Abstract
Insects produce a variety of antimicrobial peptides (AMPs). Induction of insect AMP genes is regulated by the Toll and IMD (immune deficiency) pathways via NF-κB and GATA factors. Little is known about species-specific regulation of AMP genes. In this report, we showed that activities of most Manduca sexta and Drosophila melanogaster AMP gene promoters were regulated in a species-specific manner in Drosophila (Dipteran) S2 cells and Spodoptera frugiperda (Lepidopteran) Sf9 cells. A κB-GATA element (22bp) from M. sexta moricin (MsMoricin) promoter could significantly increase activities of Drosophila AMP gene promoters in S2 cells, and an MsMoricin promoter activating element (MPAE) (140bp) could increase activity of drosomycin promoter specifically in Sf9 cells. However, κB and GATA factors alone were not sufficient for MsMoricin gene activation, suggesting that other co-regulators may be required to fully activate AMP genes. Our results suggest that induction of insect AMP genes may require a transcription complex composed of common nuclear factors (such as NF-κB and GATA factors) and species-related co-regulators, and it is the co-regulators that may confer species-specific regulation of AMP genes. In addition, we showed that activity of Drosophila drosomycin promoter could be activated cooperatively by the inserted exogenous κB-GATA element and the endogenous κB element. These findings revealed an approach of engineering AMP genes with enhanced activities, which may lead to broad applications.
Keywords: NF-κB, GATA, Drosophila melanogaster, Manduca sexta, Antimicrobial peptide, Promoter
1. Introduction
The innate immune system is conserved from insects to humans (Ganesan et al., 2011; Mogensen, 2009). Insect innate immune system relies on various germ line encoded pattern recognition receptors (PRRs) to sense pathogen-associated molecular patterns (PAMPs) and induce cellular and humoral responses (Charroux et al., 2009; Charroux and Royet, 2010; Kanost et al., 2004; Lemaitre and Hoffmann, 2007; Marmaras and Lampropoulou, 2009). An important aspect of insect humoral responses is induced production of a variety of antimicrobial peptides (AMPs) (Diamond et al., 2009). Most AMPs are small cationic peptides with activities against microorganisms and parasites (Brogden, 2005; Imler and Bulet, 2005; Kokoza et al., 2010). In Drosophila melanogaster, induced production of AMPs is regulated by the Toll and IMD (immune deficiency) pathways (Lemaitre et al., 1995; 1996; DeGregorio et al., 2002). The Toll pathway mediates recognition of fungi and Gram-positive bacteria (Ashok, 2009), while the IMD pathway senses infection by most Gram-negative bacteria (Kaneko and Silverman, 2005). The Toll pathway triggers activation of NF-κB factors Dorsal and Dif, while the IMD pathway leads to activation of Relish (Engström et al., 1993; Ganesan et al., 2011; Gross et al., 1996; Hetru and Hoffmann, 2009; Ip et al., 1993; Stöven et al., 2003).
GATA factors are a family of zinc finger containing transcription factors, which recognize the (A/T)GATA(A/G) consensus sequence, and they are involved in regulation of gene expression and differentiation (Patient and McGhee, 2002). GATA factors have been identified in vertebrates, D. melanogaster, Caenorhabditis elegans, and plants (Patient and McGhee, 2002; Reyes et al., 2004). Vertebrate GATA-1 interacts with many other regulatory partners, such as Friend of GATA (FOG), p300/CBP, PU.1 and c-myb (Lowry and Mackay, 2006). GATA factors interact with different factors to regulate gene transcription (Dai et al., 2002; Eisbacher et al., 2003; Gordon et al., 1997; Zhang et al., 2007). In D. melanogaster, Serpent (dGATAb) and dGATAe regulate induced gene expression in fat body and midgut, respectively (Petersen et al., 1999; Senger et al., 2006). GATA factors are also required for immunity in C. elegans and the silkworm Bombyx mori (Cheng et al., 2006; Kerry et al., 2006). Adjacent κB and GATA sites have been identified in many insect immune gene promoters and both sites are required for gene induction (Harshman and James, 1998; Kadalayil et al., 1997; Senger et al., 2004; Tingvall et al., 2001). Human GATA-3 and/or GATA-2 interact with NF-κB to trigger GlcNac6ST-1 transcription (Chen et al., 2008). In addition, κB and GATA sites are both required for induced expression of Drosophila cecropin A (Kadalayil et al., 1997). Therefore, κB-GATA synergy seems to be a common mechanism for immune gene regulation (Senger et al., 2004). However, little is known about synergistic effect of κB and GATA factors in insects.
In D. melanogaster, seven groups of AMPs have been identified, some AMP genes (drosomycin, diptericin, metchnikowin) have been identified only in Drosophila, others (attacin, cecropin, drosocin, defensin) are also found in other insect species (Imler and Bulet, 2005; Levashina et al., 1998). Drosomycin is an anti-fungal peptide isolated from immune challenged D. melanogaster (Fehlbaum et al., 1994; Tian et al., 2008). Expression of drosomycin is synergistically regulated by the Toll and IMD pathways (Tanji et al., 2007; Tanji et al., 2010). Diptericin is another species-related AMP gene first identified in Phormia terranovae and later in D. melanogaster (Dimarcq et al., 1988; Wicker et al., 1990). Different groups of AMP genes have also been identified in lepidopteran insects, such as B. mori and Manduca sexta (Kanost et al., 2004). Moricin, gloverin and lebocin genes have been identified only in lepidopteran insects (Axen et al., 1997; Chowdhury et al., 1995; Hara and Yamakawa, 1995; Kanost et al., 2004). Moricin was originally isolated from the hemolymph of B. mori and showed antibacterial activity against several Gram-negative and Gram-positive bacteria (Hara and Yamakawa, 1995, 1996). The N-terminal region of B. mori Moricin adopts an amphipathic alpha-helix structure that may increase permeability of the cytoplasmic membrane (Hemmi et al., 2002). Moricin analogues have been identified in other lepidopteran species, including M. sexta, Galleria mellonella, and Spodoptera litura (Brown et al., 2008; Oizumi et al., 2005; Zhu et al., 2003).
