% above control (n = 4, P < 0.05) at 30 min after the last occlusion. Thus, the increased tyrosine phosphorylation of nuclear STAT1 and STAT3 (Fig. 3) was associated with a corresponding increase in the STAT-DNA binding activity (Fig. 4A,B). Administration of AG-490 20 min before the six occlusion-reperfusion cycles reduced the is-chemic PC-induced increase in the STAT-DNA binding by >73% at 30 min (n = 5, P < 0.05 vs. PC; Fig. 4C,D). In the absence of PC, AG-490 did not change the STAT-DNA binding activity (88 ± 12% of control, n = 5 (Fig. 4D)).
Fig. 4.
Effect of ischemic PC on STAT1/3-DNA binding activity. Nuclear extracts were prepared from mice that underwent a sham operation without treatment (group I, control) or after receiving AG-490 (group V, AG-490 control), or from the ischemic-reperfused region of untreated preconditioned mice that were euthanized 0 min (group II, PC-0′), 15 min (group III, PC-15′), or 30 min (group IV, PC-30′) after the sixth occlusion, or from the ischemic-reperfused region of preconditioned mice that received AG-490 20 min before ischemic PC and were euthanized 30 min after the sixth occlusion (group VI, AG-490 + PC-30′). The nuclear extracts were subjected to EMSA for analysis of STAT1/3-DNA binding activity using the 32P-labeled GAS probe. (A) Representative EMSA showing that the shifted band corresponding to the STAT-GAS complex (indicated by the arrow) increased markedly 15 and 30 min after ischemic PC in the nuclear extracts. (C) The increased activity of STAT1/3-DNA binding was inhibited by AG-490. (B,D) Densitometric analysis of the STAT1/3-GAS complexes. Data are mean ± S.E.M.
3.1.2. Effect of ischemic PC on COX-1 and COX-2 protein expression and activity
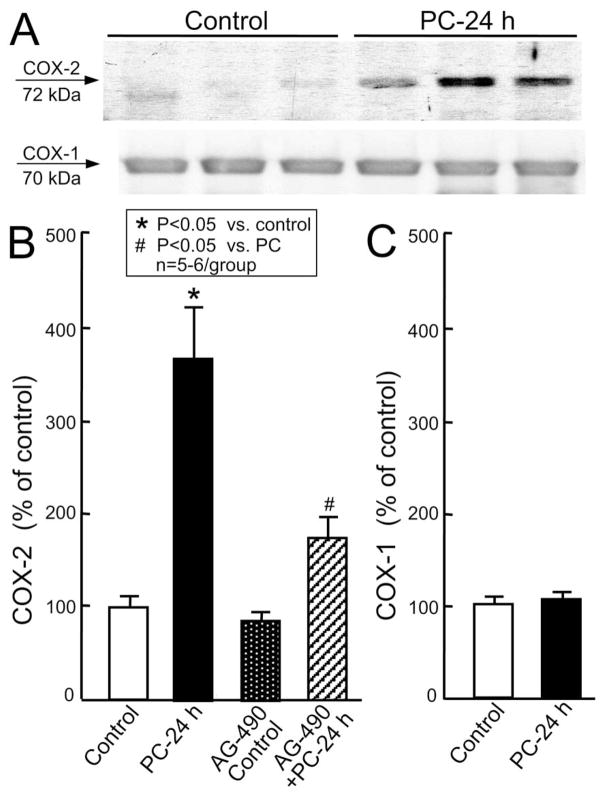
To determine whether activation of the JAK-STAT axis is involved in the regulation of COX-2 expression, we measured COX-2 and COX-1 protein expression by western immunoblotting. As illustrated in Fig. 5A,B, the six coronary occlusion-reperfusion cycles upregulated COX-2 (373 ± 60% of control, n = 6, P < 0.05) 24 h after the last occlusion but had no effect on COX-1. Pretreatment of mice with AG-490 before the six coronary occlusion-reperfusion cycles inhibited the ischemic PC-induced upregulation of COX-2 by > 71% (n = 6, P < 0.05 vs. PC) 24 h after ischemic PC (Fig. 5B). In the absence of PC, AG-490 did not change COX-2 (86 ± 9% of control, n = 5 (Fig. 5B)). The dose of AG-490 used in this study (40 μg/g i.p.) is the same that has previously been shown to block the cardioprotective effects of late PC and the concomitant upregulation of iNOS in this murine model [16].
Fig. 5.
