Abstract
Borrelia burgdorferi is the causative agent of Lyme disease, the most commonly reported arthropod-borne disease in the United States. B. burgdorferi is a highly invasive bacterium, yet lacks extracellular protease activity. In order to aid in its dissemination, B. burgdorferi binds plasminogen, a component of the hosts' fibrinolytic system. Plasminogen bound to the surface of B. burgdorferi can then be activated to the protease plasmin, facilitating the bacterium's penetration of endothelial cell layers and degradation of extracellular matrix components. Enolases are highly conserved proteins with no sorting sequences or lipoprotein anchor sites, yet many bacteria have enolases bound to their outer surfaces. B. burgdorferi enolase is both a cytoplasmic and membrane associated protein. Enolases from other pathogenic bacteria are known to bind plasminogen. We confirmed the surface localization of B. burgdorferi enolase by in situ protease degradation assay and immunoelectron microscopy. We then demonstrated that B. burgdorferi enolase binds plasminogen in a dose-dependent manner. Lysine residues were critical for binding of plasminogen to enolase, as the lysine analog εaminocaproic acid significantly inhibited binding. Ionic interactions did not play a significant role in plasminogen binding by enolase, as excess NaCl had no effects on the interaction. Plasminogen bound to recombinant enolase could be converted to active plasmin. We conclude that B. burgdorferi enolase is a moonlighting cytoplasmic protein which also associates with the bacterial outer surface and facilitates binding to host plasminogen.
Introduction
Borrelia burgdorferi, the Lyme disease spirochete, causes the most common arthropod-borne disease in the United States and many other temperate regions of the world. Lyme disease is a significant cause of morbidity and it continues to be a serious public health concern [1]. Spirochetes such as B. burgdorferi are unique pathogens in that they lack classical virulence factors such as toxins. Instead, part of their remarkable ability to cause disease and suffering lies in their ability to widely disseminate throughout host tissues. B. burgdorferi lacks surface proteases that could degrade the hosts' extracellular matrix and accelerate the spirochete's penetration of host tissues. However, B. burgdorferi can usurp a host protease, plasminogen.
Plasminogen is a serine protease present in serum as an inactive proenzyme [2]. Plasminogen is converted by tissue-type plasminogen activator (tPA) or urokinase plasminogen activator (uPA) to active plasmin. Plasmin has a critical role in host fibrinolysis and extracellular matrix remodeling and therefore its activity is tightly controlled. Binding of plasminogen by mammalian plasminogen receptors is mediated by lysine-binding Kringle domains [3]. Binding of plasminogen to a mammalian receptor, fibrin clot, or a bacterial cell facilitates its activation to plasmin and makes the molecule less susceptible to inactivation by α2 antiplasmin [4], [5].
Many bacterial species are able to bind and use host plasminogen. B. burgdorferi is known to bind host plasminogen, which can then be converted to active plasmin. B. burgdorferi with bound plasmin is able to degrade fibronectin, penetrate the endothelium, and activate matrix metalloprotease-9 (MMP-9) and MMP-1 [6], [7], [8]. Plasminogen is required for efficient dissemination in ticks, and plasminogen deficient mice have decreased spirochetemia [9]. Several plasminogen-binding proteins have been identified and characterized in B. burgdorferi, including ErpA/C/P, OspA, OspC, and a 70 kDa plasminogen binding protein [10], [11], [12], [13].
Enolases are cytosolic metalloenzymes that catalyze the conversion of 2-phospho-D-glycerate to phosphoenolpyruvate [14]. Despite the lack of classical protein sorting machinery or cell membrane anchoring moieties, enolases are expressed on the surface of a variety of eukaryotic cells (including neuronal, cancer, epithelial, endothelial and hematopoietic cells), where they can function as plasminogen receptors [14]. Remarkably, enolases are also found on the surface of bacterial cells where they can similarly function as plasminogen receptors [4], [14], [15], [16].
While several plasminogen-binding proteins have been proposed and partially characterized for B. burgdorferi, we reasoned that the B. burgdorferi enolase might also be a plasminogen-binding protein. We now demonstrate that the B. burgdorferi enolase is exposed on the bacterial outer surface, and can bind host plasminogen.
Materials and Methods
Bacteria
Virulent Borrelia burgdorferi strain B31-MI-16 [17], [18], [19] was grown at 34°C to cell densities of approximately 1×107 ml in modified Barbour-Stoenner-Kelly II (BSK-II) medium [20]. Total DNA (chromosomal and plasmids) was isolated using the DNeasy blood and tissue kit (Qiagen, Valencia, CA) according to the manufacturer's instructions.
Recombinant proteins
Polyhistidine-tagged, full-length ErpC has been described previously [10], [21], [22]. All recombinant proteins contained amino-terminal tags. The enolase gene (ORF BB0337) plus approximately 500 bp upstream and downstream DNA, was produced by PCR of B. burgdorferi B31-MI-16 DNA using primers enoF flk 5′ tgcttgtgccatgaggaata and enoR flk 5′ ataaggcacggcatttcaag and subsequent cloning into pCR2.1 (Invitrogen, Carlsbad, CA). Full-length enolase for recombinant protein production was produced by PCR using primers enoF prot 5′caccatgggttttcacatttatgaaatca and enoR prot 5′ttatttttgtttaatagaataaaagacgctc and insertion into pET200 (Invitrogen). The resultant plasmid's insert was entirely sequenced on both strands to ensure that no undesired mutations had occurred during PCR or cloning procedures. Recombinant proteins were expressed in Escherichia coli Rosetta (DE3)pLysS (Novagen, Madison, WI), upon induction with isopropyl-β-D-thiogalactopyranoside (IPTG). Induced E. coli cells were harvested and then lysed by two passages through a French pressure cell at 1,000 p.s.i in a mixture of 30 mM imidazole, 0.5 M NaCl, and 20 mM NaPO4 (pH 7.4), and debris was cleared by centrifugation. Recombinant proteins were purified from cleared lysates using MagneHis nickel-conjugated magnetic beads (Promega, Madison, WI). All recombinant proteins were dialyzed overnight against phosphate-buffered saline (PBS) using 3,500 kDa molecular-weight-cutoff Slide-A-Lyzer cassettes (Pierce, Rockford, IL) at 4°C. Protein purity was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) followed by staining with Coomassie brilliant blue. Protein concentrations were determined by bicinchoninic acid protein assays (BCA; Pierce).