Our previous research reveals that lipopolysaccharide (LPS) and lipoteichoic acid (LTA) can induce AMP gene expression in M. sexta larvae (Rao and Yu, 2010). In D. melanogaster, peptidoglycan can activation AMP genes (Werner et al., 2000; 2003), but ultrapure LPS molecules do not induce AMP expression in adult flies (Kaneko et al., 2004), indicating that there may be important differences between dipteran and lepidopteran species regarding regulation of AMP genes. It is not known whether expression of AMP genes is regulated in a species-specific manner, and whether different co-regulators are involved in regulating AMP gene expression in lepidopteran and dipteran insects. In this study, we cloned promoters for M. sexta moricin (MsMoricin), cecropin and lysozyme genes and compared activities of the three M. sexta (Lepidopteran) and seven D. melanogaster (Dipteran) AMP gene promoters in D. melanogaster S2 cells and Spodoptera frugiperda (Lepidopteran) Sf9 cells. We found that most AMP gene promoters were regulated in a species-specific manner in the two cell lines in that D. melanogaster AMP gene promoters had no or low activity in Sf9 cells and M. sexta AMP gene promoters had no or low activity in S2 cells. We then showed that κB and GATA factors alone were not sufficient to activate MsMoricin promoter, and a κB-GATA element (22bp) from the MsMoricin promoter could significantly increase activities of D. melanogaster AMP gene promoters when inserted into the promoters. We also showed that the κB-GATA element and the endogenous κB site2 of drosomycin promoter were all required to cooperatively enhance drosomycin promoter activity. More importantly, we identified an activating element, designated as MsMoricin promoter activating element (MPAE) (140bp), which could increase activity of drosomycin promoter specifically in Sf9 cells, thus MPAE may contain co-regulator binding sites for nuclear factors specifically expressed in lepidopteran species. Our results suggest that common factors such as NF-κB and GATA factors are functional in both dipteran and lepidopteran insects, while co-regulators may confer species-specific regulation of AMP genes.
2. Material and methods
2.1 Insects, bacterial peptidoglycan (PG) and insect cell lines
M. sexta eggs were kindly provided by Professor Michael Kanost, Department of Biochemistry at Kansas State University. Larvae were reared on an artificial diet at 25°C (Dunn and Drake, 1983), and the 5th instar larvae were used for hemocytes collection. Ultrapure peptidoglycan from E. coli strain K12 (Cat#: tlrl-pgnek) was purchased from InvivoGen (San Diego, California, USA) and used for activation experiments. Drosophila melanogaster S2 cells were purchased from American Type Culture Collection (ATCC). Spodoptera frugiperda Sf9 cells were purchased from Invitrogen Corporation, USA.
2.2 Genomic DNA extraction and genome walking
M. sexta genomic DNA was extracted from hemocytes collected from the 5th instar larvae with PureLink™ Genomic DNA Kit (Invitrogen, USA). D. melanogaster genomic DNA was extracted from S2 cells. Genome walking was performed to clone MsMoricin and MsCecropin promoters with GenomeWalker Universal Kit (Clontech, USA) following instructions of the manufacturer. Briefly, 2.5 μg M. sexta genomic DNA was digested with Dra I, EcoR V, Pvu II or Stu I, respectively. Digested fragments were purified and ligated to a synthetic adaptor GWAdaptor. Adaptor primers (GW-AP1 and GW-AP2) and gene specific primers (MsMoricinGSP1-4, MsCecropinGSP1 and 2) (Table S1) were used for PCR reactions.
2.3 RNA extraction and 5′ RACE
M. sexta hemocytes were collected from the 5th instar larvae at 6 h after E. coli XL1-blue injection and total RNA was prepared from hemocytes with TRI reagent (Sigma Aldrich, USA). cDNA was prepared with ImProm-II reverse transcriptase (Promega, USA). 5′ RACE was performed to determine transcription start site of M. sexta moricin promoter with SMARTer™ RACE cDNA amplification kit (Clontech, USA).
2.4 Sequence analysis
Transcription factor binding sites were predicted with Alibaba2.1 (http://www.gene-regulation.com/). Other sequences were analyzed with DNAMAN (Lynnon Corporation, Quebec, Canada).
2.5 Construction of luciferase reporter plasmids
For luciferase reporter plasmids, promoters from antimicrobial peptide (AMP) genes of M. sexta and D. melanogaster were cloned by genome walking or PCR using genomic DNAs as templates. PCR was performed with Taq DNA polymerase using gene specific primers listed in Table S1. For MsMoricin, MsLysozyme and D. melanogaster AMP genes reporters, PCR products were digested and ligated to the Kpn I/Bgl II sites of pGL3Basic vector (Promega, USA). For MsCecropin reporter, PCR product was digested and ligated to the Xho I and Hind III sites of pGL3Basic vector. MsMoricin, MsCecropin, MsLysozyme, diptericin, DmAttacin A, DmDefensin, drosomycin, DmCecropin A1, drosocin and metchnikowin luciferase reporters contained 1456bp, 877bp, 1241bp, 980bp, 977bp, 1651bp, 812bp, 670bp, 660bp, and 1560bp of 5′ upstream sequences, respectively. In these luciferase reporters, +1 indicates the translation start site (ATG) in MsCecropin, while +1 indicates transcription initiation sites for MsMoricin, drosomycin, diptericin and DmAttacin A genes. Deletion and mutation reporters were constructed by overlapping PCR. The first round of overlapping PCR was performed to amplify the 5′ and 3′ end DNA fragments individually with overlapping regions, and the second round of overlapping was done by mixing fragments amplified from the first round PCR as templates with the 5′ and 3′ primers. MsMoricin κB5 (GTAAAGTCCC) was mutated to TTAGAGTTAT, and GATA-1 (TCGTTATCTG) was mutated to TCGCGTATCG. Drosomycin κB site-1 (GGGTTTAACC) was mutated to ATTTTTAACC, κB site-2 (AGTAGTTCCC) was mutated to AGTAGTTAAT, and a predicted κB site-4 (GGACAGTCCA) was mutated to TGAGAGTTAT. MsMoricin κB-GATA element (GTAAAGTCCCTATCGTTATCTG) was mutated to mutκB-GATA (with a mutated κB site) (TTAGAGTTATTATCGTTATCTG), κB-mutGATA (with a mutated GATA site) (GTAAAGTCCCTATCGAAAAACG), or mutκB-mutGATA (with both mutated κB and GATA sites) (TTAGAGTTATTATCGAAAAACG). To make insertion constructs, κB-GATA, mutκB-GATA, κB-mutGATA, mutκB-mutGATA, GATA (TATCGTTATCTGAGAG), MPAE (−242 to −57), MPAE-κB (−242 to −47), and MPAE-κB-GATA (−242 to −35) were inserted into different promoters at positions indicated in figures, respectively. Plasmids for transfection were prepared with PureYield™ Plasmid Miniprep System (Promega, USA). Sequences of the promoters are given in Texts S1–S9, where known or predicted NF-κB elements and GATA elements are noted by single and double underlines, respectively. Transcriptional/Translational start sites are shown in boxes.