Effects of ischemic PC on myocardial COX-2 and COX-1 expression. Samples were obtained from wild-type mice that underwent a sham operation without treatment (group VII, control) or after receiving AG-490 (group IX, AG-490 control) or from the ischemic-reperfused region of preconditioned mice that received no treatment (group VIII, PC–24 h) or AG-490 (group X, AG-490 + PC – 24 h) 20 min before the first occlusion. All mice were euthanized 24 h after the sixth reperfusion. (A) Representative immunoblots of COX-2 and COX-1 expression. (B,C) Densitometric analysis of immunoreactive COX-2 and COX-1 signals, respectively. COX-2 was significantly increased 24 h after ischemic PC, and this increase was prevented by AG-490. Data are mean ± S.E.M.
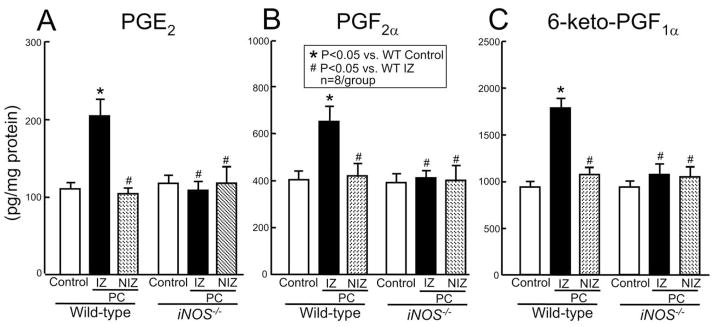
In accordance with the induction of COX-2 protein, the six coronaryocclusion-reperfusion cycles also resulted in increased myocardial levels of PGE2 (185 ± 20% of wild-type control; Fig. 6A), PGF2α (161 ± 16% of wild-type control; Fig. 6B), and 6-keto-PGF1α (190 ± 12% of wild-type control; Fig. 6C) 24 h later in wild-type mice. This increase in prostanoids was observed only in the ischemic-reperfused region; prostanoid levels in the nonischemic region did not change (Fig. 6), indicating that constitutive COX-1 was not activated. This observation, coupled with the fact that COX-1 was not upregulated in the ischemic-reperfused region (Fig. 5C), supports the conclusion that the increased levels of PGE2, PGF2α, and 6-keto-PGF1α reflected increased COX-2 activity.
Fig. 6.
Effect of targeted disruption of the iNOS gene on myocardial levels of PGE2, PGF2α, and 6-keto-PGF1α 24 h after ischemic PC. Myocardial samples were obtained from wild-type and iNOS−/− mice that underwent a sham operation (control) or from the nonischemic (NIZ) and the ischemic-reperfused (IZ) zones of wild-type and iNOS−/− mice that were preconditioned with six coronary occlusion-reperfusion cycles. Myocardial levels of PGE2, PGF2α, and 6-keto-PGF1α were analyzed with EIA kits as described in Section 2. Data are mean ± S.E.M.
3.2. Phase II: effect of targeted disruption of the iNOS and COX-2 genes
3.2.1. Effect of targeted disruption of the iNOS gene on COX-2 protein expression and activity
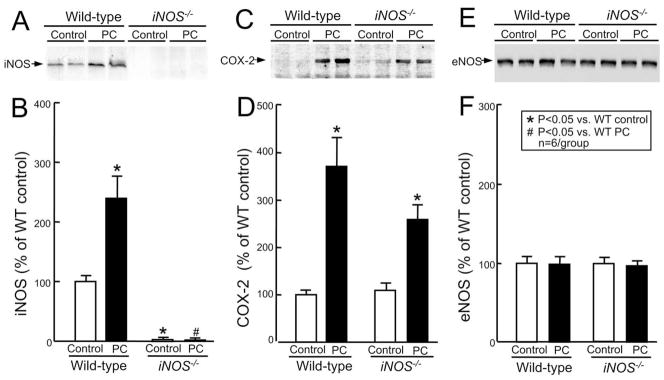
To investigate whether iNOS plays a role in the upregulation of COX-2, we determined the effect of targeted disruption of the iNOS gene (iNOS−/−) on COX-2 protein expression and activity. As shown in Fig. 7A, the six occlusion-reperfusion cycles resulted in increased expression of iNOS protein 24 h later in wild-type mice, confirming our previous findings [17]. In iNOS−/− mice, there was no detectable iNOS signal in either control or preconditioned myocardium (Fig. 7A,B). As observed previously [17], targeted disruption of the iNOS gene did not alter eNOS protein expression (Fig. 7E,F).
Fig. 7.
