Abstract
Background
The comparative importance of physical inactivity and obesity as predictors of coronary heart disease (CHD) risk remains unsettled.
Methods and Results
We followed 88 393 women, 34 to 59 years of age, in the Nurses’ Health Study from 1980 to 2000. These participants did not have cardiovascular disease and cancer at baseline. We documented 2358 incident major CHD events (including nonfatal myocardial infarction and fatal CHD) during 20 years of follow-up, including 889 cases of fatal CHD and 1469 cases of nonfatal myocardial infarction. In a multivariate model adjusting for cardiovascular risk factors, overweight and obesity were significantly associated with increased risk of CHD, whereas increasing levels of physical activity were associated with a graded reduction in CHD risk (P<0.001). In joint analyses of body mass index (BMI) and physical activity in women who had a healthy weight (BMI, 18.5 to 24.9 kg/m2) and were physically active (exercise ≥3.5 h/wk) as the reference group, the relative risks of CHD were 3.44 (95% confidence interval [CI], 2.81 to 4.21) for women who were obese (BMI ≥30 kg/m2) and sedentary (exercise <1 h/wk), 2.48 (95% CI, 1.84 to 3.34) for women who were active but obese, and 1.48 (95% CI, 1.24 to 1.77) for women who had a healthy weight but were sedentary. In combined analyses of waist-hip ratio and physical activity, both waist-hip ratio and physical activity were significant predictors of CHD, and the highest risk was among women in the lowest category of physical activity and the highest tertile of waist-hip ratio (relative risk=3.03; 95% CI, 1.96 to 4.18). Even a modest weight gain (4 to 10 kg) during adulthood was associated with 27% (95% CI, 12% to 45%) increased risk of CHD compared with women with a stable weight after adjusting for physical activity and other cardiovascular risk factors.
Conclusions
Obesity and physical inactivity independently contribute to the development of CHD in women. These data underscore the importance of both maintaining a healthy weight and regular physical activity in preventing CHD.
Keywords: obesity, women, exercise, coronary disease, epidemiology
Both obesity and physical inactivity are recognized as major risk factors for the development of coronary heart disease (CHD), and modifying these factors is considered an effective lifestyle intervention for CHD prevention.1 However, the relative importance of obesity and physical activity as predictors of CHD risk remains controversial. In a recent report, Wessel et al2 found that among 906 women undergoing coronary angiography or suspected ischemia, self-reported physical fitness scores but not measures of obesity were independently associated with CHD incidence. This study was relatively small, however, and most women had existing coronary disease at baseline. Thus, it is not clear whether the results apply to healthy women. Recently, we reported that body mass index (BMI) and physical activity independently predicted total and cause-specific mortality.3 In the present study, we examined independent and joint associations of physical activity and adiposity measures (BMI, waist circumference, and waist-to-hip ratio [WHR]) with incidence of CHD during 20 years of follow-up in the Nurses’ Health Study (NHS).
Methods
The NHS cohort was established in 1976, when 121 700 female registered nurses, 30 to 55 years of age, completed a mailed questionnaire about their medical history and lifestyle. Women have provided information regarding lifestyle and health conditions biennially since 1976. The 1980 questionnaire asked about weight at 18 years of age; about 80% of the participants provided the information. Diet and physical activity were assessed through the use of validated questionnaires starting from 1980.4 For this study, we included 88 393 women in the analyses after excluding those who reported cardiovascular disease or cancer at baseline in 1980. The study was approved by the Human Research Committees at the Brigham and Women’s Hospital.
Assessment of Overall and Abdominal Adiposity
BMI was calculated as weight in kilograms divided by the square of height in meters (kg/m2) to assess overall obesity. Self-reported weights were validated among 184 participants in the NHS living in the Boston area and were highly correlated with measured weights (r=0.96; mean difference [self-reported − measured weight]=−1.5 kg).5 Recalled weight at age 18 years was also highly correlated (r=0.87; mean difference [recalled − measured weight]=−1.4 kg) with measured weight from the physical examination records for the same age among 118 women from another cohort of female nurses.6
In 1986, NHS participants measured and reported measurements of their waist (at the umbilicus) and hip (the largest circumference) to the nearest quarter of an inch. In a validation study, the correlations between self-reported and technician-measured circumferences were 0.89 for the waist, 0.84 for the hip, and 0.70 for the WHR.7
Assessment of Physical Activity
Physical activity measures were described in detail previously.3 Women were first asked about physical activity on the 1980 questionnaire. They were asked to report the average number of hours spent each week during the past year on moderate (eg, brisk walking) and on vigorous recreational activities (eg, vigorous sports and jogging). A similar question was included in the 1982 questionnaire. In 1986, 1988, 1992, 1996, and 1998, women were asked to report the average time spent per week on the following activities: walking, jogging, running, bicycling, lap swimming, playing tennis or squash, and participating in calisthenics. Using this information, we calculated the average amount of time per week spent in moderate-to-vigorous activities (requiring 3 or more metabolic equivalents per hour, including brisk walking) at each time point.8
Our validation study indicated relatively good validity and reproducibility for the questionnaire. The correlation between physical activity reported on 1-week recalls and that reported on the questionnaire was 0.79. The correlation between moderate to vigorous activity reported in diaries and that reported on the questionnaire was 0.62. In a separate population 20 to 59 years of age, recruited from a university community (n=103), the correlation between the physical activity score on a very similar questionnaire and maximum oxygen consumption was 0.54.9
Assessment of CHD Incidence
The end point for this study was the incidence of CHD (including CHD deaths and nonfatal myocardial infarction [MI] occurring after the return of the 1980 questionnaire but before June 1, 2000). We sought to obtain medical records for all self-reported MIs. Records were reviewed by physicians with no knowledge of the self-reported risk factor status. MI was confirmed according to World Health Organization criteria: symptoms plus either diagnostic ECG changes or elevated cardiac enzymes. Deaths were reported by next of kin and the postal system or ascertained through the National Death Index. Follow-up for the deaths was more than 98% complete.10 Fatal coronary disease was defined as fatal MI if this was confirmed by hospital records or autopsy or if coronary disease was listed as the cause of death on the certificate and this was the underlying and most plausible cause and if evidence of previous coronary disease was available. Sudden death within 1 hour of onset of symptoms in women with no other plausible cause other than coronary disease (8% of fatal CHD) was also included.