2.6 Insect cell culture and transfection
Drosophila Schneider 2 (S2) cells and S. frugiperda Sf9 cells were maintained at 27°C in TNM-FH (HyClone, USA) supplemented with 10% fetal bovine serum, 1×L-Glutamine, 50 IU/mL penicillin and 0.05 mg/mL streptomycin (Hyclone, USA). For DNA transfection, 104 cells were plated in each well of a 96-well plate and transfected with 150 ng reporter plasmid and 15 ng pRL-TK renilla luciferase plasmid as an internal control (Promega, USA) for 12 h. Then fresh medium containing 10 μg/mL PG-K12 was used to stimulate cells for 48 h before measuring the luciferase activities. Even though 20-hydroxyecdysone-treated cells are more sensitive to immune challenges (Dimarcq et al., 1997; Silverman et al., 2000), we did not use the hormone to treat cells in our experiments because our purpose was to observe increase in promoter activities activated by PG-K12. Each transfection was performed in three wells independently and transfection experiments were repeated three times. Firefly and renilla luciferase activities were measured using Dual-luciferase Reporter Assay system (Promega, USA). Briefly, S2 cells or Sf9 cells in 96-well plates were washed once with sterile PBS solution (137 mM NaCl, 2.7 mM KCl, 4.3 mM Na2HPO4, 1.47 mM KH2PO4, pH 7.4), and then lysed with 30 μL 1× lysis buffer (Promega, USA) at room temperature for 15 min with shaking. The cell lysate (30 μL) was transferred to a tube containing 100 μL luciferase assay reagent II and mixed well, then reading was recorded immediately, 100 μL stop & glo reagent was added to quench the first reaction and the control renilla luciferase activity was measured using a Liquid Scintillation Counter (Cat #: 425-034, HIDEX, Turku, Finland).
2.7 Data analysis
Figures were made with the GraphPad Prism software with one representative set of data. Significance of difference was determined by an unpaired t-test or by one way ANOVA followed by a Tukey’s multiple comparison test using the same software (GraphPad, San Diego, CA).
3. Results
3.1 Species-specific regulation of AMP gene promoters in S2 and Sf9 cells
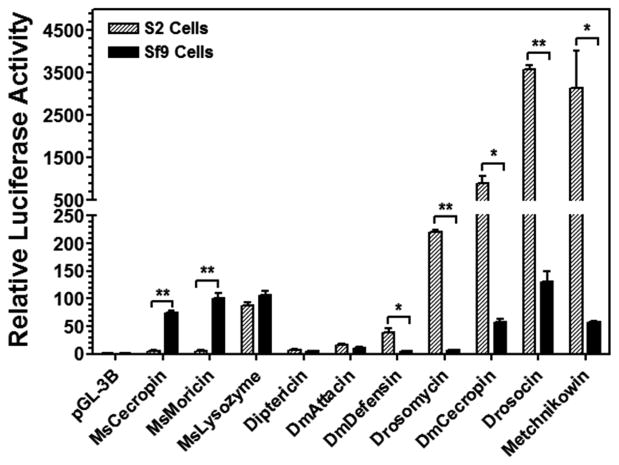
We cloned a 1.4-kb 5′-regulatory fragment of M. sexta moricin (MsMoricin) gene (GenBank accession number: JF309316.1) and constructed a luciferase reporter. Initial activity assay in D. melanogaster S2 cells (a dipteran cell line) and S. frugiperda Sf9 cells (a lepidopteran cell line) showed that MsMoricin promoter did not have activity in S2 cells, but had relatively high activity in Sf9 cells after peptidoglycan (PG) stimulation (Figure 1). This result suggests that insect AMP genes may be regulated in a species-specific manner. We used PG-K12 (from E. coli K12) to directly stimulate S2 and Sf9 cells since we did not overexpress any Rel/NF-κB proteins in these cells, and PG-K12 can bind to cell surface peptidoglycan-recognition proteins (PGRPs) to activate the IMD pathway in Drosophila (Kaneko et al., 2004). Thus, the activity observed is due to activation of promoters by endogenous transcription factors in S2 or Sf9 cells. Activation of the Toll pathway by Lys-type peptidoglycan from Gram-positive bacteria not only requires PGRPs but also involves activation of proteinases and Spätzle (ligand for the Toll receptor) (Ganesan et al., 2011), thus we did not use Gram-positive peptidoglycan in our study. All the following experiments in S2 and Sf9 cells were stimulated with PG-K12.
Figure 1. Species-specific regulation of AMP gene promoters in S2 and Sf9 cells.
S2 and Sf9 cells were transiently cotransfected with different reporter vectors using pRL-TK as an internal control. PG-K12 (peptidoglycan from E. coli K12 strain, 10 μg/ml final concentration) was used to stimulate cells for 48 h before luciferase activities were measured. Relative luciferase activity was normalized to the pGL-3B control group (arbitrarily set as 1). Bars represent the mean of three individual measurements ± S.E.M. Significance of difference between S2 and Sf9 cells in each group was determined by an unpaired t-test (* p<0.05; ** p<0.001).
To further test whether insect AMP gene promoters indeed are regulated in a species-specific manner, we cloned two more M. sexta AMP promoters (MsCecropin and MsLysozyme) and seven D. melanogaster AMP promoters (drosomycin (Drs), diptericin (Dpt), drosocin, metchnikowin, DmCecropin A1, DmAttacin A, and DmDefensin), and constructed these promoters as luciferase reporters. Results from dual luciferase assays showed that MsMoricin and MsCecropin promoters were active only in Sf9 cells; drosomycin, DmDefensin, DmCecropin A1, metchnikowin and drosocin promoters were either active only in S2 cells or showed significantly higher activities in S2 cells than in Sf9 cells (Figure 1). DmAttacin A and diptericin promoters had lower activities in both S2 and Sf9 cells, and MsLysozyme promoter showed similarly high activities in both cell lines (Figure 1). These results suggest that some AMP gene promoters indeed are regulated in a species-specific manner.
3.2 Identification of an active κB-GATA element in MsMoricin promoter
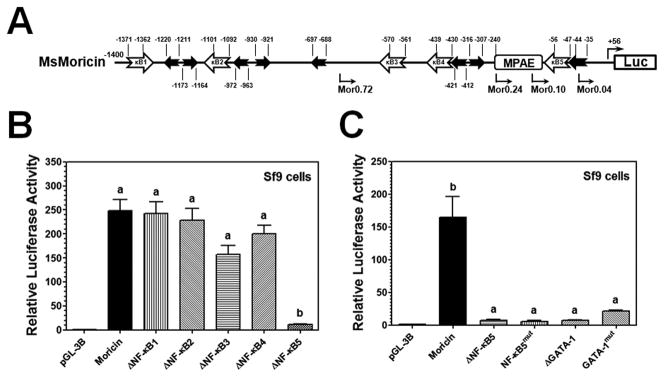
In order to identify promoter regulatory elements, we first studied regulation of MsMoricin gene. Analysis of MsMoricin promoter sequence showed that there are five predicted κB sites and eight GATA sites with one GATA-1 site only 2bp downstream of the κB5 (Figure 2A). Deletion of the κB1, 2, 3 or 4 did not have an effect on MsMoricin promoter activity; however, deletion of the κB5 almost completely abolished promoter activity in Sf9 cells (Figure 2B). Deletion or mutation of either the κB5 or the adjacent GATA-1 site significantly decreased MsMoricin promoter activity in Sf9 cells (Figure 2C), indicating that the κB5 and GATA sites function together as an active element required for activation of MsMoricin promoter.