Effect of targeted disruption of the iNOSgene on ischemic PC-induced expression of COX-2 and eNOS protein. Samples were obtained from wild-type (group XI, wild-type control) and iNOS−/− (group XIII, iNOS−/− control) mice that underwent a sham operation or from the ischemic-reperfused region of both wild-type (group XII, wild-type PC) and iNOS−/− (group XIV, iNOS−/− PC) mice that were preconditioned with six coronary occlusion-reperfusion cycles 24 h earlier. (A) Western blot confirming that the immunoreactivity of iNOS protein was upregulated 24 h after ischemic PC in the wild-type mice and was completely ablated in control and preconditioned iNOS−/− mice. (C) Immunoblotting indicating that COX-2 expression was increased 24 h after ischemic PC in both wild-type and iNOS−/− mice. (E) Immunoblotting showing that eNOS expression was not affected in control and preconditioned mice with targeted disruption of the iNOS gene (iNOS−/−). (B,D,F) Densitometric analysis of immunoreactive iNOS, COX-2, and eNOS signals. Data are mean ± S.E.M.
In wild-type mice, the six coronary occlusion-reperfusion cycles upregulated COX-2 protein 24 h after the last occlusion (373 ± 60% of wild-type control, P < 0.05) (Fig. 7C,D). In iNOS−/− mice, the same six occlusion-reperfusion cycles still upregulated COX-2 protein 24 h later (260 ± 32% of wild-type control, P < 0.05) (Fig. 7C,D). There was no significant difference between these two groups (P = 0.128) indicating that expression of COX-2 following ischemic PC does not require iNOS. However, targeted disruption of the iNOS gene completely blocked the increase in myocardial levels of PGE2, PGF2α, and 6-keto-PGF1α (Fig. 6A–C) 24 h following ischemic PC, demonstrating that the increase in COX-2 activity in preconditioned myocardium requires up-regulation of iNOS. Prostanoid levels in the nonischemic myocardium were not different in wild-type and iNOS−/− mice (Fig. 6) indicating that constitutive COX-1 activity does not require iNOS.
3.2.2. Effect of targeted disruption of the COX-2 gene on iNOS protein expression and activity
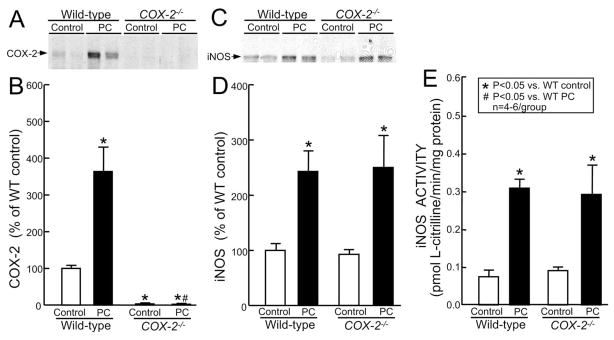
To investigate the role of COX-2 in iNOS upregulation, the effect of ischemic PC on iNOS expression and activity was studied in mice with disruption of the COX-2 gene (COX-2−/−). In these mice, there was no detectable COX-2 expression in either control or preconditioned myocardium, confirming that COX-2 was completely absent (Fig. 8A,B). iNOS expression did not differ between preconditioned wild-type mice (239 ± 37% of wild-type control) and preconditioned COX-2−/− mice (COX-2−/− PC-24 h: 249 ± 59% of wild-type control), demonstrating that transcriptional regulation of iNOS following ischemic PC does not require COX-2 (Fig. 8C,D). Consistent with iNOS protein expression, there was no difference of iNOS activity between preconditioned wild-type and preconditioned COX-2−/− mice, either in the cytosolic fraction (0.311 ± 0.022 vs. 0.282 ± 0.077 pmol L-citrilline/min/mg protein, respectively) (Fig. 8E) or in the membranous fraction (0.050 ± 0.002 vs. 0.061 ± 0.006 pmol L-citrilline/min/mg protein, respectively).
Fig. 8.
Effect of targeted disruption of the COX-2 gene on iNOS protein expression and activity. Samples were obtained from wild-type (group XI, wild-type control) and COX-2−/− (group XVII, COX-2−/− control) mice that underwent a sham operation or from the ischemic-reperfused region of wild-type (group XVI, wild-type PC) and COX-2−/− (group XVIII, COX-2−/− PC) mice that were preconditioned with six coronary occlusion-reperfusion cycles. All mice were euthanized 24 h after the sixth reperfusion. (A) Western blot confirming that the immunoreactivity of COX-2 was completely ablated in COX-2−/− mice. (C) Representative immunoblots showing that iNOS expression was upregulated 24 h after ischemic PC in wild-type mice, and that this increase was not affected by deletion of the COX-2 gene. (B,D) Densitometric analysis of immunoreactive COX-2 and iNOS signals. (E) iNOS activity in the cytosolic fraction was increased in both preconditioned wild-type and preconditioned COX-2−/− mice. Data are mean ± S.E.M.