Statistical Analysis
We grouped women into 9 categories of BMI measured in 1980, which included standard cutoffs for underweight (BMI <18.5), overweight (BMI ≥25), class 1 obesity (BMI ≥30), class 2 obesity (BMI ≥35), and class 3 obesity (BMI ≥40). We calculated weight change between age 18 and 1980 and grouped women into 5 categories [loss ≥4 kg, stable (weight change <4 kg), gain 4 to 10 kg, gain 10.1 to 19.9 kg, gain 20 to 39.9 kg, gain ≥40 kg]. For all analyses, we excluded women with reported cancer or cardiovascular disease at baseline. Women with cancer who died during follow-up also were excluded. Participants contributed person-time from the date of return of the 1980 (BMI analyses) or 1986 (waist and hip circumference analyses) questionnaires until the date of death or June 1, 2000, whichever came first. The relative risk (RR) was calculated as the rate for a given category of BMI as compared with the referent category. Age-adjusted analyses were conducted through the use of 5-year age categories by the Mantel-Haenszel method.11 Cox proportional hazard regression12 was used to adjust for age and other potential confounders, including smoking, alcohol use, menopausal status/postmenopausal hormone use, aspirin use, and parental history of MI before 60 years of age.
To best represent long-term physical activity levels and to reduce measurement error, we created measures of cumulative average of hours of moderate to vigorous activities from all available questionnaires up to the start of each 2-year follow-up interval.13 Consistent with our previous study,3 physical activity was divided into 3 categories: <1 h/wk, 1 to 3.49 h/wk, and ≥3.5 h/wk. In a secondary analysis, we additionally controlled for a dietary score reflecting higher intakes of the ratio of polyunsaturated fat as opposed to saturated fat, marine omega-3 fatty acids, folate, and cereal fiber and low intakes of trans fat and glycemic load.14 To examine the joint effects of physical activity and abdominal adiposity on CHD, we used 1986 (when waist and hip circumferences were first assessed) as the baseline. In these analyses, waist circumference and the ratio of WHR circumferences were divided into tertiles (cut-points were ≤28 inches, 28.1 to 31.9, and ≥32 for waist circumference and <0.74, 0.74 to 0.79, and ≥0.80 for WHR). Likelihood ratio tests were used to examine statistical interactions between physical activity and overall or abdominal obesity on mortality by comparing −2 log likelihood χ2 between nested models with and without the cross-product terms of physical activity and obesity measures. Additionally, to assess the effect of recent BMI with risk of CHD and to account for latency of the disease, we did an analysis by using updated BMI every 4 years as main exposure.
The population-attributable risk conferred by being overweight or less than optimally physical active (<3.5 h/wk of moderate to vigorous physical activity) was calculated to estimate the percentage of CHD in our cohort that would theoretically not have occurred if all women had been in the low-risk group (not overweight and engaged in regular exercise), assuming a causal relation between the risk factors and CHD.15 Statistical analyses were conducted with the use of SAS, version 8.2. All probability values were 2-sided.
Results
During 20 years of follow-up, 2358 incident cases of CHD were identified, including 889 cases of fatal CHD and 1469 cases of nonfatal MI. Table 1 shows RRs of CHD according to baseline BMI categories in 1980. As compared with women with BMI 18.5 and 22.9, those with BMI ≥40 had 5-fold increased risk of CHD. The risk of CHD increased monotonically with increasing BMI. Further adjustment of dietary score did not change the association. For each 1 unit of BMI increment, risk of CHD was increased by 8% (95% CI, 7% to 9%). In the analyses stratified by smoking, the association remained J-shaped for past smokers. A positive association between BMI and risk of CHD emerged for never-smokers and current smokers. A secondary analysis using updated BMI with a 4-year lag between BMI measurements and CHD events showed similar results; the multivariate RRs for the top 4 categories of BMI were 1.96(1.69 to 2.27) for BMI 30 to 32.9, 2.24 (95% CI, 1.83 to 2.75) for BMI 33 to 34.9, 2.63 (95% CI, 2.19 to 3.16) for BMI 35 to 39, and 3.22 (95% CI, 2.53 to 4.10) for BMI ≥40 compared with BMI 18.5 to 22.9 (P for trend <0.0001). In multivariate analysis adjusting for history of hypertension, high cholesterol, and diabetes, the association between CHD risk and increasing BMI was somewhat attenuated (the RRs across categories of BMI in Table 1 were 1.04 [0.74 to 1.46], 1.0 [ref], 1.19 [1.06 to 1.35], 1.17 [1.02 to 1.34], 1.66 [1.46 to 1.89], 1.78 [1.53 to 2.08], 1.90 [1.52 to 2.37], 2.25 [1.86 to 2.73], and 2.48 [1.91 to 3.22], P for trend <0.001).
TABLE 1.