Figure 2. Identification of an active κB-GATA element in MsMoricin promoter.
MsMoricin promoter was cloned by genome walking. Luciferase activities were measured in PG-K12 stimulated Sf9 cells. (A) Schematic representation of MsMoricin promoter. In silico analysis showed five predicted κB sites (open arrowed boxes) and eight GATA binding sites (filled arrowed boxes) with one GATA-1 site adjacent to the κB5. MPAE: MsMoricin Promoter Activating Element. (B) The κB5 site is required for promoter activity. Each of the five κB sites was deleted seperately, and only deletion of the κB5 caused a complete loss of promoter activity. (C) Both the κB5 and the adjacent GATA-1 sites are required for promoter activity. The κB5 or GATA-1 site was deleted or mutated separately. Deletion or mutation of either the κB5 or the adjacent GATA-1 site caused a significantly loss in promoter activity. Bars represent the mean of three individual measurements ± S.E.M. Identical letters are not significant difference between groups (p>0.05), while different letters indicate significant difference between groups (p<0.05) determined by one way ANOVA followed by a Tukey’s multiple comparison test.
3.3 Identification of an activating element upstream of the MsMoricin κB-GATA element
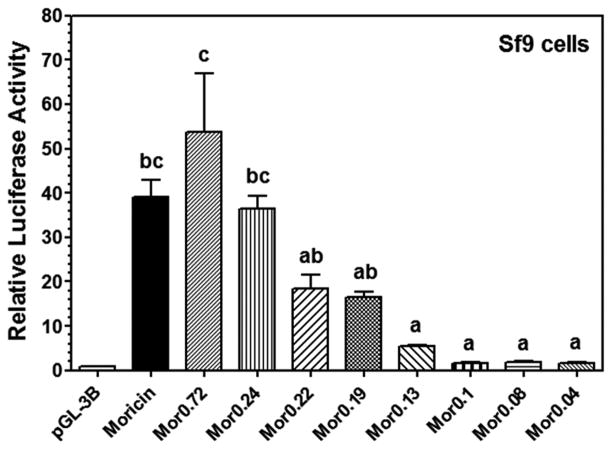
To further identify active elements in MsMoricin promoter, we made several deletion constructs and found that a short promoter of about 240 bp (Mor0.24) was fully active as 1.4kb MsMoricin promoter (Figure 3). Further deletions to the 240-bp promoter caused gradually loss in promoter activities. Noticeably, Mor0.1 (0.1kb), a construct with a complete κB5-GATA element, was inactive. The region between Mor0.24 and Mor0.1 was obviously critical to promoter activity as Mor0.24 (0.24kb) had similarly high activity as MsMoricin promoter (1.4kb) did, but Mor0.1 (0.1kb) almost had no activity (Figure 3). These results indicated that the κB-GATA element was necessary but not sufficient to activate MsMoricin promoter, and the region between -240bp and −100bp in the promoter (designated as Moricin Promoter Activating Element, MPAE) may contain important co-regulator binding sites, and these co-regulators are also required to fully activate moricin gene.
Figure 3. Identification of an MsMoricin Promoter Activating Element (MPAE).
MsMoricin promoter deletion mutants were constructed by shortening the 5′ end. Luciferase activities of these promoters were measured in Sf9 cells as described in Figure 2 (Refer to Figure 2A for the schematic representation).
3.4 The κB-GATA element of MsMoricin can enhance promoter activities of AMP genes
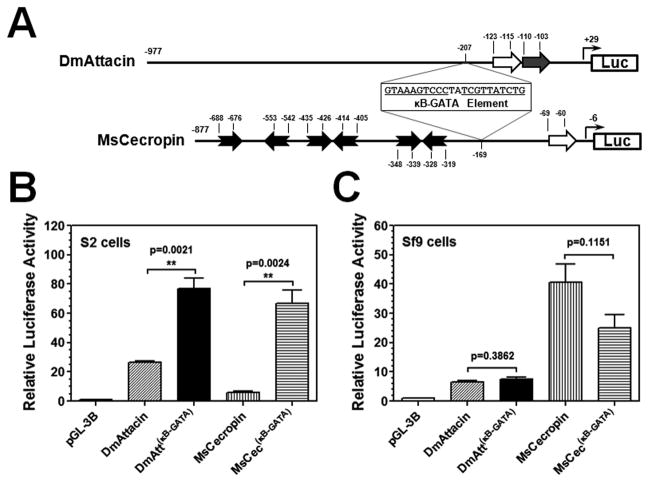
We showed that the κB-GATA element was essential but not sufficient to activate MsMoricin promoter (Figures 2 and 3). To test whether the κB-GATA element (22bp) from MsMoricin promoter can increase promoter activities of M. sexta and D. melanogaster AMP genes in S2 and/or Sf9 cells, the κB-GATA element was inserted into MsCecropin, diptericin and DmAttacin A promoters. Diptericin and DmAttacin A promoters were selected for the experiments since they had low activities in S2 cells (Figure 1). MsCecropin promoter contains six predicted GATA sites (Figure 5A). Diptericin and DmAttacin A promoters both contain a GATA site adjacent to a κB site (Senger et al., 2004) (Figures 4A and 5A), and the two κB sites in diptericin promoter have identical sequences (Kappler et al., 1993). When an MsMoricin κB-GATA element was inserted 40bp upstream of the endogenous GATA site (30bp downstream of the κB1) in diptericin promoter, a significantly increase in promoter activity was observed in both S2 and Sf9 cells, but insertion of mutant κB-GATA elements (either with a mutated κB or GATA site, or both) did not significantly change promoter activity (Figure 4B and C). In addition, insertion of a κB-GATA element increased diptericin promoter activity to a significantly higher level in S2 cells (~24-fold of diptericin promoter) than in Sf9 cells (~15-fold) (Figure 4B and C). Similarly, insertion of a κB-GATA element into DmAttacin A and MsCecropin promoters also significantly increased activities of the two promoters in S2 cells (Figure 5B), but did not increase promoter activities in Sf9 cells (Figure 5C). Together, these results suggest that the MsMoricin κB-GATA element can increase AMP promoter activity, and this element may not be species-related.
Figure 5. The κB5-GATA element can increase DmAttacin A and MsCecropin promoter activities in S2 cells.