3.2.3. Physical association of iNOS with COX-2 protein
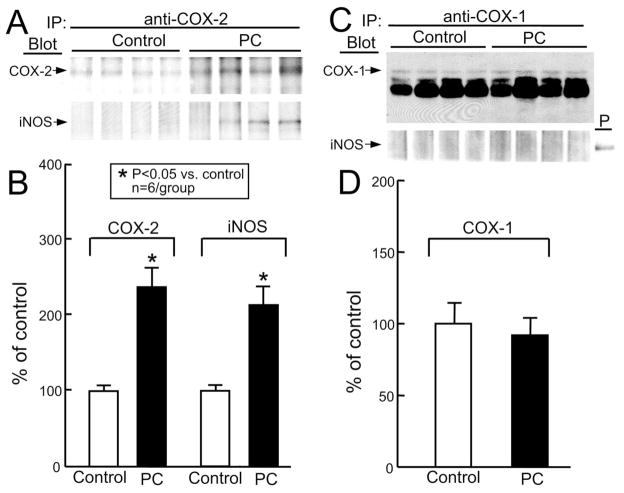
Since COX-2 activity requires upregulation of iNOS (Figs. 6 and 7) and since both COX-2 and iNOS are upregulated in preconditioned myocardium (Figs. 5 and 7), we examined whether COX-2 forms complexes with iNOS. Myocardial homogenates from control and preconditioned mice were immunoprecipitated with anti-COX-2 antibodies and then immunoblotted for COX-2 or iNOS protein. Fig. 9A,B shows that the upregulated COX-2 protein co-precipitated with the upregulated iNOS protein, demonstrating that COX-2 is physically associated with iNOS in preconditioned myocardium. In contrast, when myocardial homogenates from control and preconditioned mice were immunoprecipitated with anti-COX-1 antibodies and then immunoblotted for COX-1 or iNOS, iNOS did not co-precipitate with COX-1 (Fig. 9C,D). These data show that iNOS forms complexes with COX-2, supporting a direct interaction between these two cardioprotective proteins.
Fig. 9.
Immunoprecipitation of iNOS with COX-2 in preconditioned myocardium. Samples were taken from wild-type mice that underwent a sham operation (group XV, control) or from the ischemic-reperfused region of the mice that were preconditioned with six coronary occlusion-reperfusion cycles (group XIX, wild-type PC). All mice were euthanized 24 h after the sixth reperfusion and the homogenates were immunoprecipitated either with anti-COX-2 antibodies followed by immunoblotting with anti-COX-2 and anti-iNOS antibodies (A) or with anti-COX-1 antibodies followed by immunoblotting with anti-COX-1 and anti-iNOS antibodies (C). (A,C) Immunoblotting showing that increased iNOS co-immunoprecipitated with COX-2 (A) but not with COX-1 (C). Positive (P) iNOS control: LPS-treated macrophages (C). (B,D) Densitometric analysis of COX-2, iNOS, and COX-1 signals. Data are mean ± S.E.M.
4. Discussion
The salient findings of this study can be summarized as follows: (i) the upregulation of COX-2 protein expression 24 h after ischemic PC is mediated by the JAK-STAT-signaling pathway; (ii) the synthesis of COX-2 protein is independent of iNOS but the activity of COX-2 requires the presence of upregulated iNOS; (iii) in contrast, neither iNOS protein expression nor iNOS activity is dependent on COX-2 upregulation; and (iv) the upregulated iNOS and COX-2 are physically associated. To our knowledge, this is the first demonstration that the JAK-STAT signaling pathway plays an essential role in the upregulation of COX-2 protein in the heart. This is also the first report that COX-2 forms complexes with iNOS in preconditioned myocardium, supporting a direct interaction between these two inducible cardioprotective proteins in the late phase of ischemic PC and in the response of the heart to stress in general.
Although activation of the JAK-STAT pathway after is-chemic PC has been demonstrated to be necessary for iNOS induction and for late PC [16], no information is presently available regarding the role of this axis in regulating COX-2 protein expression in the heart. We found that, in the mouse, ischemic PC results in a robust increase in COX-2 protein expression (almost 300% above control (Fig. 5)) 24 h later, concomitant with increased COX-2 activity as evidenced by the increased myocardial content of three COX-2-derived prostanoids (PGE2, PGF2α, and 6-keto-PGF1α) (Fig. 6). These are the first data to document upregulation of COX-2 following ischemic PC in the mouse, and are consistent with our previous observations in rabbits [11], except for the fact that the increase in PGE2and 6-keto-PGF1α was less pronounced than that observed in the rabbit model. Pretreatment with the JAK inhibitor AG-490 blunted not only the tyrosine phosphorylation and DNA binding activity of STAT1 and STAT3 (Figs. 3 and 4), indicating inhibition of the JAK-STAT pathway, but also the increase in COX-2 protein expression (Fig. 5), indicating that STAT1 and/or STAT3 play an essential role in the transcriptional activation of the COX-2 gene. As the JAK-STAT pathway also plays an essential role in the induction of iNOS during late PC [16], the present results imply that the recruitment of the JAK-STAT axis by the initial ischemic stress controls the expression of both iNOS and COX-2, a concept which is consistent with the fact that the GAS-binding sequence is present in the promoters of both the mouse iNOS [43] and the mouse COX-2 genes [21]. Thus, our data support a pivotal function of JAK-STAT signaling in the shift of the heart to a preconditioned phenotype.