Body Mass Index and Relative Risk of CHD Among Women Who Were 39 to 61 Years of Age in 1980 and Were Followed From 1980 Through 2000 in the Nurses’ Health Study
| Body Mass Index (kg/m2)
|
P for Trend | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <18.5 | 18.5 to 22.9 | 23 to 24.9 | 25 to 26.9 | 27 to 29.9 | 30 to 32.9 | 33 to 34.9 | 35 to 39.9 | ≥40 | ||
| All participants | ||||||||||
| Total CHD | ||||||||||
| No. of cases | 35 | 659 | 430 | 290 | 401 | 240 | 94 | 141 | 68 | |
| Person-years | 33 970 | 755 961 | 343 355 | 206 294 | 178 246 | 92 561 | 30 736 | 36 802 | 13 959 | |
| Incidence (/100 000 PY) | 103 | 87 | 125 | 141 | 225 | 259 | 306 | 383 | 487 | |
| Age-adjusted RR (95% CI) | 1.27 (0.90–1.78) | 1.00 | 1.24 (1.10–1.40) | 1.31 (1.14–1.51) | 2.12 (1.87–2.40) | 2.47 (2.13– 2.87) | 3.07 (2.47– 3.81) | 3.99 (3.32– 4.78) | 5.10 (3.97–6.55) | <0.001 |
| Multivariate RR (95% CI)* | 1.05 (0.74–1.47) | 1.00 | 1.25 (1.10– 1.41) | 1.28 (1.11–1.47) | 2.01 (1.77–2.28) | 2.36 (2.03–2.74) | 2.76 (2.22–3.43) | 3.59 (2.98–4.33) | 4.56 (3.54–5.88) | <0.001 |
| Women who never smoked | ||||||||||
| No. of cases | 3 | 132 | 127 | 94 | 119 | 86 | 42 | 55 | 27 | |
| Person-years | 12 470 | 315 264 | 151 793 | 94 336 | 83 940 | 45 904 | 15 303 | 18 275 | 6767 | |
| Incidence (/100 000 PY) | 24 | 42 | 84 | 100 | 142 | 187 | 274 | 301 | 399 | |
| Age-adjusted RR (95% CI) | 0.65 (0.21–2.03) | 1.00 | 1.63 (1.28–2.08) | 1.82 (1.40–2.38) | 2.60 (2.03–3.33) | 3.47 (2.65–4.56) | 5.35 (3.78–7.58) | 6.24 (4.55–8.55) | 8.23 (5.43–12.45) | <0.001 |
| Multivariate RR (95% CI)† | 0.63 (0.20–1.98) | 1.00 | 1.55 (1.22–1.99) | 1.66 (1.27–2.16) | 2.24 (1.74–2.88) | 2.89 (2.19–3.82) | 4.17 (2.93–5.94) | 4.75 (3.44–6.56) | 6.30 (4.13–9.60) | <0.001 |
| Past smokers | ||||||||||
| No. of cases | 19 | 234 | 155 | 98 | 137 | 88 | 36 | 56 | 29 | |
| Person-years | 10 523 | 272 721 | 124 772 | 74 019 | 62 456 | 33 028 | 10 923 | 13 459 | 5249 | |
| Incidence (/100 000 PY) | 181 | 86 | 124 | 132 | 219 | 266 | 330 | 416 | 553 | |
| Age-adjusted RR (95% CI) | 2.16 (1.35–3.44) | 1.00 | 1.26 (1.03–1.54) | 1.25 (0.99–1.59) | 2.12 (1.72–2.62) | 2.64 (2.06–3.37) | 3.48 (2.45–4.95) | 4.46 (3.33–5.97) | 6.10 (4.15–8.97) | <0.001 |
| Multivariate RR (95% CI)† | 1.99 (1.25–3.18) | 1.00 | 1.18 (0.96–1.45) | 1.13 (0.89–1.43) | 1.78 (1.43–2.20) | 2.09 (1.63–2.68) | 2.58 (1.81–3.68) | 3.24 (2.40–4.36) | 4.26 (2.87–6.30) | <0.001 |
| Current smokers | ||||||||||
| No. of cases | 11 | 284 | 141 | 97 | 138 | 65 | 15 | 29 | 12 | |
| Person years | 10 572 | 161 558 | 64 480 | 36 334 | 30 476 | 12 960 | 4284 | 4829 | 1800 | |
| Incidence (/100 000 PY) | 104 | 176 | 219 | 267 | 453 | 502 | 350 | 600 | 667 | |
| Age-adjusted RR (95% CI) | 0.61 (0.33–1.12) | 1.00 | 1.17 (0.96–1.44) | 1.36 (1.08–1.71) | 2.37 (1.93–2.90) | 2.76 (2.11– 3.62) | 2.01 (1.19–3.37) | 3.70 (2.53–5.43) | 4.11 (2.31–7.33) | <0.001 |
| Multivariate RR (95% CI)‡ | 0.58 (0.32–1.05) | 1.00 | 1.12 (0.91–1.37) | 1.24 (0.98–1.56) | 2.08 (1.70–2.56) | 2.33 (1.78–3.07) | 1.59 (0.94–2.68) | 2.98 (2.02–4.39) | 3.23 (1.80–5.78) | <0.001 |
Adjusted for age (<50, 50 to 54, 55 to 59, 60 to 64, ≥65), smoking status (never, past, current [1 to 14, 15 to 24, ≥25 cigarettes/d], adjusted for the analyses for all women only), parental history of coronary heart disease, and postmenopausal status and hormone use (never use, past, current), physical activity (5 categories), alcohol consumption (0, 0.1 to 4.9, 5 to 14.9, ≥15 g/d), and aspirin use (<1, 1 to 2, 3 to 6, 7 to 14, 15+/wk).
Adjusted the same as *, except for smoking status.
The number of smoked cigarettes [1 to 14, 15 to 24, ≥25 cigarettes/d] was controlled for current smokers.