(A) Schematic representation of DmAttacin A and MsCecropin promoters. An NF-κB and a GATA sites in DmAttacin A promoter have been identified (Senger et al., 2004; Tanji et al., 2007), and an NF-κB site and six GATA sites in MsCecropin promoter were predicted. An MsMoricin κB5-GATA element was inserted into DmAttacin A promoter at the −207 position and into MsCecropin promoter at the −169 position. Activities of these promoters were determined in S2 cells (B) or Sf9 cells (C) as described in Figures 1 and 2.
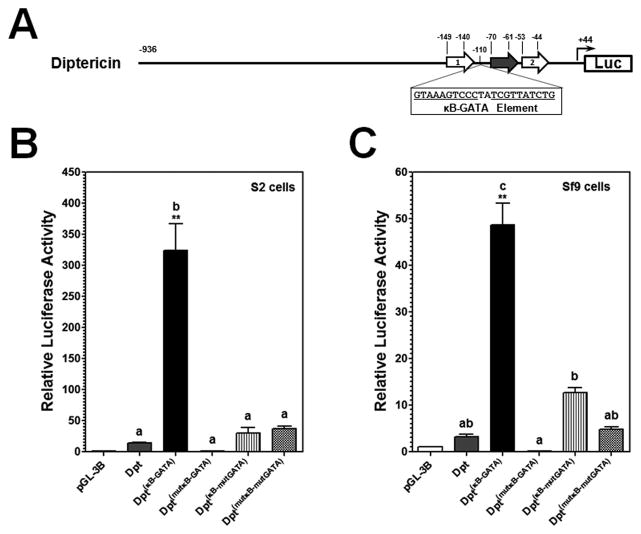
Figure 4. MsMoricin κB5-GATA element can increase Drosophila diptericin promoter activity in S2 and Sf9 cells.
(A) Schematic representation of diptericin promoter. Open arrowed boxes indicate κB sites and filled arrowed box indicates GATA-1 site in diptericin promoter (Kappler et al., 1993; Reichhart et al., 1992; Senger et al., 2004). An MsMoricin κB5-GATA element or a mutant κB5-GATA element (mutation in either the κB5 or the GATA-1 site, or both sites) was inserted into diptericin promoter at the −110 position (represented by Dpt(κB-GATA), Dpt(mutκB-GATA), Dpt(κB-mutGATA), or Dpt(mutκB-mtGATA)). Activities of these diptericin promoters were determined in S2 cells (B) or Sf9 cells (C) as described in Figures 1 and 2.
3.5 The κB-GATA element and the κB site2 cooperatively increase drosomycin promoter activity
D. melanogaster drosomycin promoter contains three κB sites, the κB site1 and site2 are mediated by the Toll and IMD pathways, respectively, and the two κB sites have synergic effects on drosomycin activation (Tanji et al., 2007; Tanji et al., 2010), but the κB site3 is not essential for promoter activity (Tanji et al., 2007). Drosomycin promoter also contains four GATA binding sites (Senger et al., 2004). Analysis of drosomycin promoter sequence showed another predicted κB site in between the κB site1 and site2, which we named the κB site4 (Figure 6A). In the following experiments, we focused our experiments on drosomycin promoter since its two κB sites are regulated differently. To test whether the predicted κB site4 is essential for drosomycin promoter, the κB site4 was deleted or mutated, and the results showed that deletion and mutation of the κB site4 did not decrease drosomycin promoter activity in S2 and Sf9 cells (Figure S1, panel A), indicating that the predicted κB site4 is not required for drosomycin promoter. We then replaced the κB site4 (10bp) with an MsMoricin κB-GATA element (22bp). Replacement of the κB site4 with a κB-GATA element significantly increased promoter activity in S2 cells (~21 folds, indicated by 4rep(κB-GATA)-Drs), but similar replacement with mutant κB-GATA elements (with a mutated κB or GATA site or both, indicated by 4rep(mutκB-GATA)-Drs, 4rep(κB-mutGATA)-Drs, or 4rep(mutκB-mutGATA)-Drs) did not significantly change activity of these promoters compared to drosomycin promoter (Figure 6B). These results demonstrated that an additional κB-GATA element can increase drosomycin promoter activity, and both the κB and GATA sites are required to increase promoter activity.
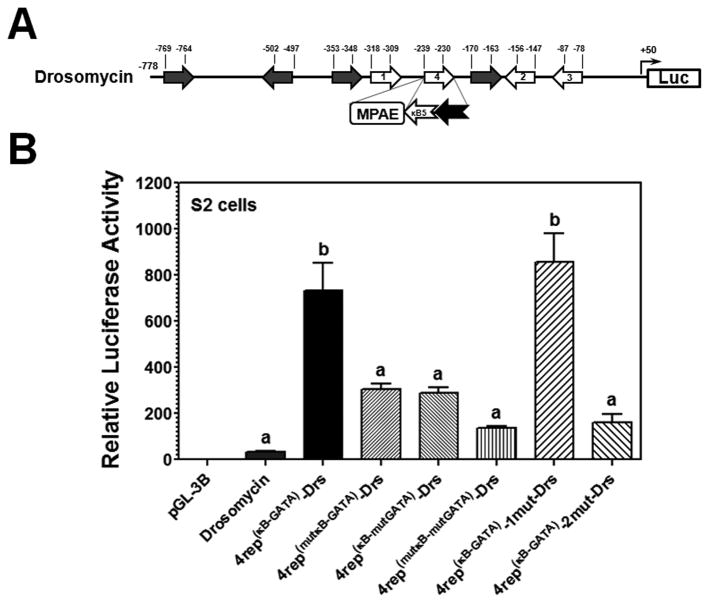
Figure 6. The κB5 and GATA elements act cooperatively with drosomycin κB site2 to increase drosomycin promoter activity.
(A) Schematic representation of drosomycin promoter. The NF-κB site1, site2 and site3 have been experimentally confirmed (Tanji et al., 2007; Tanji et al., 2010), and four GATA binding sites have been identified (Senger et al., 2004). A κB site4 was predicted. The predicted κB site4 of drosomycin promoter was deleted or mutated (represented by 4del-Drs or 4mut-Drs), or replaced with an MsMoricin κB5-GATA element or a mutant κB5-GATA element (mutation in either the κB5 or the GATA-1 site, or both sites) (represented by 4rep(κB-GATA)-Drs, 4rep(mutκB-GATA)-Drs, 4rep(κB-mutGATA)-Drs, or 4rep(mutκB-mtGATA)-Drs). The κB site1 or site2 in the κB5-GATA replaced promoter was also mutated (represented by 4rep(κB-GATA)-1mut-Drs or 4rep(κB-GATA)-2mut-Drs). Activities of these promoters were determined in S2 cells (B) or in Sf9 cells (Figure S1, panels A and B) as described in Figures 1 and 2.