The interaction between iNOS and COX-2 has been the focus of much investigation, and the results have varied in different tissues, cell lines, and pathophysiological conditions [24–36]. In a conscious rabbit model of ischemic PC, the increase in myocardial PGE2 and 6-keto-PGF1α levels was blocked by the iNOS inhibitors SMT and 1400W [15], supporting a requirement for iNOS in driving cardiac COX-2 activity. The reason for testing this concept in the present study was twofold: (1) to determine whether this concept is applicable to another species (mouse vs. rabbit) and (2) to verify this concept using a genetic approach as apposed to a pharmacologic approach. Our finding that disruption of the iNOS gene had no effect on the induction of COX-2 protein (Fig. 7) but completely abrogated the increase in myocardial PGE2, PGF2α, and 6-keto-PGF1α (Fig. 6) provides conclusive evidence that the activity of COX-2, but not its protein expression, requires upregulation of iNOS. The reason(s) why COX-2 but not COX-1 is dependent on iNOS-derived NO is unclear. We postulate that a physical association between these two proteins may be important. There is increasing evidence that the function of NOS isoforms is related to their specific subcellular location, and that deletion of one isoform cannot be compensated for by other isoforms expressed in the same cell [44]. If this is the case for iNOS-dependent activation of COX-2, then one might expect a direct interaction between these two proteins. To test this concept, we immunoprecipitated preconditioned heart tissues with anti-COX-2 antibodies followed by immunoblotting for iNOS. As illustrated in Fig. 9, we found that COX-2 co-precipitated with iNOS, whereas COX-1 did not, supporting the hypothesis that a direct interaction between these two proteins may be important to drive COX-2 activity.
We also tested whether COX-2 affects iNOS upregulation. Our previous studies in conscious rabbits showed that the COX-2-selective inhibitors NS-398 and celecoxib had no effect on iNOS activity or myocardial NOx levels [15]. In the present study, we examined this concept using COX-2−/− mice. We found that the deletion of COX-2 had no effect on the induction of iNOS protein (Fig. 8C,D) or iNOS activity (Fig. 8E), corroborating our previous findings in rabbits. Taken together, the results of this investigation indicate that iNOS upregulation occurs independently of COX-2 but COX-2 upregulation is dependent on iNOS, providing further support to our previous hypothesis [15] that COX-2 is located downstream of iNOS in the protective pathway of late PC.
The precise cell type(s) that express(es) COX-2 in the heart in response to ischemic PC remain(s) unclear. Virtually all the mammalian cells are capable of expressing COX-2 [45]. In vitro exposure to hypoxia, oxidative stress, or cytokines has been shown to induce COX-2 in endothelial cells and cardiac myocytes [46–48]. Further studies using in situ hybridization and/or immunohistochemistry will be needed to determine the cellular specificity of COX-2 protein expression in the preconditioned heart in vivo.
Acknowledgments
This study was supported by National AHA grant 0150074N (Y.-T.X.), by NIH grants R01 HL-65660 (Y.-T.X.), HL-43151, HL-55757, HL-68088, and HL-70897 (R.B.), by the Jewish Hospital Research Foundation, Louis-ville, KY, and by the Commonwealth of Kentucky Research Challenge Trust Fund.
Footnotes
Guest Editor, Rakesh Kukreja, handled the review process for this article.