As compared with women of normal weight, overweight women had an RR of CHD of 1.43 (95% CI, 1.26 to 1.63) and obese women had an RR of 2.44 (95% CI, 2.17 to 2.74) (Table 2). This association was stronger among never-smokers than among current smokers. Underweight was not significantly associated with risk except among past smokers. Lower physical activity levels were associated with a graded increased risk in CHD (P for trend <0.001). Further adjustment for BMI somewhat attenuated the inverse association, but the association remained significant. This association was slightly stronger among never-smokers and past smokers than current smokers (Table 3).
TABLE 2.
Multivariate Relative Risks of CHD According to Categories of BMI in 1980
| BMI (kg/m2)* |
||||
|---|---|---|---|---|
| <18.5 | 18.5–24.9 | 25–29.9 | ≥30 | |
| All participants | 1.01 (0.71, 1.44) | 1.00 | 1.43 (1.28, 1.59) | 2.44 (2.17, 2.74) |
| Women who never smoked | 0.60 (0.19, 1.87) | 1.00 | 1.51 (1.24, 1.85) | 2.83 (2.30, 3.48) |
| Past smokers | 1.85 (1.13, 3.01) | 1.00 | 1.27 (1.06, 1.51) | 2.20 (1.82, 2.67) |
| Current smokers | 0.60 (0.32, 1.12) | 1.00 | 1.55 (1.30, 1.84) | 2.28 (1.83, 2.85) |
Multivariate analyses were adjusted for age (<50, 50 to 54, 55 to 59, 60 to 64, ≥65), smoking status (never, past, current [1 to 14, 15 to 24, ≥25 cigarettes/d], adjusted for the analyses for all women only), parental history of coronary heart disease, and postmenopausal status and hormone use (never use, past, current), alcohol consumption (0, 0.1 to 4.9, 5 to 14.9, ≥15 g/d), and aspirin use (<1, 1 to 2, 3 to 6, 7 to 14, 15+/wk). The number of smoked cigarettes [1 to 14, 15 to 24, ≥25 cigarettes/d] was controlled for current smokers. P for trend <0.001.
For analyses of BMI, we also controlled for quintiles of physical activity (defined as moderate to vigorous activities including brisk walking).
BMI was adjusted in 3 categories (<25, 25 to 29.9, ≥30 kg/m2).
TABLE 3.
Multivariate Relative Risks of CHD According to Categories of Updated Physical Activity Levels, Nurses’ Health Study, 1980 to 2000
| Physical Activity (h/wk)
|
P | |||
|---|---|---|---|---|
| ≥3.5 | 1–3.49 | <1 | ||
| All participants | ||||
| Multivariate RR without BMI | 1.00 | 1.43 (1.27, 1.61) | 1.58 (1.39, 1.80) | <0.001 |
| Multivariate RR with BMI* | 1.00 | 1.34 (1.18, 1.51) | 1.43 (1.26, 1.63) | <0.001 |
| Women who never smoked | ||||
| Multivariate RR without BMI | 1.00 | 1.39 (1.11, 1.74) | 1.75 (1.38, 2.21) | <0.001 |
| Multivariate RR with BMI | 1.00 | 1.28 (1.02, 1.60) | 1.51 (1.19, 1.92) | <0.001 |
| Past smokers | ||||
| Multivariate RR without BMI | 1.00 | 1.47 (1.20, 1.79) | 1.79 (1.44, 2.21) | 0.001 |
| Multivariate RR with BMI | 1.00 | 1.37 (1.12, 1.68) | 1.63 (1.32, 2.02) | <0.001 |
| Current smokers | ||||
| Multivariate RR without BMI | 1.00 | 1.43 (1.16, 1.76) | 1.31 (1.05, 1.65) | 0.009 |
| Multivariate RR with BMI | 1.00 | 1.38 (1.12, 1.70) | 1.24 (0.99, 1.56) | 0.04 |
Multivariate analyses were adjusted for age (<50, 50 to 54, 55 to 59, 60 to 64, ≥65), smoking status (never, past, current [1 to 14, 15 to 24, ≥25 cigarettes/d], adjusted for the analyses for all women only), parental history of coronary heart disease, and postmenopausal status and hormone use (never use, past, current), alcohol consumption (0, 0.1 to 4.9, 5 to 14.9, ≥15 g/d), and aspirin use (<1, 1 to 2, 3 to 6, 7 to 14, 15+/wk). The number of smoked cigarettes [1 to 14, 15 to 24, ≥25 cigarettes/d] was controlled for current smokers.
BMI was adjusted in 3 categories (<25, 25 to 29.9, ≥30 kg/m2).
In Table 4, we present joint associations of BMI and physical activity with risk of CHD. Both increasing BMI and decreasing physical activity levels were associated with risk of CHD. Obese women who were sedentary had the highest CHD risk (RR=3.44; 95% CI, 2.81 to 4.21; P for interaction between BMI and physical activity=0.98). Cigarette smoking in combination with obesity and physical inactivity dramatically increased the risk of CHD (multivariate RR for obese sedentary smokers versus normal-weight active nonsmokers=9.37; 95% CI, 6.45,13.60).
TABLE 4.