SDS-PAGE and Western blotting
Fresh BSKII medium and spent BSKII medium from late log phase cultures of B. burgdorferi were centrifuged briefly (12,000 x g, 10 minutes) and the supernatants, recombinant enolase (1.25 µg/ml) or whole-cell lysate from log-phase B. burgdorferi (2×107 bacteria/ml) were separated by SDS-PAGE and proteins were either transferred to nitrocellulose membranes or stained with Coomassie brilliant blue. Membranes were blocked overnight at 4°C with 5% (w/v) non-fat dried milk in Tris-buffered saline-Tween 20 (TBS-T; 20 mM Tris (pH7.5), 150 mM NaCl, 0.05% (v/v) Tween 20). Membranes were next washed with TBS-T, and incubated for 2 h at room temperature with rabbit antiserum raised against human α-enolase (Pierce) diluted 1∶500 in TBS-T. After extensive washing with TBS-T, membranes were incubated for 1 h at room temperature with horseradish-peroxidase-conjugated donkey anti-rabbit IgG antibody (GE Healthcare, Piscataway, NJ), diluted 1: 20,000 in TBS-T. After a final series of washes with TBS-T, bound antibodies were detected using SuperSignal West Pico enhanced chemiluminescence substrate (Pierce).
In situ protease analysis
B. burgdorferi were grown to mid-exponential phase in BSK-II, pelleted by centrifugation, washed once with PBS, and resuspended in PBS to a final concentration of approximately 2×109 bacteria/ml. Examination of bacterial suspensions by darkfield microscopy did not indicate detectable lysis of the bacteria. Bacteria were then incubated at room temperature in PBS containing 40 µg of proteinase K (Fisher Scientific, Pittsburgh, PA) for 30, 60 or 120 minutes, whereupon digestion was terminated by addition of paramethylsulfonyl fluoride (PMSF; Sigma) to a final concentration of 1.6 mg/ml, followed by sample boiling. Control aliquots of bacteria were incubated in buffer for 2 h at room temperature without added protease, followed by the addition of inhibitor and boiling as with the protease-treated bacteria. Equal volumes of each bacterial lysate were subjected to SDS-PAGE and transferred to nitrocellulose membranes, and the susceptibility of enolase to protease digestion was assessed by immunoblot analysis with rabbit polyclonal anti-human α-enolase (Pierce) as described above. As experimental controls, lysates were also immunoblotted with monoclonal antibodies directed against OspC (located on the bacterial outer surface and thus susceptible to proteolysis [22], [23]) and FlaB (located in the periplasmic space and thus protected against protease digestion in intact bacteria [24]). Densitometric analysis was performed using ImageJ software (http://imageJ.nih.gov/ij).
Immunoelectron microscopy
For immunogold localization of enolase and OspC, cultures of B. burgdorferi were grown to mid-exponential phase then harvested by centrifugation and washed three times in sterile PBS, pH 7.4. TEM grids were washed repeatedly in PBS both before processing and between all incubation steps. All solutions were microfiltered using 0.4 µm syringe filters prior to use. Fifty microliter droplets containing non-diluted B. burgdorferi. were placed on the surface of parafilm covered plastic petri dishes. Gold 400 mesh formvar coated carbon-stabilized TEM grids (Ted Pella, Redding, CA) were then placed on each droplet for 90 mins at room temperature followed by a 20 minute fixation with buffered 4% paraformaldehyde. Sections were then incubated sequentially in the appropriate blocking buffer (4% normal serum/PBS, 1 hr. at room temperature), polyclonal rabbit anti-human α-enolase (1∶50 in blocking buffer, Pierce) or monoclonal mouse anti-OspC ([25] kind gift of Brian Stevenson), for 2 hours followed by biotinylated goat anti-rabbit or horse anti mouse IgG (1∶500 in blocking buffer, 2 hr.; Vector Laboratories, Burlingame, CA), respectively. This was followed by a 30 min incubation on droplets of streptavidin-conjugated 10 nm gold particles (Ted Pella). Grids were stabilized by post-fixation on droplets of 4% paraformaldehyde/2% glutaraldehyde for 10 minutes and washed repeatedly in PBS. To further assess specificity of the monoclonal anti-enolase antibody, additional spirochete grids were processed as described above with omission of the primary antibody. Grids were then counterstained by placing on droplets of a 5% molybdate solution for 60 seconds, washed repeatedly in PBS and viewed using a Hitachi 7500 transmission electron microscope.
ELISA
For the enzyme-linked immunosorbent assay (ELISA), Maxisorp 96- well plates (Nalge-Nunc, Rochester, NY) were coated overnight with 10 µg/ml human plasminogen (Sigma-Aldrich, St. Louis, MO), 10 µg/ml recombinant enolase, 10 µγ/ml recombinant ErpC (positive control for plasminogen binding), or 10 µg/ml bovine serum albumin (BSA [negative control]) in PBS at 4°C. Plates were brought to room temperature and washed once with PBS supplemented with 0.05% (vol/vol) Tween 20 (PBS-T). Wells were blocked for 2 h at room temperature with PBS with 1% (mass/vol) gelatin and then washed three times with PBS-T. Afterwards, 100 µl/well of either recombinant enolase, ErpC or plasminogen (Sigma-Aldrich) [see text for concentrations]) was added and incubated for 2 h at 37°C. Wells were washed three times with PBS-T and then incubated for 1 h at room temperature with rabbit antiserum raised against human α-enolase (which cross-reacts with the highly similar Borrelia burgdorferi protein; Pierce), diluted 1∶500 in PBS, or goat anti-human plasminogen (Novus Biologicals, Littleton, CO). Plates were washed three times with PBS-T, and then wells were incubated for 1 h at room temperature with horseradish peroxidase-conjugated anti-goat immunoglobulin G (IgG; Jackson Immunochemicals, West Grove, PA), horseradish peroxidase-conjugated anti-rabbit IgG (GE Healthcare), or horseradish peroxidase-conjugated protein G (Invitrogen), diluted 1∶5,000. Wells were again washed three times with PBS-T, 100 µl/well. Tetramethylbenzidine substrate (Thermo Scientific, Rockford, IL) was added, and then reactions were stopped by addition of 100 µl/well of 2 N H2SO4. Absorbance was read at 450 nm using a BioTek plate reader utilizing KC4 software (BioTek, Winooski, VT). To determine the role of lysines in plasminogen-enolase interaction, the lysine analog ε-aminocaproic acid (final concentration, 3–30 mM [Sigma-Aldrich]) was added with plasminogen to enolase-coated wells. Protein binding affinities (Kd) were calculated as that concentration of ligand required for half-maximal binding activity. For experiments examining the role of ionic interactions in enolase binding to plasminogen, increasing concentrations of NaCl were added to the PBS-based binding buffer with enolase. For experiments analyzing the ability of whole B. burgdorferi to bind recombinant enolase, plates were coated overnight with intact B. burgdorferi (2×107/ml; washed 3X in PBS); after washing and blocking with SeaBlock (Pierce) for 2 hours, recombinant B. burgdorferi enolase (0–50 µg/ml) was added for 2 hours at 37°C, plates washed, and enolase detected as described above.