To test whether increase in drosomycin promoter activity by the MsMoricin κB-GATA element requires the endogenous κB site1 or site2, the κB site1 or site2 in the κB-GATA replaced promoter was mutated (indicated by 4rep(κB-GATA)-1mut-Drs or 4rep(κB-GATA)-2mut-Drs). Mutation of the site1 did not impair the κB-GATA element to increase promoter activity; however, mutation of the site2 completely abolished the ability of the κB-GATA element to enhance promoter activity in S2 cells (Figure 6B). These results suggest that the κB-GATA element and the endogenous κB site2 act cooperatively to increase drosomycin promoter activity. In Sf9 cells, all the site4 replacement and mutation constructs showed similar low activities as drosomycin promoter did (Figure S1, panel B), suggesting that the κB-GATA element cannot increase drosomycin promoter activity in Sf9 cells.
3.6 MPAE may contain lepidoptera-related co-regulator binding sites
We also mutated the κB site1 and site2 in drosomycin promoter and found that mutation of the κB site1 did not cause a loss of promoter activity in S2 cells, and mutation of the κB site2 significantly decreased promoter activity in S2 cells, but both mutations did not have an effect on promoter activity in Sf9 cells (Figure 7). These results in S2 cells were consistent with those reported previously that the κB site1 and site2 are activated by the Toll and IMD pathways, respectively (Tanji et al., 2007; Tanji et al., 2010), as PG-K12 (peptidoglycan from E. coli strain K12) that can activate the IMD pathway in Drosophila was used to stimulate S2 cells in our experiments. To test whether the MPAE (140bp), κB, GATA, or the whole MPAE-κB-GATA element (205bp, from −240 to −35bp) from MsMoricin promoter can increase drosomycin promoter activity in S2 cells and/or Sf9 cells in particular, an MPAE was inserted prior to the κB site1 or site2, or the κB site1 or site2 was replaced with an MPAE, MPAE-κB or MPAE-κB-GATA. Our results showed that insertion of an MPAE before the κB site1 did not significantly change promoter activity in S2 cells, but did significantly increase promoter activity in Sf9 cells (1.8-fold of drosomycin promoter) (Figure 7A and B). Replacement of the κB site1 with an MPAE increased promoter activity significantly in both S2 cells (3.5-fold) and Sf9 cells (1.7-fold) (Figure 7A and B). Replacement of the κB site1 with an MPAE-κB did not further increase promoter activity compared to the MPAE replacement. However, replacement of the κB site1 with a whole MPAE-κB-GATA element increased promoter activity to a significantly higher level in both S2 cells (11.6-fold) and Sf9 cells (3.8-fold) (Figure 7A and B). Since the κB site 1 is not activated by PG and drosomycin promoter did not have activity in Sf9 cells (Figure 1), these results suggest that MPAE could specifically increase drosomycin promoter activity in Sf9 cells.
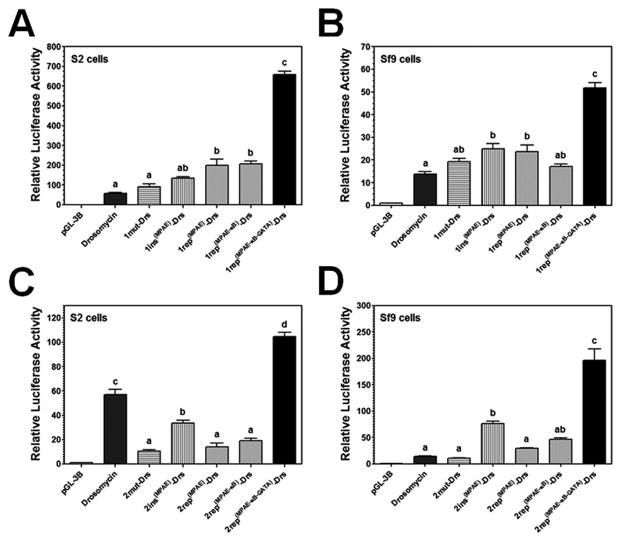
Figure 7. MPAE can specifically increase drosomycin promoter activity in Sf9 cells.
The κB site1 or site2 in drosomycin promoter was mutated, or replaced with an MPAE, MPAE-κB5 or MPAE-κB5-GATA (represented by 1mut-Drs, 1rep(MPAE)-Drs, 1rep(MPAE-κB)-Drs, or 1rep(MPAE-κB-GATA)-Drs for the site1, and the same for the site2). An MPAE was also inserted just before the site 1 or site2 (represented by 1ins(MPAE)-Drs or 2ins(MPAE)-Drs). Activities of these promoters were determined in S2 cells (A and C) or Sf9 cells (B and D) as described in Figures 1 and 2.
Drosomycin κB site2 is activated by Gram-negative peptidoglycan via the IMD pathway (Tanji et al., 2007; Tanji et al., 2010), thus mutation of the κB site2 significantly decreased promoter activity in S2 cells (0.2-fold of drosomycin promoter) (Figure 7C). Insertion of an MPAE prior to the κB site2 and replacement of the κB site2 with an MPAE and MPAE-κB significantly decreased promoter activity in S2 cells (0.6-, 0.25- and 0.3-fold of drosomycin promoter, respectively) (Figure 7C), suggesting that an MPAE or MPAE-κB could not replace the κB site2 to activate drosomycin in S2 cells. But insertion of an MPAE before the κB site2 significantly increased promoter activity in Sf9 cells (5.6-fold), while replacement of the κB site 2 with an MPAE and an MPAE-κB did not have an effect on promoter activity in Sf9 cells (Figure 7D). However, replacement of the κB site2 with a whole MPAE-κB-GATA element increased promoter activity to a significantly higher level in both S2 cells (1.8-fold) and Sf9 cells in particular (14.3-fold) (Figure 7C and D). Since the κB-GATA element could increase drosomycin promoter activity in S2 cells but not in Sf9 cells (Figures 6B and S1), the κB site2 but not the κB site1 is required for peptidoglycan stimulation (Tanji et al., 2007; Tanji et al., 2010), these results indicated that MPAE may contain some binding sites for nuclear factors expressed specifically in Sf9 cells (a lepidopteran cell line), which can increase drosomycin promoter activity in Sf9 cells.