Multivariate Relative Risks of CHD According to Categories of BMI and Physical Activity, Nurses’ Health Study, 1980 to 2000
| Physical Activity (h/wk)
|
|||
|---|---|---|---|
| ≥3.5 | 1–3.5 | <1 | |
| All participants | |||
| BMI 18.5–24.9 | |||
| No. of cases | 218 | 447 | 279 |
| Person-years | 331 900 | 427 647 | 198 547 |
| RR | 1.00 (reference) | 1.32 (1.12, 1.56) | 1.48 (1.24, 1.77) |
| BMI 25–30 | |||
| No. of cases | 100 | 285 | 185 |
| Person-years | 87 448 | 157 727 | 83 957 |
| RR | 1.43 (1.13, 1.82) | 1.92 (1.61, 2.30) | 2.04 (1.67, 2.48) |
| BMI ≥30 | |||
| No. of cases | 55 | 220 | 175 |
| Person-years | 29 454 | 72 800 | 48 075 |
| RR | 2.48 (1.84, 3.34) | 3.32 (2.74, 4.01) | 3.44 (2.81, 4.21) |
| Never smokers | |||
| BMI 18.5–24.9 | |||
| No. of cases | 58 | 103 | 64 |
| Person-years | 140 788 | 183 956 | 81 942 |
| RR | 1.00 (reference) | 1.19 (0.86, 1.65) | 1.50 (1.05, 2.15) |
| BMI 25–30 | |||
| No. of cases | 28 | 81 | 65 |
| Person-years | 40 812 | 73 016 | 38 545 |
| RR | 1.29 (0.82, 2.03) | 1.80 (1.28, 2.53) | 2.44 (1.71, 3.49) |
| BMI ≥30 | |||
| No. of cases | 23 | 83 | 64 |
| Person-years | 14 757 | 35 946 | 23 822 |
| RR | 2.88 (1.77, 4.69) | 3.75 (2.67, 5.28) | 3.67 (2.56, 5.28) |
| Past smokers | |||
| BMI 18.5–24.9 | |||
| No. of cases | 71 | 163 | 108 |
| Person-years | 127 321 | 152 298 | 67 802 |
| RR | 1.00 (reference) | 1.59 (1.20, 2.10) | 2.03 (1.50, 2.75) |
| BMI 25–30 | |||
| No. of cases | 43 | 92 | 59 |
| Person-years | 31 276 | 56 116 | 29 325 |
| RR | 1.85 (1.27, 2.71) | 1.94 (1.42, 2.65) | 2.11 (1.49, 2.99) |
| BMI ≥30 | |||
| No. of cases | 20 | 83 | 68 |
| Person-years | 10 322 | 26 698 | 16 962 |
| RR | 2.73 (1.65, 4.49) | 3.42 (2.48, 4.72) | 4.07 (2.90, 5.71) |
| Current smokers | |||
| BMI <25 | |||
| No. of cases | 81 | 176 | 104 |
| Person-years | 60 701 | 88 553 | 47 168 |
| RR | 1.00 (reference) | 1.24 (0.95, 1.61) | 1.16 (0.87, 1.56) |
| BMI 25–30 | |||
| No. of cases | 29 | 109 | 59 |
| Person-years | 14 727 | 27 431 | 15 392 |
| RR | 1.28 (0.83, 1.95) | 2.08 (1.55, 2.78) | 1.74 (1.24, 2.45) |
| BMI ≥30 | |||
| No. of cases | 12 | 53 | 41 |
| Person-years | 4209 | 9644 | 6889 |
| RR | 1.88 (1.03, 3.46) | 2.96 (2.08, 4.20) | 2.67 (1.82, 3.92) |
The number of smoked cigarettes (1 to 14, 15 to 24, ≥25 cigarettes/d) was controlled for current smokers.
Adjusted for age (<50, 50 to 54, 55 to 59, 60 to 64, ≥65), smoking status (never, past, current 1 to 14, 15 to 24, ≥25 cigarettes/d), parental history of CHD, postmenopausal status and hormone use (never use, past, current), aspirin use (<1, 1 to 2, 3 to 6, 7 to 14, 15+/wk), and alcohol consumption (0, 0.1 to 4.9, 5 to 14.9, ≥15 g/d).
Table 5 shows RRs of CHD according to categories of weight change from age 18 through 1980. Compared with women with a stable weight (gain or lose <4 kg), women with even a modest weight gain (4 to 10 kg) had a significantly increased risk of CHD from 1980 to 2000 (RR=1.27; 95% CI, 1.12 to 1.45). Those women who gained ≥40 kg had an RR of 3.86 (95% CI, 3.02 to 4.94) of developing CHD. The association between weight gain and CHD was persistent among both sedentary and active women (P for interaction between physical activity and weight change=0.79).
TABLE 5.
Weight Change Since the Age of 18 Through 1980 and Relative Risk of CHD According to Physical Activity Levels, Nurses’ Health Study, 1980 to 2000
| Loss ≥4 kg | Stable | Gain 4 to 10 kg | Gain 10.1 to 19.9 kg | Gain 20 to 39.9 kg | Gain ≥40 kg | P for Trend | |
|---|---|---|---|---|---|---|---|
| All participants | |||||||
| No. of cases | 181 | 407 | 581 | 631 | 456 | 78 | |
| Person-years | 133 751 | 465 754 | 508 478 | 376 781 | 174 487 | 17 745 | |
| Age-adjusted RR | 1.49 (1.25, 1.77) | 1.00 | 1.20 (1.06, 1.36) | 1.56 (1.37, 1.76) | 2.38 (2.08, 2.72) | 4.28 (3.36, 5.46) | <0.001 |
| Multivariate RR* | 0.94 (0.78, 1.13) | 1.00 | 1.27 (1.12, 1.45) | 1.65 (1.45, 1.87) | 2.33 (2.03, 2.67) | 3.86 (3.02, 4.94) | <0.001 |
| Stratified by physical activity(h/wk) | |||||||
| <3.5 | 0.92 (0.75, 1.13) | 1.00 | 1.26 (1.09, 1.45) | 1.59 (1.38, 1.82) | 2.27 (1.96, 2.64) | 3.48 (2.66, 4.54) | <0.001 |
| ≥3.5 | 1.07 (0.69, 1.64) | 1.00 | 1.33 (0.99, 1.78) | 1.97 (1.46, 2.65) | 2.62 (1.83, 3.75) | 8.93 (4.69, 16.99) | <0.001 |
Multivariate analyses was adjusted for age (<50, 50 to 54, 55 to 59, 60 to 64, ≥65), BMI at age of 18, smoking status (never, past, current [1 to 14, 15 to 24,
≥25 cigarettes/d], adjusted for the analyses for all women only), parental history of coronary heart disease, postmenopausal status and hormone use (never use, past, current), physical activity (5 categories), aspirin use (<1, 1 to 2, 3 to 6, 7 to 14, 15+/wk), and alcohol consumption (0, 0.1 to 4.9, 5 to 14.9, ≥15 g/d). Physical activity and alcohol consumption were adjusted using 1980 data.