Plasminogen activation assay
Maxisorp 96-well plates (Nalge-Nunc) were coated overnight with 10 µg/ml recombinant enolase or 10 µg/ml BSA in PBS at 4°C. Plates were brought to room temperature and washed once with PBS-T. Wells were blocked for 2 h at room temperature with PBS with 2% (mass/vol) BSA and then washed three times with PBS-T. Afterwards 100 µl/well of 10 µg/ml human plasminogen was added and incubated for 2 h at 37°C. Wells were washed three times with PBS-T, and then 4 ng/well of human uPA (Chemicon, Temecula, CA) was added. Next, the plasmin-specific substrate D-valyl-leucyl-lysine-p-nitroanilide dihydrochloride (Sigma-Aldrich) was added at a final concentration of 0.3 mM in 64 mM Tris, 350 mM NaCl, 0.15% Triton X-100 (pH 7.5). Plates were incubated overnight at 37°C, and absorbance was read at 405 nm.
Results
B. burgdorferi enolase is surface exposed
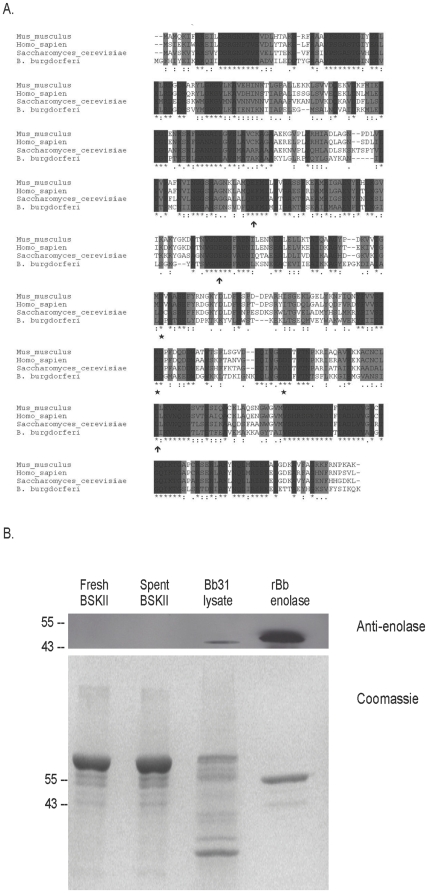
Enolases are highly conserved among organisms as diverse as bacteria and humans [14]. Alignment of enolases from diverse species suggested that an anti-human antibody might recognize B. burgdorferi enolase (Figure 1A). Notably, the amino acid residues required for binding the cofactor, manganese (165E, 206E, 339L), as well as the residues of the enzymatic active site (243D, 289E, 317D), were also conserved (Figure 1A and [27]). Recombinant B. burgdorferi enolase or whole cell lysate were separated by SDS-PAGE and transferred to nitrocellulose. As anticipated, an anti-human α-enolase polyclonal antibody recognized purified recombinant B. burgdorferi enolase as well as the native enolase in whole cell lysates (Figure 1B). Since the B. burgdorferi medium is rich in bovine serum albumin and rabbit serum, we confirmed that the medium itself was not recognized by the anti-human α-enolase antibody (Figure 1B, fresh BSK-II lane).
Figure 1. Conservation of enolase.
(A) Alignment of enolases from mouse, human, yeast and B. burgdorferi. Dark shaded residues indicate identity; light gray residues indicate similarity. Arrows indicate the 3 conserved residues that make up the enzymatic active site; stars indicate the three conserved residues required for binding the cofactor manganese. (B) A rabbit polyclonal antiserum directed against human enolase recognizes enolase from B. burgdorferi. Top panel: Western blot; Bottom panel: Coomassie-stained SDS-PAGE gel. Molecular weight markers are indicated on the left. Lane 1: fresh BSKII medium; Lane 2: spent BSKII medium from late-log phase cultures of B. burgdorferi; Lane 3: B. burgdorferi cell lysate; Lane 4: recombinant enolase from B. burgdorferi.
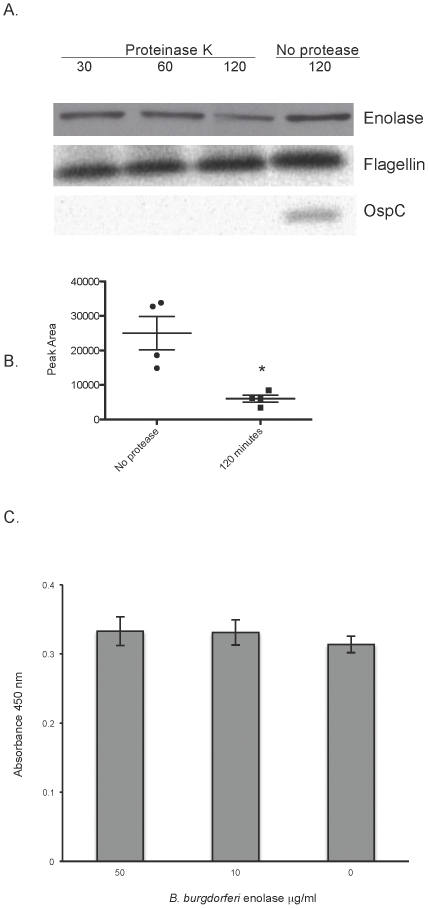
Despite the lack of canonical protein export sequences, enolases of many species, both bacteria and eukaryote, are localized on the cell surface [14], [16], [27], [28], [29], [30], [31], [32], [33], [34], [35]. The B. burgdorferi enolase was previously shown to fractionate with both the soluble and membrane fractions [36]. To address the possibility of surface exposure of enolase, in situ protease degradation assays were performed. Intact B. burgdorferi were incubated in the presence of proteinase K for 30, 60 or 120 minutes. Protease was then inactivated, bacteria were lysed and proteins separated by SDS-PAGE. After transfer to nitrocellulose membranes, blots were incubated with antisera specific for OspC, an outer surface protein, FlaB, a periplasmic protein, or enolase. As shown in Figure 2A, OspC was totally degraded, indicating surface exposure. FlaB was not affected by the protease, indicating that the bacteria were intact. There was a considerable reduction in the amount of enolase detected by 120 minutes of incubation with proteinase K, compared to the no protease control. Densitometric analysis of band intensities revealed a significant reduction in peak area between the 120-minute proteinase K treated band and the no protease control (Figure 2B). Although enolase is a glycolytic enzyme, and found within the cytoplasm, these data indicate that a proportion of B. burgdorferi enolase is also surface exposed.
Figure 2. B. burgdorferi enolase is surface exposed.