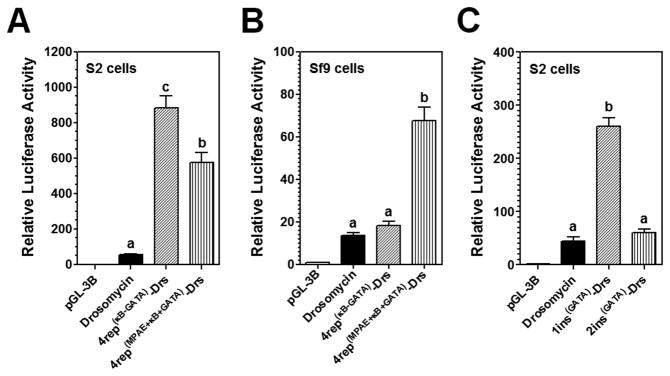
To further confirm that the MPAE can increase drosomycin promoter activity in Sf9 cells, we replaced the predicted κB site4 (10bp), a non-essential site, with an MsMoricin κB-GATA or an MPAE-κB-GATA (Figure 8A and B). Replacement of the κB site4 with a κB-GATA significantly increased promoter activity in S2 cells (Figure 8A), but did not have an effect on promoter activity in Sf9 cells (Figure 8B). Replacement of the site4 with an MPAE-κB-GATA though also significantly increased promoter activity in S2 cells compared to the control drosomycin promoter, but decreased promoter activity compared to the κB-GATA replacement (Figure 8A), suggesting that an MPAE did not further increase (actually decreased) promoter activity in S2 cells. However, the MPAE-κB-GATA replacement significantly increased promoter activity in Sf9 cells (Figure 8B), indicating that MPAE indeed contains lepidoptera-related co-regulator binding sites that can increase drosomycin promoter activity in Sf9 cells. We also inserted an MsMoricin GATA site just after the κB site1 and the site2 of drosomycin promoter and our results showed that a GATA insertion after the κB site1 significantly increased promoter activity in S2 cells (5.8-fold), but did not increase promoter activity in Sf9 cells, and a GATA insertion after the κB site2 did not have an effect on promoter activity in both S2 and Sf9 cells (Figures 8C and S1).
Figure 8. Effects of the GATA, κB-GATA and MPAE-κB-GATA elements on drosomycin promoter activity.
MsMoricin GATA-1 element was inserted immediately after the κB site1 or site2 of drosomycin promoter (representing by 1ins(GATA)-Drs or 2ins(GATA)-Drs), or the predicted κB site4 of drosomycin promoter was replaced with an MsMoricin κB5-GATA or an MPAE-κB5-GATA element (represented by 4rep(κB-GATA)-Drs or 4rep(MPAE-κB-GATA)-Drs). Activities of these promoters were determined in S2 cells (A and C) or Sf9 cells (B and Figure S1, panel C) as described in Figures 1 and 2.
4 Discussion
4.1 Species-specific regulation of AMP genes
Most AMP promoters investigated in this study showed species-specific regulation (Figure 1), suggesting that certain components in the transcription complex may account for the species-specific regulation. Since the κB and GATA transcription factors bind to similar consensus sequences across different species, M. sexta κB-GATA element may not be species-related and therefore is functional in D. melanogaster S2 cells. We hypothesize that transcription of insect AMP genes may involve formation of a transcription complex composed of both common factors (NF-κB and GATA) and species-related co-regulators, and it is the co-regulator that confers species-specific regulation. Indeed, some AMPs are species-specific. For example, moricin, gloverin and lebocin genes have been identified only in lepidopteran insects so far (Axen et al., 1997; Chowdhury et al., 1995; Hara and Yamakawa, 1995). D. melanogaster deformed epidermal autoregulatory factor-1 (DEAF-1) has been identified as a new factor that contributes to induced expression of metchnikowin and drosomycin (Gross and McGinnis, 1996; Reed et al., 2008), two species-specific AMP genes in Drosophila. In addition, Dorsal interacting proteins have been identified (Li et al., 2007; Ratnaparkhi et al., 2008). These factors/proteins may function as species-related co-regulators. MsLysozyme promoter showed similarly high activity in both S2 and Sf9 cells (Figure 1). We did not identify κB sites in MsLysozyme promoter by in silico analysis. Moreover, induced expression level of MsLysozyme mRNA by different bacterial components was always significantly lower than that of other AMP genes that are regulated by NF-κB factors (X-J Rao and X-Q Yu, unpublished data). Therefore, MsLysozyme is likely not regulated by NF-κB factors. It is also possible that regulation of AMP promoters may be tissue-specific, since S2 and Sf9 cells were used in this study, and S2 cells were hemocyte origin whereas Sf9 cells were developed from the ovary.
4.2 The κB, GATA and MPAE elements cooperatively induce gene transcription
MsMoricin κB-GATA element is necessary but not sufficient to activate MsMoricin promoter induced by E. coli peptidoglycan (Figures 2 and 3). This result indicates that other co-regulators are required to cooperate with NF-κB and GATA factors to activate transcription of MsMoricin. These co-regulators likely bind to the 140bp MPAE (MsMoricin Promoter Activating Element) region (Figures 2A and 3), which contains predicted binding sites for nuclear factors such as YY-1, Pit-1, Oct-1, and C/EBP. However, deletion of a predicted YY-1 (−201 to −192bp) and a Pit-1 (−185 to −176bp) site in MPAE did not have an effect on MsMoricin promoter activity (X-J Rao and X-Q Yu, unpublished data), suggesting that there may be novel co-regulator binding sites in the MPAE element.
4.3 The κB-GATA and MPAE elements activate D. melanogaster AMP gene promoters in S2 and SF9 cells differently
It has been reported that when Drosophila Toll and IMD pathways are stimulated simultaneously at a low level, there is a synergic effect on activation of drosomycin gene probably due to formation of Dorsal-Relish and/or Dif-Relish heterodimers that bind to the κB site2 (Tanji et al., 2007; Tanji et al., 2010). However, how Dorsal-Relish and/or Dif-Relish heterodimers synergistically activate drosomycin promoter is not well understood. We showed that the κB-GATA element from MsMoricin promoter could enhance activities of Drosophila AMP promoters (Figures 4–6) in S2 cells. We also showed that drosomycin promoter could be activated cooperatively by the endogenous κB site2 and the exogenous κB-GATA element (Figures 6B). Nonetheless, the same set of reporters consistently showed low activities in Sf9 cells (Figure S1), indicating that the κB-GATA element is not a species-related activating element.
MPAE specifically increased drosomycin promoter activity in Sf9 cells (Figure 8B), but not in S2 cells (Figure 8A), strongly suggesting that MPAE indeed contains lepidoptera-related co-regulator binding sites. Since the κB site1 is not activated by the IMD pathway (Tanji et al., 2007; Tanji et al., 2010), high activity of the 1rep(MPAE-κB-GATA)-Drs reporter in S2 cells (Figure 7A) may result from synergic effect of the κB site2 and the κB-GATA element. But high activity of the 1rep(MPAE-κB-GATA)-Drs reporter in Sf9 cells (Figure 7B) is due to both the MPAE and the κB-GATA elements. The κB site2 is activated by the IMD pathway (Tanji et al., 2007; Tanji et al., 2010), low activities of the MPAE and MPAE-κB replaced reporters (2rep(MPAE)-Drs and the 2rep(MPAE-κB)-Drs) (Figure 7C) suggest that an MPAE or an exogenous κB cannot substitute for the κB site2 to activate drosomycin in S2 cells. However, a whole MPAE-κB-GATA element is a stronger element than the κB site2, since it could increase drosomycin activity to a significantly higher level in S2 cells (Figure 7A and C). Insertion of an MPAE alone before the site2 already caused a significant increase in promoter activity in Sf9 cells (2ins(MPAE)-Drs reporter in Figure 7D). A whole MPAE-κB-GATA element increased activity in Sf9 cells to the highest level (2rep(MPAE-κB-GATA)-Drs reporter in Figure 7D). These results altogether indicate that MPAE contains lepidoptera-related regulators that can activate drosomycin promoter in Sf9 cells, and the MsMoricin κB-GATA is a stronger element than the endogenous κB site2 in activation of drosomycin promoter.