References
- 1.Kuzuya T, Hoshida S, Yamashita N, Fuji H, Oe H, Hori M, et al. Delayed effects of sublethal ischemia on the acquisition of tolerance to ischemia. Circ Res. 1993;72:1293–9. doi: 10.1161/01.res.72.6.1293. [DOI] [PubMed] [Google Scholar]
- 2.Marber MS, Latchman DS, Walker JM, Yellon DM. Cardiac stress protein elevation 24 hours after brief ischemia or heat stress is associated with resistance to myocardial infarction. Circulation. 1993;88:1264–72. doi: 10.1161/01.cir.88.3.1264. [DOI] [PubMed] [Google Scholar]
- 3.Sun JZ, Tang XL, Park SW, Qiu Y, Turrens JF, Bolli R. Evidence for an essential role of reactive oxygen species in the genesis of late preconditioning against myocardial stunning in conscious pigs. J Clin Invest. 1996;97:562–76. doi: 10.1172/JCI118449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolli R, Manchikalapudi S, Tang XL, Takano H, Qiu Y, Guo Y, et al. The protective effect of late preconditioning against myocardial stunning in conscious rabbits is mediated by nitric oxide synthase: evidence that nitric oxide acts both as a trigger and as a mediator of the late phase of ischemic preconditioning. Circ Res. 1997;81:1094–107. doi: 10.1161/01.res.81.6.1094. [DOI] [PubMed] [Google Scholar]
- 5.Bolli R, Bhatti ZA, Tang XL, Qiu Y, Zhang Q, Guo Y, et al. Evidence that late preconditioning against myocardial stunning in conscious rabbits is triggered by the generation of nitric oxide. Circ Res. 1997;81:42–52. doi: 10.1161/01.res.81.1.42. [DOI] [PubMed] [Google Scholar]
- 6.Qiu Y, Ping P, Tang XL, Manchikalapudi S, Rizvi A, Zhang J, et al. Direct evidence that protein kinase C plays an essential role in the development of late preconditioning against myocardial stunning in conscious rabbits and that ε is the isoform involved. J Clin Invest. 1998;101:2182–98. doi: 10.1172/JCI1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dawn B, Xuan YT, Qiu Y, Takano H, Tang XL, Ping P, et al. Bifunctional role of protein tyrosine kinases in late preconditioning against myocardial stunning in conscious rabbits. Circ Res. 1999;85:1154–63. doi: 10.1161/01.res.85.12.1154. [DOI] [PubMed] [Google Scholar]
- 8.Jones WK, Flaherty MP, Tang XL, Takano H, Qiu Y, Banerjee S, et al. Ischemic preconditioning increases iNOS transcript levels in conscious rabbits via a nitric oxide-dependent mechanism. J Mol Cell Cardiol. 1999;31:1469–81. doi: 10.1006/jmcc.1999.0983. [DOI] [PubMed] [Google Scholar]
- 9.Ping P, Zhang J, Zheng YT, Li RC, Dawn B, Tang XL, et al. Demonstration of selective protein kinase C-dependent activation of Src and Lck tyrosine kinases during ischemic preconditioning in conscious rabbits. Circ Res. 1999;85:542–50. doi: 10.1161/01.res.85.6.542. [DOI] [PubMed] [Google Scholar]
- 10.Xuan YT, Tang XL, Banerjee S, Takano H, Li RC, Han H, et al. Nuclear factor-KB plays an essential role in the late phase of ischemic preconditioning in conscious rabbits. Circ Res. 1999;84:1095–109. doi: 10.1161/01.res.84.9.1095. [DOI] [PubMed] [Google Scholar]
- 11.Shinmura K, Tang XL, Wang Y, Xuan YT, Liu SQ, Takano H, et al. Cyclooxygenase-2 mediates the cardioprotective effects of the late phase of ischemic preconditioning in conscious rabbits. Proc Natl Acad Sci USA. 2000;97:10197–202. doi: 10.1073/pnas.97.18.10197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo Y, Bao W, Wu WJ, Shinmura K, Tang XL, Bolli R. Evidence for an essential role of cyclooxygenase-2 as a mediator of the late phase of ischemic preconditioning in mice. Basic Res Cardiol. 2000;95:479–84. doi: 10.1007/s003950070024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972–83. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- 14.Xuan YT, Tang XL, Qiu Y, Banerjee S, Takano H, Han H, et al. Biphasic response of cardiac NO synthase isoforms to ischemic preconditioning in conscious rabbits. Am J Physiol. 2000;279:H2360–2371. doi: 10.1152/ajpheart.2000.279.5.H2360. [DOI] [PubMed] [Google Scholar]
- 15.Shinmura K, Xuan YT, Tang XL, Kodani E, Han H, Zhu Y, et al. Inducible nitric oxide synthase modulates cyclooxygenase-2 activity in the heart of conscious rabbits during the late phase of ischemic preconditioning. Circ Res. 2002;90:602–8. doi: 10.1161/01.res.0000012202.52809.40. [DOI] [PubMed] [Google Scholar]