(A) Demonstration of outer surface exposure of enolase by in situ protease degradation. Whole B. burgdorferi were incubated with proteinase K for 30, 60 or 120 minutes or 120 minutes in buffer with no protease. Proteases were then inactivated, bacteria were lysed, proteins were separated by SDS-PAGE, and the integrity of enolase, OspC (outer surface protein), and FlaB (periplasmic protein) were analyzed by immunoblot. Two separate protease degradation experiments were performed, with blots repeated 4 times. A representative blot is shown. (B) Densitometric analysis of in situ protease degradation immunoblots. Images from blots were scanned and peak areas for 120-minute proteinase K treatment and no protease control were assessed using ImageJ software (http://imageJ.nih.gov/ij). Data represent the means and standard errors from 4 separate blots. *, P = 0.03, Student's t test assuming unequal variances. (C) Binding of exogenous B. burgdorferi enolase to the surface of whole bacteria. Binding of B. burgdorferi enolase (0–50 µg/ml) to immobilized B. burgdorferi (2×107/ml) was analyzed by ELISA, with bound enolase detected by rabbit polyclonal antiserum directed against human enolase. Data represent the means and standard errors from three separate experiments with six replicates per enolase concentration.
In Gram-positive bacteria such as Streptococcus pneumoniae, it has been suggested that autolysins are involved in liberating enolase from the bacterial cytoplasm, and that streptococci scavenge enolase from lysed neighbor cells [37], [38]. However, we were unable to detect enolase in spent culture medium from late log phase B. burgdorferi (Figure 1B, spent BSKII lane). In addition, we incubated intact B. burgdorferi (2×107 bacteria/ml) with recombinant B. burgdorferi enolase and assayed the amount of surface-associated enolase by ELISA. We did not detect any significant increase in surface-bound enolase compared to bacteria incubated in the absence of exogenous B. burgdorferi enolase (Figure 2C).
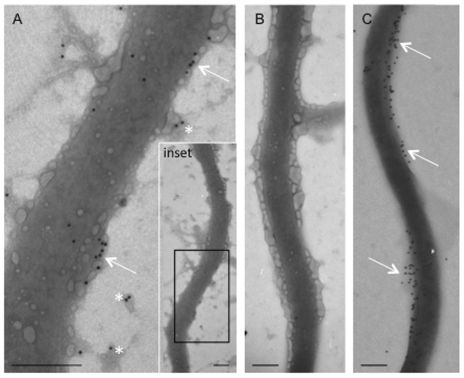
We then used immunoelectron microscopy to confirm the presence of enolase on the spirochete outer membrane. Our analysis revealed that gold-labeled anti-human α-enolase was scattered sporadically over the outer surface of the majority of spirochetes (Figure 3A). The density of labeling was quite variable cell-to-cell suggesting some variability in surface exposure of enolase among individual spirochetes. It is noteworthy that labeling was routinely observed on isolated patches of material resembling outer membrane (Figure 3A. asterisks). Omission of the anti-enolase antibody resulted in the complete absence of gold labeling on all control grids examined (Figure 3B). Antibody against an abundant outer surface protein, OspC, was used as a positive control. Anti-OspC labeling was generally denser than enolase, and tended to localize in large patches covering the surface of the spirochete (Figure 3C). The dense labeling of OspC is consistent with the presence of a well-preserved outer membrane and supports our data indicating the localization of enolase to the outer surface of B. burgdorferi.
Figure 3. Immunoelectron microscopic analysis of B. burgdorferi enolase.
A) Anti-enolase was localized intermittently across the outer surface of B. burgdorferi (arrows). Gold particles were also observed on membrane blebs in proximity to the spirochete (asterisks). The boxed area in the inset indicates the region demonstrated in image (A). B) Omission of the primary antibody resulted in a complete loss of immunoreactivity. C) Anti-OspC immunolabeling demonstrated moderately heavy labeling on the outer surface of the spirochete (Arrows). Magnification bar = 0.2 µm.
Enolase binds human plasminogen
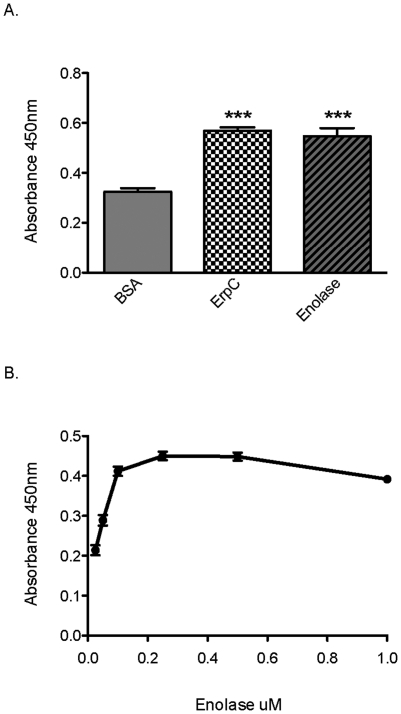
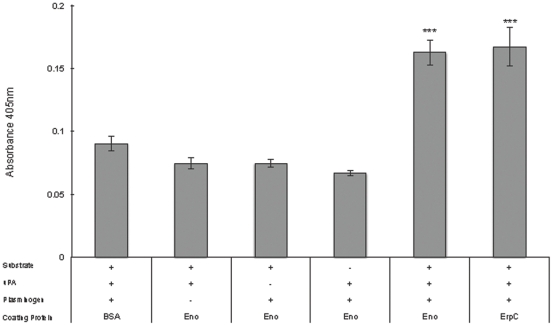
Previous studies have demonstrated that enolases of various gram-positive and gram-negative bacteria bind plasminogen (reviewed in [4]), and this prompted us to investigate whether the enolase of B. burgdorferi was capable of binding human plasminogen. Microtiter plates were coated with recombinant enolase, bovine serum albumin (negative control protein) or ErpC (a known B. burgdorferi plasminogen-binding protein [10]), and then plasminogen binding was assayed by ELISA. Enolase demonstrated significant binding of human plasminogen (Figure 4A). Analyses using various concentrations of recombinant enolase demonstrated that B. burgdorferi enolase bound plasminogen in a dose-dependent manner, with an apparent Kd of 125 nM (Figure 4B).
Figure 4. B. burgdorferi enolase binds plasminogen.
(A) Binding of plasminogen (25 µg/ml) to immobilized proteins (10 µg/ml) was analyzed by ELISA, with bound plasminogen detected using specific antiserum. BSA was used as a negative control for nonspecific binding. ErpC is a known plasminogen binding protein of B. burgdorferi [10]. Data represent the means and standard errors from three separate experiments with six replicates per protein. ***, P<0.001, Student's t test assuming unequal variances. (B) Enolase binds plasminogen in a dose-dependent manner. Binding of plasminogen (10 µg/ml) to immobilized enolase (0–1 µM) was analyzed by ELISA, with bound plasminogen detected using specific antiserum. BSA was used as a negative control for nonspecific binding. Values represent plasminogen binding to enolase minus background absorbance for BSA. Data represent the means and standard errors from a representative experiment (1 of 4) with six replicates per concentration of enolase.