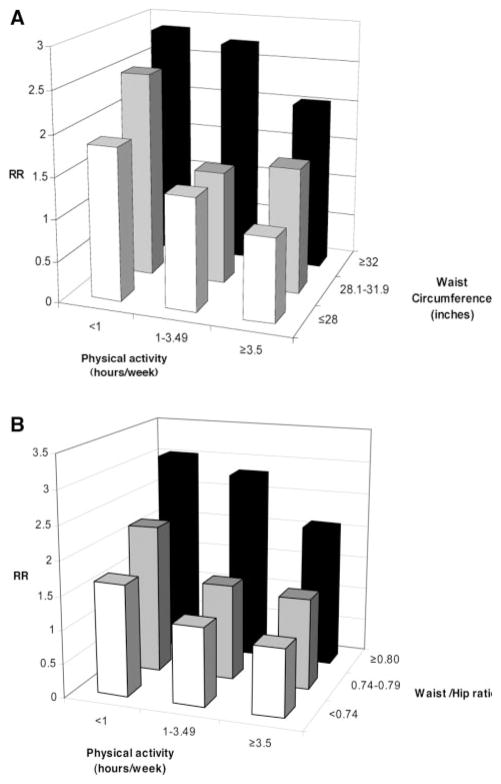
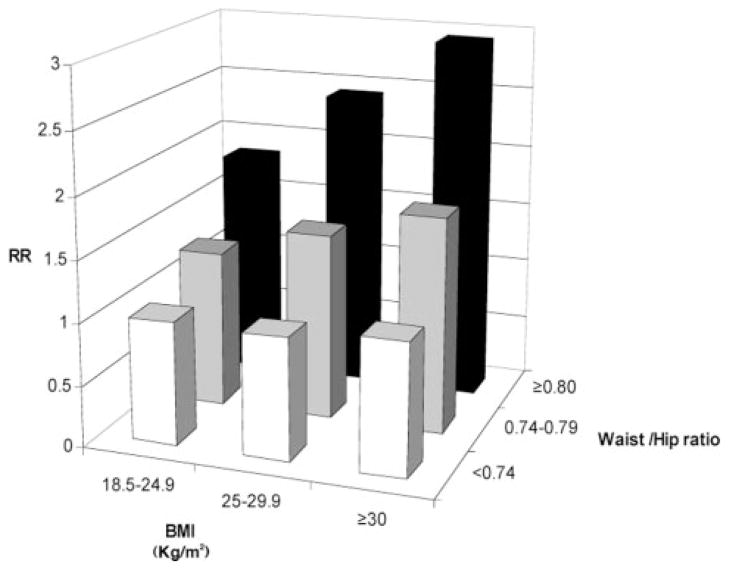
In joint analyses of physical activity and abdominal adiposity, we excluded underweight participants due to the small number of cases in the underweight category. The highest risk of CHD was among women in the lowest category of physical activity and the highest tertile of waist circumference (RR=2.85; 95% CI, 1.84 to 4.43) or WHR (RR=3.03; 95% CI, 1.96 to 4.68) (Figure 1). The associations of physical activity and abdominal obesity with CHD were independent of each other (P for interaction=0.13 between physical activity and waist circumference; P for interaction=0.48 between physical activity and WHR). In a model adjusted for physical activity levels and cardiovascular risk factors, the associations of WHR and BMI with CHD were also independent of each other (Figure 2). Compared with women with BMI 18.5 to 24.9 and in the lowest tertile of WHR, women with BMI ≥30 and in the highest tertile of WHR had a RR of 2.94 (95% CI, 2.21 to 3.90) for developing CHD (P for interaction=0.74). To rule out residual confounding, we controlled for continuous BMI and quadratic term of BMI, and the results of joint effect of physical activity with both waist circumference and WHR did not appreciably change.
Figure 1.
A, Joint associations of waist circumference and physical activity with CHD, the Nurses’ Health Study 1986 to 2000. *Adjusted for age (<50, 50 to 54, 55 to 59, 60 to 64, ≥65), parental history of CHD, postmenopausal status and hormone use (never-use, past, current), physical activity (5 categories), aspirin use (<1, 1 to 2, 3 to 6, 7 to 14, 15+/wk), BMI (<25, 25 to 29.9, ≥30 kg/m2), and alcohol consumption (0, 0.1 to 4.9, 5 to 14.9, ≥15 g/d). B, Joint associations of WHR and physical activity with CHD, the Nurses’ Health Study 1986 to 2000. *Adjusted for age (<50, 50 to 54, 55 to 59, 60 to 64, ≥65), parental history of CHD, postmenopausal status and hormone use (never-use, past, current), physical activity (5 categories), aspirin use (<1, 1 to 2, 3 to 6, 7 to 14, 15+/wk), BMI (<25, 25 to 29.9, ≥30 kg/m2), and alcohol consumption (0, 0.1 to 4.9, 5 to 14.9, ≥15 g/d).
Figure 2.
Joint associations of WHR and BMI and CHD, the Nurses’ Health Study 1986 to 2000. *Adjusted for age (<50, 50 to 54, 55 to 59, 60 to 64, ≥65), parental history of CHD, post-menopausal status and hormone use (never-use, past, current), physical activity (5 categories), aspirin use (<1, 1 to 2, 3 to 6, 7 to 14, 15+/wk), and alcohol consumption (0, 0.1 to 4.9, 5 to 14.9, ≥15 g/d).