- 16.Xuan YT, Yiru G, Hui H, Yanqing Z, Bolli R. An essential role of the JAK-STAT pathway in ischemic preconditioning. Proc Natl Acad Sci USA. 2001;98:9050–5. doi: 10.1073/pnas.161283798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guo Y, Jones WK, Xuan YT, Bao W, Wu WJ, Han H, et al. The late phase of ischemic preconditioning is abrogated by targeted disruption of the iNOS gene. Proc Natl Acad Sci USA. 1999;96:11507–12. doi: 10.1073/pnas.96.20.11507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Appleby SB, Ristimaki A, Neilson K, Narko K, Hla T. Structure of the human cyclo-oxygenase-2 gene. Biochem J. 1994;302:723–7. doi: 10.1042/bj3020723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto K, Arakawa T, Ueda N, Yamamoto S. Transcriptional roles of nuclear factor kappa B and nuclear factor-interleukin-6 in the tumor necrosis factor alpha-dependent induction of cyclooxygenase-2 in MC3T3-E1 cells. J Biol Chem. 1995;270:31315–20. doi: 10.1074/jbc.270.52.31315. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y, Fischer SM. Transcriptional regulation of cyclooxygenase-2 in mouse skin carcinoma cells. Regulatory role of CCAAT/enhancer-binding proteins in the differential expression of cyclooxygenase-2 in normal and neoplastic tissues. J Biol Chem. 1998;273:27686–94. doi: 10.1074/jbc.273.42.27686. [DOI] [PubMed] [Google Scholar]
- 21.Yamaoka K, Otsuka T, Niiro H, Nakashima H, Tanaka Y, Nagano S, et al. Selective DNA-binding activity of interleukin-10-stimulated STAT molecules in human monocytes. J Interferon Cytokine Res. 1999;19:679–85. doi: 10.1089/107999099313839. [DOI] [PubMed] [Google Scholar]
- 22.Horvath CM, Wen Z, Darnell JE. A Stat protein domain that determines DNA sequence recognition suggests a novel DNA-binding domain. Gene Dev. 1995;9:984–94. doi: 10.1101/gad.9.8.984. [DOI] [PubMed] [Google Scholar]
- 23.Decker T, Kovarik P, Meinke A. GAS elements: a few nucleotides with a major impact on cytokine-induced gene expression. J Interferon Cytokine Res. 1997;17:121–34. doi: 10.1089/jir.1997.17.121. [DOI] [PubMed] [Google Scholar]
- 24.Salvemini D, Misko TP, Masferrer JL, Seibert K, Currie MG, Needleman P. Nitric oxide activates cyclooxygenase enzymes. Proc Natl Acad Sci USA. 1993;90:7240–4. doi: 10.1073/pnas.90.15.7240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Salvemini D, Seibert K, Masferrer JL, Misko TP, Currie MG, Needleman P. Endogenous nitric oxide enhances prostaglandin production in a model of renal inflammation. J Clin Invest. 1994;93:1940–7. doi: 10.1172/JCI117185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davidge ST, Baker PN, Laughlin MK, Roberts JM. Nitric oxide produced by endothelial cells increases production of eicosanoids through activation of prostaglandin H synthase. Circ Res. 1995;77:274–83. doi: 10.1161/01.res.77.2.274. [DOI] [PubMed] [Google Scholar]
- 27.Nogawa S, Forster C, Zhang F, Nagayama M, Ross ME, Iadecola C. Interaction between inducible nitric oxide synthase and cyclooxygenase-2 after cerebral ischemia. Proc Natl Acad Sci USA. 1998;95:10966–71. doi: 10.1073/pnas.95.18.10966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marnett LJ, Wright TL, Crews BC, Tannenbaum SR, Morrow JD. Regulation of prostaglandin biosynthesis by nitric oxide is revealed by targeted deletion of inducible nitric-oxide synthase. J Biol Chem. 2000;275:13427–30. doi: 10.1074/jbc.275.18.13427. [DOI] [PubMed] [Google Scholar]
- 29.Stadler J, Harbrecht BG, Di Silvio M, Curran RD, Jordan ML, Simmons RL, et al. Endogenous nitric oxide inhibits the synthesis of cyclooxygenase products and interleukin-6 by rat Kupffer cells. J Leukoc Biol. 1993;53:165–72. doi: 10.1002/jlb.53.2.165. [DOI] [PubMed] [Google Scholar]
- 30.Amin AR, Attur M, Patel RN, Thakker GD, Marshall PJ, Rediske J, et al. Superinduction of cyclooxygenase-2 activity in human osteoarthritis-affected cartilage: influence of nitric oxide. J Clin Invest. 1997;99:1231–7. doi: 10.1172/JCI119280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Habib A, Bernard C, Lebret M, Creminon C, Esposito B, Tedgui A, et al. Regulation of the expression of cyclooxygenase-2 by nitric oxide in rat peritoneal macrophages. J Immunol. 1997;158:3845–51. [PubMed] [Google Scholar]