Role of lysines in enolase-plasminogen binding
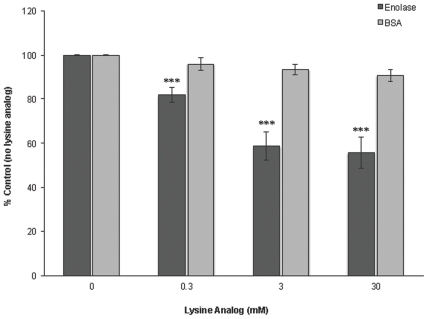
Plasminogen receptors, both mammalian and bacterial, often bind plasminogen through lysine residues that interact with the Kringle domains of plasminogen [14], [39], [40], [41]. The enolase of B. burgdorferi is comprised of 5.7% lysine residues. Addition of the lysine analog ε-aminocaproic acid significantly reduced the interaction between enolase and plasminogen, while the lysine analog had no effect on the background binding of plasminogen to BSA (Figure 5). These data indicate a role for lysines in enolase-plasminogen binding.
Figure 5. Role of lysines in enolase/plasminogen binding activity.
Binding of plasminogen to immobilized enolase (10 µg/ml) was analyzed by ELISA. Plasminogen (25 µg/ml) was added to enolase-coated wells in the presence or absence of 0–30 mM ε-aminocaproic acid (lysine analog). Bound plasminogen was detected using a specific antiserum. BSA was used as a negative control. Data represent the means and standard errors from three separate experiments with 12 replicates per condition. ***, P<0.001, Student's t test assuming unequal variances.
Role of ionic interactions in enolase-plasminogen binding
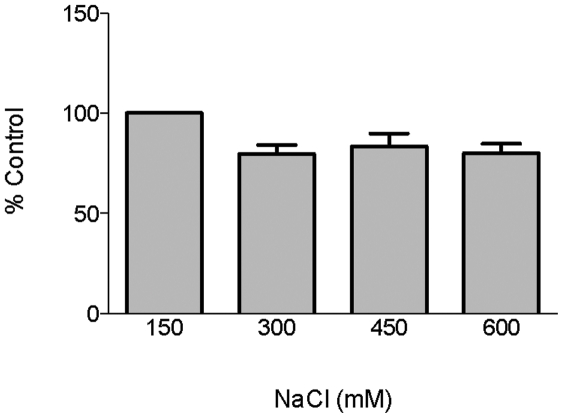
The enolase of B. burgdorferi is highly charged, with a theoretical pI of 5.38. To assess the role of ionic interactions in enolase binding of plasminogen, binding assays were performed in the presence of various concentrations of NaCl. NaCl at up to four times the normal physiological concentration had a small but insignificant negative effect on binding (Figure 6). These data suggest that ionic interactions are not required for the enolase-plasminogen interaction.
Figure 6. Role of ionic interactions in enolase binding of plasminogen.
Binding of plasminogen to immobilized enolase (10 µg/ml) was analyzed by ELISA. Plasminogen (25 µg/ml) was incubated in the presence of increasing concentrations of NaCl. Bound plasminogen was detected using a specific antiserum. BSA was used as a negative control. Data are presented as % control (physiological NaCl, 150 mM) from the means and standard errors from three experiments with six replicates per concentration of NaCl.
Plasminogen bound to enolase can be converted to active plasmin
B. burgdorferi-bound plasminogen is converted to plasmin [11]. To determine if enolase-bound plasminogen can also be converted to the active enzyme, microtiter plates were coated with enolase, blocked, plasminogen and the activator uPA were added, and proteolytic activity was quantified using a plasmin-specific chromogenic substrate. As shown in Figure 7, enolase-bound plasminogen was converted to active plasmin. These data suggest that, in vivo, enolase-bound plasminogen would be accessible to plasminogen activators.
Figure 7. Enolase-bound plasminogen is converted into plasmin.
Enolase-coated wells of microtiter plates were incubated with plasminogen, urokinase (uPA), and/or a plasmin-specific chromogenic substrate. Proteolytic activity was measured by absorbance at 405 nM. Data represent the means and standard errors from three different experiments with six replicates per condition. ***, P<0.001 compared to the activation of plasminogen bound to the control protein, BSA, Student's t test assuming unequal variances.
Discussion
Enolases belong to a class of proteins referred to as moonlighting proteins: proteins that have multiple functions and often exist in distinct locations within a cell [42]. Along with its role as an essential glycolytic enzyme, we have demonstrated that B. burgdorferi enolase has an additional function as a cell-surface plasminogen receptor. Enolases lack traditional sorting signals and how these proteins become surface exposed is unknown. The enolase of B. burgdorferi is both cytoplasmic and surface exposed (Figures 2 and 3, and [36]), and humans and animals produce antibodies to the B. burgdorferi enolase during natural infection [43]. Proteins destined for the outer surface are expensive for a cell to produce [44], and the amino acid sequence of enolase certainly suggests an inexpensive protein. The B. burgdorferi protein, like many enolases, is rich in “cheap” amino acids such as alanine (8.3%) and glycine (7.2%) while low in ‘expensive’ amino acids such as phenylalanine (3.9%) and tryptophan (1.3%). Intriguingly, a hydrophobic domain in human αenolase suggested to play a role in its membrane association (AAVPSGASTGI) is identical in the B. burgdorferi enolase [14]. It is clear, however, that for both eukaryotic and bacterial enolases, surface exposure is not unusual [16], [27], [28], [29], [30], [32], [33], [34], [35], [45]. Other cytoplasmic proteins, such as DnaK, have also been identified as plasminogen-binding proteins [46], [47].
A potential consequence of the surface exposure of the B. burgdorferi enolase is its possible contribution to autoimmunity. Streptococcal enolases are cross-reactive with human enolase and are thought to play a role in post-streptococcal sequelae such as rheumatic heart disease [48]. Eukaryotic enolases are also associated with autoimmune conditions. The identified cross-reactive epitopes in cancer-associated retinopathy (FRAAVPSG and RYMGKGVS) are almost identical to the residues present in the B. burgdorferi enolase [49].
Lysine residues play a critical role in the interaction of plasminogen receptors with their ligand. A number of bacterial plasminogen-binding proteins contain essential lysines [4], [6], [10], [50]. The lysine analog ε-aminocaproic acid significantly inhibited enolase binding to plasminogen. For many plasminogen receptors, it is the C-terminal lysines that are critical for binding [16], [39]. The lysine residues in B. burgdorferi enolase, however, are dispersed. Internal plasminogen binding sites have been identified for other pathogen's receptors [51], [52], [53]. We are continuing to define the plasminogen-binding site(s) of the B. burgdorferi enolase.
Plasminogen binding by B. burgdorferi is clearly important in vivo.