The prevalence of women with either BMI ≥25 or physical activity less than 3.5 hours per week in the cohort was 82% among all participants and 83% among never-smokers as of 1990. Overweight and physical inactivity together could account for approximately 32% of total CHD in the overall cohort (95% CI, 23% to 40%) and 46% among never-smokers (95% CI, 29% to 58%).
Discussion
In this large cohort study of middle-aged women, physical activity and adiposity independently predicted risk of CHD. Being physically active moderately attenuated but did not eliminate the adverse effect of obesity on coronary health, and being lean did not counteract the increased risk associated with physical inactivity. The lowest risk of CHD was observed among physically active, lean women. Weight gain during adulthood was associated with a significantly increased risk of CHD, independent of physical activity levels.
These results are consistent with and extend our recent findings on total mortality and cardiovascular mortality.3 Although mortality is a commonly used and appealing end point, the studies of BMI and mortality are particularly susceptible to reverse causation (eg, lower BMI may be caused by underlying disease) and confounding by smoking.16 Thus, it is equally important to study incidence of chronic diseases as end points. In our previous analyses,3 the linear relation between BMI and mortality became evident only among never-smokers, whereas in the present study, the linear relation between BMI and incident CHD events was demonstrated in never-smokers and current smokers, although a J-shaped relation was evident among past smokers. This may be due to residual confounding by different durations of past smoking and smoking cessation among past smokers that were controlled for in the analyses. In our primary analyses, we did not control for history of hypertension, high cholesterol, and diabetes because these variables are on the biological pathway between obesity and CHD.16 As expected, the association between BMI and CHD risk was somewhat attenuated after adjustment for these variables but remained statistically significant.
Only a few previous studies have compared the relations of physical activity or “fitness” and obesity with cardiovascular disease risk. In an 8-year follow-up of 21 925 men, 30 to 83 years of age, in the Aerobics Center Longitudinal Study, Lee et al17 found that being physically fit completely abrogated excess total and cardiovascular mortality associated with body fatness. Moreover, lean men with lower cardiorespiratory fitness actually had a higher risk of all-cause and CVD mortality than did men who had higher overall or abdominal fat mass but had higher physical fitness. However, this study included only 428 deaths. More recently, Stevens et al18 examined joint effects of fitness and fatness on mortality among 2506 women and 2860 men in the Lipid Research Clinics Study with more than 22 years of follow-up. In contrast to the findings from the Aerobics Center Longitudinal Study, both fitness and fatness predicted total and CVD mortality, and being physically fit did not eliminate the association between obesity and excess mortality. However, neither study included nonfatal MI in the end point assessment.
Recently, Wessel et al2 reported that among 906 women undergoing coronary angiography for suspected ischemia, self-reported physical fitness instead of lower BMI was significantly associated with lower incidence of coronary artery disease. Besides its relatively small sample size and short follow-up (4 years), it is not clear whether the results are generalizable to healthy women. In that study, many women already had coronary disease at baseline, which might have inhibited exercise. Although the study controlled for a cardiovascular disease severity score, residual confounding remains a potential explanation for the observations. In our study, we excluded women who reported cardiovascular disease or cancer at baseline to avoid the impact of these diseases on weight or physical activity levels. Our results are consistent with findings from a recent study of 18 892 Finnish men and women, 25 to 74 years of age without a history of cardiovascular disease at baseline, in which adiposity and physical activity independently predicted incidence of CHD during 10 years of follow-up and physical activity attenuated in part excess risk caused by obesity.19
In the present study, we did not assess cardiorespiratory fitness. However, physical activity is the primary modifiable determinant of fitness, and even modest levels of physical activity (eg, 30 min/d brisk walking) can achieve levels of cardiorespiratory fitness that have been associated with a significant reduction in mortality risk.20 Moreover, activity is the relevant modifiable behavior. Thus, our analyses of the combined effects of physical activity and obesity have direct public health implications. In our study, the adverse effects of body fatness on CHD risk were persistent in both lower and higher physical activity categories. Conversely, the benefits of physical activity were not limited to lean women; among those who were overweight and obese, physically active women tended to have lower CHD risk as compared with sedentary women. The combination of physical inactivity, obesity, and smoking dramatically increased risk of CHD in women, but increasing exercise would also be beneficial even among obese smokers.
Although our study suggests that obesity confers a greater magnitude of coronary risk than does physical inactivity, limitations of our physical activity assessment should be considered. Measurement errors in self-reported physical activity are inevitable, and nondifferential mis-classification might have biased the association of physical activity with risk of CHD toward the null. However, this should not substantially affect the analyses stratified according to physical activity levels. Our validation studies using physical activity diaries indicated good reproducibility and validity of self-reported physical activity. Moreover, physical activity was assessed periodically during follow-up, and the use of the repeated measures in the analyses not only dampened measurement errors but also took into account real changes in physical activity levels over time. Because our study population is primarily white and is composed of registered nurses, the relative homogeneity of the cohort with respect to socioeconomic status and education reduces confounding and enhances internal validity. On the other hand, our results may not apply to other ethnic groups.
Conclusions
Our study indicates that BMI, WHR, and physical inactivity are independently associated with risk of CHD. The present study shows that the beneficial effects of physical activity on CHD do not appear to be enough to offset the deleterious effects of obesity. Therefore, the avoidance of weight gain and participation in regular physical activity for the prevention of CHD should both be emphasized.
Acknowledgments
This study was supported by research grants HL-24074, HL-34594, P30-DK46200, and CA-87969 from the National Institutes of Health. Dr Hu is a recipient of the American Heart Association Established Investigator Award.
Footnotes
Disclosures
None.