- 32.Patel R, Attur MG, Dave M, Abramson SB, Amin AR. Regulation of cytosolic COX-2 and prostaglandin E2 production by nitric oxide in activated murine macrophages. J Immunol. 1999;162:4191–7. [PubMed] [Google Scholar]
- 33.Clancy R, Varenika B, Huang W, Ballou L, Attur M, Amin AR, et al. Nitric oxide synthase/COX cross-talk: nitric oxide activates COX-1 but inhibits COX-2-derived prostaglandin production. J Immunol. 2000;165:1582–7. doi: 10.4049/jimmunol.165.3.1582. [DOI] [PubMed] [Google Scholar]
- 34.Tsai AL, Wei C, Kulmacz RJ. Interaction between nitric oxide and prostaglandin H synthase. Arch Biochem Biophys. 1994;313:367–72. doi: 10.1006/abbi.1994.1400. [DOI] [PubMed] [Google Scholar]
- 35.Curtis JF, Reddy NG, Mason RP, Kalyanaraman B, Eling TE. Nitric oxide: a prostaglandin H synthase 1 and 2 reducing cosubstrate that does not stimulate cyclooxygenase activity or prostaglandin H synthase expression in murine macrophages. Arch Biochem Biophys. 1996;335:369–76. doi: 10.1006/abbi.1996.0518. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton LC, Warner TD. Interactions between inducible isoforms of nitric oxide synthase and cyclo-oxygenase in vivo: investigations using the selective inhibitors, 1400W and celecoxib. Br J Pharmacol. 1998;125:335–40. doi: 10.1038/sj.bjp.0702077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoving S, Bar-Shimon M, Tijmes JJ, Goldshleger R, Tal DM, Karlish SJ. Novel aromatic isothiouronium derivatives which act as high affinity competitive antagonists of alkali metal cations on Na/K-ATPase. J Biol Chem. 1995;270:29788–93. doi: 10.1074/jbc.270.50.29788. [DOI] [PubMed] [Google Scholar]
- 38.Southan GJ, Szabo C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem Pharmacol. 1996;51:383–94. doi: 10.1016/0006-2952(95)02099-3. [DOI] [PubMed] [Google Scholar]
- 39.Garvey EP, Oplinger JA, Furfine ES, Kiff RJ, Laszlo F, Whittle BJ, et al. 1400W is a slow, tight binding, and highly selective inhibitor of inducible nitric-oxide synthase in vitro and in vivo. J Biol Chem. 1997;272:4959–63. doi: 10.1074/jbc.272.8.4959. [DOI] [PubMed] [Google Scholar]
- 40.Guo Y, Wu WJ, Qiu Y, Tang XL, Yang Z, Bolli R. Demonstration of an early and a late phase of ischemic preconditioning in mice. Am J Physiol. 1998;275:H1375–1387. doi: 10.1152/ajpheart.1998.275.4.H1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morham SG, Langenbach R, Loftin CD, Tiano HF, Vouloumanos N, Jennette JC, et al. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995;83:473–82. doi: 10.1016/0092-8674(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 42.Singh K, Balligand JL, Fischer TA, Smith TW, Kelly RA. Regulation of cytokine-inducible nitric oxide synthase in cardiac myocytes and microvascular endothelial cells. Role of extracellular signal-regulated kinases 1 and 2 (ERK1/ERK2) and STAT1 alpha. J Biol Chem. 1996;271:1111–7. doi: 10.1074/jbc.271.2.1111. [DOI] [PubMed] [Google Scholar]
- 43.Xie Q, Kashiwabara Y, Nathan C. Role of transcription factor NF-κB/Rel in induction of nitric oxide synthase. J Biol Chem. 1994;269:4705–8. [PubMed] [Google Scholar]
- 44.Barouch LA, Harrison RW, Skaf MW, Rosas GO, Cappola TP, Kobeissi ZA, et al. Nitric oxide regulates the heart by spatial confinement of nitric oxide synthase isoforms. Nature. 2002;416:337–40. doi: 10.1038/416337a. [DOI] [PubMed] [Google Scholar]
- 45.Vane JR, Bakhle YS, Botting RM. Cyclooxygenases 1 and 2. Annu Rev Pharmacol Toxicol. 1998;38:97–120. doi: 10.1146/annurev.pharmtox.38.1.97. [DOI] [PubMed] [Google Scholar]
- 46.Adderley SR, Fitzgerald DJ. Oxidative damage of cardiomyocytes is limited by extracellular regulated kinases 1/2-mediated induction of cyclooxygenase-2. J Biol Chem. 1999;274:5038–46. doi: 10.1074/jbc.274.8.5038. [DOI] [PubMed] [Google Scholar]
- 47.Schmedtje JF, Jr, Ji YS, Liu WL, DuBois RN, Runge MS. Hypoxia induces cyclooxygenase-2 via the NF-κB p65 transcription factor in human vascular endothelial cells. J Biol Chem. 1997;272:601–8. doi: 10.1074/jbc.272.1.601. [DOI] [PubMed] [Google Scholar]
- 48.LaPointe MC, Sitkins JR. Phospholipase metabolites regulate A2 inducible nitric oxide synthase in myocytes. Hypertension. 1998;31:218–24. doi: 10.1161/01.hyp.31.1.218. [DOI] [PubMed] [Google Scholar]