Plasminogen-deficient mice exhibit reduced spirochetemia, and plasminogen is required for efficient dissemination of B. burgdorferi within the tick vector [9]. B. burgdorferi-bound plasminogen can degrade extracellular matrix proteins and activate matrix metalloproteases, facilitating the spirochete's spread through host tissues [6], [7], [8]. Interestingly, B. burgdorferi upregulates expression of both the plasminogen activator uPA and the plasmin inhibitor PAI-2 from monocytes. The upregulation of uPA could activate plasminogen bound to the surface of B. burgdorferi. The upregulation of PAI-2 had no effect on B. burgdorferi transmigration but did decrease monocyte migration across Matrigel [54]. In addition to the suppression of leukocyte function, B. burgdorferi may capitalize on plasminogen binding for other immune evasive strategies. For instance, binding of plasminogen is anti-opsonic for Staphylococcus aureus, and the enolase of Streptococcus sobrinus increases IL-10 expression in mice [55], [56].
It is not surprising that B. burgdorferi may express multiple plasminogen-binding proteins on its surface [10], [11], [12], [13]. Redundancy in bacterial adhesins is not unusual. Indeed, B. burgdorferi possesses at least three distinct fibronectin-binding proteins [57], [58], [59]. Many bacterial pathogens possess both high and low-affinity plasminogen-binding proteins on their cell surface [60], [61]. The B. burgdorferi plasminogen-binding protein ErpP, for instance, has a Kd of ∼25 nM, while we calculate that the enolase has a Kd of ∼125 nM (Figure 4B, and [10]. In addition, B. burgdorferi may utilize different plasminogen-binding receptors at different stages in its infectious life cycle, i.e., tick versus mammal. Plasminogen binding may not be the only function of extracellular enolase; the enolase of S. aureus binds laminin, and LenA, a surface receptor of Leptospira interrogans, interacts with plasminogen, complement Factor H, and laminin [26], [31], [62]. The plasminogen-binding OspA protein of B. burgdorferi also functions in invasion of the tick salivary glands, and ErpP of B. burgdorferi binds both plasminogen and complement factor H [10], [63].
B. burgdorferi is a successful extracellular pathogen that co-opts host proteins for its own advantage. We have identified that the enolase of B. burgdorferi moonlights as a surface-exposed, plasminogen-binding protein.
Acknowledgments
The authors thank Amy Bowman for technical assistance, and Brian Stevenson and Ann Flower for helpful discussion.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: ND EPSCoR (UND0014095; www.ndepscor.nodak.edu). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.CDC Summary of notifiable diseases – United States, 2009. Morb Mortal Wkly Rep. 2011;58:1–100. [PubMed] [Google Scholar]
- 2.Sun H. The interaction between pathogens and the host coagulation system. Physiology. 2006;21:281–288. doi: 10.1152/physiol.00059.2005. [DOI] [PubMed] [Google Scholar]
- 3.Wiman B, Lijnen HR, Collen D. On the specific interaction between the lysine-binding sites in plasmin and complementary sites in alpha2-antiplasmin and in fibrinogen. Biochem Biophys Acta. 1979;579:142–154. doi: 10.1016/0005-2795(79)90094-1. [DOI] [PubMed] [Google Scholar]
- 4.Lahteenmaki K, Kuusela P, Korhonen TK. Bacterial plasminogen activators and receptors. FEMS Microbiol Rev. 2001;25:531–552. doi: 10.1111/j.1574-6976.2001.tb00590.x. [DOI] [PubMed] [Google Scholar]
- 5.Lottenberg R, Broder CC, Boyle MD. Identification of a specific receptor for plasmin on a group A streptococcus. Infect Immun. 1987;55:1914–1918. doi: 10.1128/iai.55.8.1914-1918.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coleman JL, Roemer EJ, Benach JL. Plasmin-coated Borrelia burgdorferi degrades soluble and insoluble components of the mammalian extracellular matrix. Infect Immun. 1999;67:3929–3936. doi: 10.1128/iai.67.8.3929-3936.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman JL, Sellati TJ, Testa JE, Kew RR, Furie MB, et al. Borrelia burgdorferi binds plasminogen, resulting in enhanced penetration of endothelial monolayers. Infect Immun. 1995;63:2478–2484. doi: 10.1128/iai.63.7.2478-2484.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gebbia JA, Coleman JL, Benach JL. Borrelia spirochetes upregulate release and activation of matrix metalloproteinase gelatinase B (MMP-9) and collagenase 1 (MMP-1) in human cells. Infect Immun. 2001;69:456–462. doi: 10.1128/IAI.69.1.456-462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Coleman JL, Gebbia JA, Piesman J, Degen JL, Bugge TH, et al. Plasminogen is required for efficient dissemination of B. burgdorferi in ticks and for enhancement of spirochetemia in mice. Cell. 1997;89:1111–1119. doi: 10.1016/s0092-8674(00)80298-6. [DOI] [PubMed] [Google Scholar]
- 10.Brissette CA, Haupt K, Barthel D, Cooley AE, Bowman A, et al. The Borrelia burgdorferi infection-associated surface proteins ErpP, ErpA, and ErpC bind human plasminogen. Infect Immun. 2009;77:300–306. doi: 10.1128/IAI.01133-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuchs H, Wallich R, Simon MM, Kramer MD. The outer surface protein A of the spirochete Borrelia burgdorferi is a plasmin(ogen) receptor. Proc Natl Acad Sci U S A. 1994;91:12594–12598. doi: 10.1073/pnas.91.26.12594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hu LT, Pratt SD, Perides G, Katz L, Rogers RA, et al. Isolation, cloning and expression of a 70-kilodalton plasminogen binding protein of Borrelia burgdorferi. Infect Immun. 1997;65:4989–4995. doi: 10.1128/iai.65.12.4989-4995.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lagal V, Portnoi D, Faure G, Postic D, Baranton G. Borrelia burgdorferi sensu stricto invasiveness is correlated with OspC-plasminogen affinity. Microbes Infect. 2006;8:645–652. doi: 10.1016/j.micinf.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 14.Pancholi V. Multifunctional alpha-enolase: its role in diseases. Cell Mol Life Sci. 2001;58:902–920. doi: 10.1007/PL00000910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lahteenmaki K, Edelman S, Korhonen TK. Bacterial metastasis: the host plasminogen system in bacterial invasion. Trends Microbiol. 2005;13:79–85. doi: 10.1016/j.tim.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Pancholi V, Fischetti VA. alpha-enolase, a novel strong plasmin(ogen) binding protein on the surface of pathogenic streptococci. J Biol Chem. 1998;273:14503–14515. doi: 10.1074/jbc.273.23.14503. [DOI] [PubMed] [Google Scholar]