References
- 1.Mosca L, Appel LJ, Benjamin EJ, Berra K, Chandra-Strobos N, Fabunmi RP, Grady D, Haan CK, Hayes SN, Judelson DR, Keenan NL, McBride P, Oparil S, Ouyang P, Oz MC, Mendelsohn ME, Pasternak RC, Pinn VW, Robertson RM, Schenck-Gustafsson K, Sila CA, Smith SC, Jr, Sopko G, Taylor AL, Walsh BW, Wenger NK, Williams CL. Evidence-based guidelines for cardiovascular disease prevention in women. Arterioscler Thromb Vasc Biol. 2004;24:e29–e50. doi: 10.1161/01.ATV.0000114834.85476.81. [DOI] [PubMed] [Google Scholar]
- 2.Wessel TR, Arant CB, Olson MB, Johnson BD, Reis SE, Sharaf BL, Shaw LJ, Handberg E, Sopko G, Kelsey SF, Pepine CJ, Merz NB. Relationship of physical fitness vs body mass index with coronary artery disease and cardiovascular events in women. JAMA. 2004;292:1179–1187. doi: 10.1001/jama.292.10.1179. [DOI] [PubMed] [Google Scholar]
- 3.Hu FB, Willett WC, Li T, Stampfer MJ, Colditz GA, Manson JE. Adiposity as compared with physical activity in predicting mortality among women. N Engl J Med. 2004;351:2694–2703. doi: 10.1056/NEJMoa042135. [DOI] [PubMed] [Google Scholar]
- 4.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, Speizer FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 5.Willett W, Stampfer MJ, Bain C, Lipnick R, Speizer FE, Rosner B, Cramer D, Hennekens CH. Cigarette smoking, relative weight, and menopause. Am J Epidemiol. 1983;117:651–658. doi: 10.1093/oxfordjournals.aje.a113598. [DOI] [PubMed] [Google Scholar]
- 6.Troy LM, Hunter DJ, Manson JE, Colditz GA, Stampfer MJ, Willett WC. The validity of recalled weight among younger women. Int J Obes Relat Metab Disord. 1995;19:570–572. [PubMed] [Google Scholar]
- 7.Rimm EB, Stampfer MJ, Colditz GA, Chute CG, Litin LB, Willett WC. Validity of self-reported waist and hip circumferences in men and women. Epidemiology. 1990;1:466–473. doi: 10.1097/00001648-199011000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Rockhill B, Willett WC, Manson JE, Leitzmann MF, Stampfer MJ, Hunter DJ, Colditz GA. Physical activity and mortality: a prospective study among women. Am J Public Health. 2001;91:578–583. doi: 10.2105/ajph.91.4.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobs DR, Jr, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical activity questionnaires. Med Sci Sports Exerc. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 10.Hu FB, Cho E, Rexrode KM, Albert CM, Manson JE. Fish and long-chain omega-3 fatty acid intake and risk of coronary heart disease and total mortality in diabetic women. Circulation. 2003;107:1852–1857. doi: 10.1161/01.CIR.0000062644.42133.5F. [DOI] [PubMed] [Google Scholar]
- 11.Mantel N. Chi-square tests with one degree of freedom: extensions of the Mantel-Haenszel procedure. J Am Stat Assoc. 1963;58:690–700. [Google Scholar]
- 12.Cox DR, Oakes D. Analysis of Survival Data. London: Chapman and Hall; 1987. [Google Scholar]
- 13.Hu FB, Stampfer MJ, Colditz GA, Ascherio A, Rexrode KM, Willett WC, Manson JE. Physical activity and risk of stroke in women. JAMA. 2000;283:2961–2967. doi: 10.1001/jama.283.22.2961. [DOI] [PubMed] [Google Scholar]
- 14.Stampfer MJ, Hu FB, Manson JE, Rimm EB, Willett WC. Primary prevention of coronary heart disease in women through diet and lifestyle. N Engl J Med. 2000;343:16–22. doi: 10.1056/NEJM200007063430103. [DOI] [PubMed] [Google Scholar]
- 15.Rothman KJ, Greenland S. Modern Epidemiology. Philadelphia, Pa: Lippincott-Raven; 1998. [Google Scholar]
- 16.Manson JE, Willett WC, Stampfer MJ, Colditz GA, Hunter DJ, Hankinson SE, Hennekens CH, Speizer FE. Body weight and mortality among women. N Engl J Med. 1995;333:677–685. doi: 10.1056/NEJM199509143331101. [DOI] [PubMed] [Google Scholar]
- 17.Lee CD, Blair SN, Jackson AS. Cardiorespiratory fitness, body composition, and all-cause and cardiovascular disease mortality in men. Am J Clin Nutr. 1999;69:373–380. doi: 10.1093/ajcn/69.3.373. [DOI] [PubMed] [Google Scholar]
- 18.Stevens J, Cai J, Evenson KR, Thomas R. Fitness and fatness as predictors of mortality from all causes and from cardiovascular disease in men and women in the lipid research clinics study. Am J Epidemiol. 2002;156:832–841. doi: 10.1093/aje/kwf114. [DOI] [PubMed] [Google Scholar]
- 19.Hu G, Tuomilehto J, Silventoinen K, Barengo N, Jousilahti P. Joint effects of physical activity, body mass index, waist circumference and waist-to-hip ratio with the risk of cardiovascular disease among middle-aged Finnish men and women. Eur Heart J. 2004;25:2212–2219. doi: 10.1016/j.ehj.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 20.Stofan JR, DiPietro L, Davis D, Kohl HW, III, Blair SN. Physical activity patterns associated with cardiorespiratory fitness and reduced mortality: the Aerobics Center Longitudinal Study. Am J Public Health. 1998;88:1807–1813. doi: 10.2105/ajph.88.12.1807. [DOI] [PMC free article] [PubMed] [Google Scholar]