- 17.Casjens S, Palmer N, van Vugt R, Huang WM, Stevenson B, et al. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs of an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol Microbiol. 2000;35:490–516. doi: 10.1046/j.1365-2958.2000.01698.x. [DOI] [PubMed] [Google Scholar]
- 18.Fraser CM, Casjens S, Huang WM, Sutton GG, Clayton R, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 19.Miller JC, von Lackum K, Babb K, McAlister JD, Stevenson B. Temporal analysis of Borrelia burgdorferi Erp protein expression throughout the mammal-tick infectious cycle. Infect Immun. 2003;71:6943–6952. doi: 10.1128/IAI.71.12.6943-6952.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zückert WR. Laboratory maintenance of Borrelia burgdorferi. In: Coico RT, Kowalik TF, Quarles J, Stevenson B, Taylor R, editors. Current Protocols In Microbiology. Hoboken, N.J.: J. Wiley and Sons; 2007. pp. 12C.11.11–12C.11.10. [DOI] [PubMed] [Google Scholar]
- 21.Stevenson B, Bono JL, Schwan TG, Rosa P. Borrelia burgdorferi Erp proteins are immunogenic in mammals infected by tick bite, and their synthesis is inducible in cultured bacteria. Infect Immun. 1998;66:2648–2654. doi: 10.1128/iai.66.6.2648-2654.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Hage N, Babb K, Carroll JA, Lindstrom N, Fischer ER, et al. Surface exposure and protease insensitivity of Borrelia burgdorferi Erp (OspEF-related) lipoproteins. Microbiology. 2001;147:821–830. doi: 10.1099/00221287-147-4-821. [DOI] [PubMed] [Google Scholar]
- 23.Fuchs R, Jauris S, Lottspeich F, Preac-Mursic V, Wilske B, et al. Molecular analysis and expression of a Borrelia burgdorferi gene encoding a 22 kDa protein (pC) in Escherichia coli. Mol Microbiol. 1992;6:503–509. doi: 10.1111/j.1365-2958.1992.tb01495.x. [DOI] [PubMed] [Google Scholar]
- 24.Holt SC. Anatomy and chemistry of spirochetes. Microbiol Rev. 1978;42:114–160. doi: 10.1128/mr.42.1.114-160.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mbow ML, Gilmore RD, Jr, Stevenson B, Golde WT, Piesman J, et al. Borrelia burgdorferi-specific monoclonal antibodies derived from mice primed with Lyme disease spirochete-infected Ixodes scapularis ticks. Hybrid Hybridomics. 2002;21:179–182. doi: 10.1089/153685902760173890. [DOI] [PubMed] [Google Scholar]
- 26.Stevenson B, Choy HA, Pinne M, Rotondi ML, Miller MC, et al. Leptospira interrogans endostatin-like outer membrane proteins bind host fibronectin, laminin and regulators of complement. PLoS ONE. 2007;2:e1188. doi: 10.1371/journal.pone.0001188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng Y, Pan X, Sun W, Wang C, Zhang H, et al. Streptococcus suis enolase functions as a protective antigen displayed on the bacterial cell surface. J Infect Dis. 2009;200:1583–1592. doi: 10.1086/644602. [DOI] [PubMed] [Google Scholar]
- 28.Agarwal S, Kulshreshtha P, Bambah Mukku D, Bhatnagar R. alpha-Enolase binds to human plasminogen on the surface of Bacillus anthracis. Biochim Biophys Acta. 2008;1784:986–994. doi: 10.1016/j.bbapap.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 29.Bergmann S, Rohde M, Chhatwal GS, Hammerschmidt S. alpha-Enolase of Streptococcus pneumoniae is a plasmin(ogen)-binding protein displayed on the bacterial cell surface. Mol Microbiol. 2001;40:1273–1287. doi: 10.1046/j.1365-2958.2001.02448.x. [DOI] [PubMed] [Google Scholar]
- 30.Candela M, Biagi E, Centanni M, Turroni S, Vici M, et al. Bifidobacterial enolase, a cell surface receptor for human plasminogen involved in the interaction with the host. Microbiology. 2009;155:3294–3303. doi: 10.1099/mic.0.028795-0. [DOI] [PubMed] [Google Scholar]
- 31.Carneiro CR, Postol E, Nomizo R, Reis LF, Brentani RR. Identification of enolase as a laminin-binding protein on the surface of Staphylococcus aureus. Microbes Infect. 2004;6:604–608. doi: 10.1016/j.micinf.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Hara H, Ohta H, Inoue T, Ohashi T, Takashiba S, et al. Cell surface-associated enolase in Actinobacillus actinomycetemcomitans. Microbiol Immunol. 2000;44:349–356. doi: 10.1111/j.1348-0421.2000.tb02505.x. [DOI] [PubMed] [Google Scholar]
- 33.Sha J, Erova TE, Alyea RA, Wang S, Olano JP, et al. Surface-expressed enolase contributes to the pathogenesis of clinical isolate SSU of Aeromonas hydrophila. J Bacteriol. 2009;191:3095–3107. doi: 10.1128/JB.00005-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moscato S, Pratesi F, Sabbatini A, Chimenti D, Scavuzzo M, et al. Surface expression of a glycolytic enzyme, alpha-enolase, recognized by autoantibodies in connective tissue disorders. Eur J Immunol. 2000;30:3575–3584. doi: 10.1002/1521-4141(200012)30:12<3575::AID-IMMU3575>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 35.Pal-Bhowmick I, Mehta M, Coppens I, Sharma S, Jarori GK. Protective properties and surface localization of Plasmodium falciparum enolase. Infect Immun. 2007;75:5500–5508. doi: 10.1128/IAI.00551-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nowalk AJ, Nolder C, Clifton DR, Carroll JA. Comparative proteome analysis of subcellular fractions from Borrelia burgdorferi by NEPHGE and IPG. Proteomics. 2006;6:2121–2134. doi: 10.1002/pmic.200500187. [DOI] [PubMed] [Google Scholar]
- 37.Jedrzejas MJ. Pneumococcal virulence factors: structure and function. Microbiol Mol Biol Rev. 2001;65:187–207. doi: 10.1128/MMBR.65.2.187-207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guiral S, Mitchell TJ, Martin B, Claverys JP. Competence-programmed predation of noncompetent cells in the human pathogen Streptococcus pneumoniae: genetic requirements. Proc Natl Acad Sci U S A. 2005;102:8710–8715. doi: 10.1073/pnas.0500879102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miles LA, Dahlberg CM, Plescia J, Felez J, Kato K, et al. Role of cell-surface lysines in plasminogen binding to cells: identification of alpha-enolase as a candidate plasminogen receptor. Biochemistry. 1991;30:1682–1691. doi: 10.1021/bi00220a034. [DOI] [PubMed] [Google Scholar]
- 40.Plow EF, Herren T, Redlitz A, Miles LA, Hoover-Plow JL. The cell biology of the plasminogen system. FASEB J. 1995;9:939–945. doi: 10.1096/fasebj.9.10.7615163. [DOI] [PubMed] [Google Scholar]
- 41.Castellino FJ, McCance SG. The kringle domains of human plasminogen. Ciba Found Symp. 1997;212:46–60. doi: 10.1002/9780470515457.ch4. [DOI] [PubMed] [Google Scholar]
- 42.Jeffery CJ. Moonlighting proteins. Trends Biochem Sci. 1999;24:8–11. doi: 10.1016/s0968-0004(98)01335-8. [DOI] [PubMed] [Google Scholar]
- 43.Barbour AG, Jasinskas A, Kayala MA, Davies DH, Steere AC, et al. A genome-wide proteome array reveals a limited set of immunogens in natural infections of humans and white-footed mice with Borrelia burgdorferi. Infect Immun. 2008;76:3374–3389. doi: 10.1128/IAI.00048-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith DR, Chapman MR. Economical evolution: microbes reduce the synthetic cost of extracellular proteins. mBio. 2010;1:1–9. doi: 10.1128/mBio.00131-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eroles P, Sentandreu M, Elorza MV, Sentandreu R. The highly immunogenic enolase and Hsp70p are adventitious Candida albicans cell wall proteins. Microbiology. 1997;143(Pt 2):313–320. doi: 10.1099/00221287-143-2-313. [DOI] [PubMed] [Google Scholar]
- 46.Kunert A, Losse J, Gruszin C, Huhn M, Kaendler K, et al. Immune evasion of the human pathogen Pseudomonas aeruginosa: elongation factor Tuf is a factor H and plasminogen binding protein. J Immunol. 2007;179:2979–2988. doi: 10.4049/jimmunol.179.5.2979. [DOI] [PubMed] [Google Scholar]
- 47.Xolalpa W, Vallecillo AJ, Lara M, Mendoza-Hernandez G, Comini M, et al. Identification of novel bacterial plasminogen-binding proteins in the human pathogen Mycobacterium tuberculosis. Proteomics. 2007;7:3332–3341. doi: 10.1002/pmic.200600876. [DOI] [PubMed] [Google Scholar]
- 48.Fontan PA, Pancholi V, Nociari MM, Fischetti VA. Antibodies to streptococcal surface enolase react with human alpha-enolase: implications in poststreptococcal sequelae. J Infect Dis. 2000;182:1712–1721. doi: 10.1086/317604. [DOI] [PubMed] [Google Scholar]
- 49.Adamus G, Amundson D, Seigel GM, Machnicki M. Anti-enolase-alpha autoantibodies in cancer-associated retinopathy: epitope mapping and cytotoxicity on retinal cells. J Autoimmun. 1998;11:671–677. doi: 10.1006/jaut.1998.0239. [DOI] [PubMed] [Google Scholar]
- 50.Boyle MD, Lottenberg R. Plasminogen activation by invasive human pathogens. Thromb Haemost. 1997;77:1–10. [PubMed] [Google Scholar]
- 51.Bergmann S, Wild D, Diekmann O, Frank R, Bracht D, et al. Identification of a novel plasmin(ogen)-binding motif in surface displayed alpha-enolase of Streptococcus pneumoniae. Mol Microbiol. 2003;49:411–423. doi: 10.1046/j.1365-2958.2003.03557.x. [DOI] [PubMed] [Google Scholar]
- 52.Ehinger S, Schubert WD, Bergmann S, Hammerschmidt S, Heinz DW. Plasmin(ogen)-binding alpha-enolase from Streptococcus pneumoniae: crystal structure and evaluation of plasmin(ogen)-binding sites. J Mol Biol. 2004;343:997–1005. doi: 10.1016/j.jmb.2004.08.088. [DOI] [PubMed] [Google Scholar]
- 53.Knaust A, Weber MV, Hammerschmidt S, Bergmann S, Frosch M, et al. Cytosolic proteins contribute to surface plasminogen recruitment of Neisseria meningitidis. J Bacteriol. 2007;189:3246–3255. doi: 10.1128/JB.01966-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Haile WB, Coleman JL, Benach JL. Reciprocal upregulation of urokinase plasminogen activator and its inhibitor, PAI-2, by Borrelia burgdorferi affects bacterial penetration and host-inflammatory response. Cell Microbiol. 2006;8:1349–1360. doi: 10.1111/j.1462-5822.2006.00717.x. [DOI] [PubMed] [Google Scholar]
- 55.Veiga-Malta I, Duarte M, Dinis M, Tavares D, Videira A, et al. Enolase from Streptococcus sobrinus is an immunosuppressive protein. Cell Microbiol. 2004;6:79–88. doi: 10.1046/j.1462-5822.2003.00344.x. [DOI] [PubMed] [Google Scholar]
- 56.Rooijakkers SH, van Wamel WJ, Ruyken M, van Kessel KP, van Strijp JA. Anti-opsonic properties of staphylokinase. Microbes Infect. 2005;7:476–484. doi: 10.1016/j.micinf.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 57.Brissette CA, Bykowski T, Cooley AE, Bowman A, Stevenson B. Borrelia burgdorferi RevA antigen binds host fibronectin. Infect Immun. 2009;77:2802–2812. doi: 10.1128/IAI.00227-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Probert WS, Johnson BJB. Identification of a 47 kDA fibronectin-binding protein expressed by Borrelia burgdorferi isolate B31. Mol Microbiol. 1998;30:1003–1015. doi: 10.1046/j.1365-2958.1998.01127.x. [DOI] [PubMed] [Google Scholar]
- 59.Seshu J, Esteve-Gassent MD, Labandeira-Rey M, Kim JH, Trzeciakowski JP, et al. Inactivation of the fibronectin-binding adhesin gene bbk32 significantly attenuates the infectivity potential of Borrelia burgdorferi. Mol Microbiol. 2006;59:1591–1601. doi: 10.1111/j.1365-2958.2005.05042.x. [DOI] [PubMed] [Google Scholar]
- 60.Berge A, Sjobring U. PAM, a novel plasminogen-binding protein from Streptococcus pyogenes. J Biol Chem. 1993;268:25417–25424. [PubMed] [Google Scholar]
- 61.Ullberg M, Kronvall G, Karlsson I, Wiman B. Receptors for human plasminogen on gram-negative bacteria. Infect Immun. 1990;58:21–25. doi: 10.1128/iai.58.1.21-25.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verma A, Brissette CA, Bowman AA, Shah ST, Zipfel PF, et al. Leptospiral endostatin-like protein A is a bacterial cell surface receptor for human plasminogen. Infect Immun. 2010;78:2053–2059. doi: 10.1128/IAI.01282-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pal U, Yang X, Chen M, Bockenstedt LK, Anderson JF, et al. OspC facilitates Borrelia burgdorferi invasion of Ixodes scapularis salivary glands. J Clin Invest. 2004;113:220–230. doi: 10.1172/JCI19894. [DOI] [PMC free article] [PubMed] [Google Scholar]