The κB-GATA element from MsMoricin increased activity of diptericin promoter in both S2 and Sf9 cells, and increased activity of drosomycin promoter only in S2 cells but not in Sf9 cells (Figures 4, 6 and S1). Diptericin is activated by the IMD pathway, while drosomycin is mainly activated by the Toll pathway. MsMoricin κB-GATA was also activated by the IMD pathway (Figure 3) as we used PG-K12 to stimulate cells. Thus, it is possible that an extra κB-GATA element and the endogenous κB sites in diptericin promoter act cooperatively to increase promoter activity in Sf9 cells.
4.4 Effect of the position, direction and consensus sequence of κB and GATA sites on AMP gene promoter activity
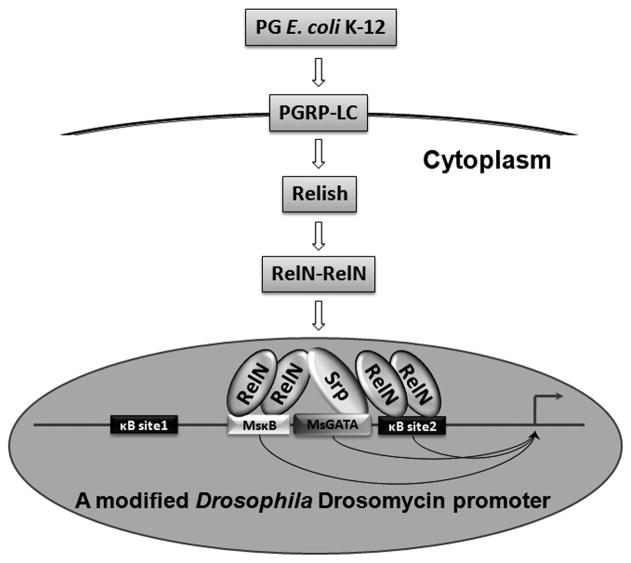
D. melanogaster Dorsal binds consensus sequence of GGG(A/T)(A/T)(T/A)(A/T/C)(C/A/T)(T/G/C); Relish binds consensus sequence of GGGA(A/T/C)N(C/T)(C/A)(C/T); Dif/Relish heterodimer binds consensus sequence of GGGA(A/T)TC(C/A)C (Busse et al., 2007; Senger et al., 2004). Drosomycin κB site1 (GGGTTTAACC) is consistent with Dorsal binding consensus; the κB site2 (GGGAACTACT) is consistent with Relish binding consensus; MsMoricin κB5 (GGGACTTTAC) is not completely consistent with any of the three consensus sequences. MsMoricin GATA site (CAGATAACGA) is consistent with Drosophila Serpent consensus sequence [(A/T/C)GATA(A/G)(C/T/G)] (Senger et al., 2004). Based on previous reports and our data, we propose that active RelN/RelN homodimer may bind to MsMoricin κB5 site and drosomycin κB site2, and a Drosophila GATA factor (Serpent, for example) may bind to MsMoricin GATA site to achieve maximal synergy in drosomycin promoter in S2 cells (Figure 9).
Figure 9. A model for synergistic effect of an exogenous κB and GATA elements with the endogenous κB element on drosomycin promoter activity.
PG-K12 activates the Imd pathway, leading to formation of active RelN/RelN homodimers that bind to the exogenous and endogenous κB sites. A Drosophila GATA factor (Serpent) binds to the exogenous GATA-1 site and probably brings the two κB sites together to facilitate formation of a transcription complex.
The position, direction and distance between the κB and GATA sites are also important for Rel-GATA synergy (Senger et al., 2004; Vardhanabhuti et al., 2007). The features of MsMoricin κB-GATA element used in our experiments are consistent with those reported previously (Figure 2A and Text S1) (Kadalayil et al., 1997; Senger et al., 2004). An MsMoricin GATA element alone could significantly increase drosomycin promoter activity in S2 cells when inserted after the endogenous κB site1 but did not show this effect when inserted after the κB site2 (Figure 8C). This may be due to special needs for the position, orientation and/or spacing of the inserted GATA element.
4.5 Potential applications of our finding
What we report here may have broad applications in transgenic engineering to increase antibacterial activities in different organisms. A κB-GATA element may be inserted into a promoter to drive expression of a transgene only after induction by bacteria, and a species-specific element, if identified, may be inserted into a promoter to drive expression of an exogenous gene in an organism. Plants do not have NF-κB factors, but they do have GATA factors and AP-1 like WRKY factors (Reyes et al., 2004; Ronald and Beutler, 2010). AP-1 and GATA-2 cooperatively regulate expression of Endothelin-1, a vasoactive peptide from endothelial cells (Kawana et al., 1995). Thus, WRKY-GATA synergy might exist in plants too, although there have been no reports so far. More research is needed to further identify unknown co-regulators in the transcription complex as this will shed light on molecular mechanisms of immune gene regulation.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health Grant GM066356. The nucleotide sequence of M. sexta moricin promoter reported in this paper has been submitted to the Genbank with accession number JF309316.1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashok Y. Drosophila toll pathway: the new model. Sci Signal. 2009;2:jc1. doi: 10.1126/scisignal.252jc1. [DOI] [PubMed] [Google Scholar]
- Axen A, Carlsson A, Engström A, Bennich H. Gloverin, an antibacterial protein from the immune hemolymph of Hyalophora pupae. Eur J Biochem. 1997;247:614–619. doi: 10.1111/j.1432-1033.1997.00614.x. [DOI] [PubMed] [Google Scholar]
- Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat Rev Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- Brown SE, Howard A, Kasprzak AB, Gordon KH, East PD. The discovery and analysis of a diverged family of novel antifungal moricin-like peptides in the wax moth Galleria mellonella. Insect Biochem Mol Biol. 2008;38:201–212. doi: 10.1016/j.ibmb.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Busse MS, Arnold CP, Towb P, Katrivesis J, Wasserman SA. A kappaB sequence code for pathway-specific innate immune responses. EMBO J. 2007;26:3826–3835. doi: 10.1038/sj.emboj.7601798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charroux B, Rival T, Narbonne-Reveau K, Royet J. Bacterial detection by Drosophila peptidoglycan recognition proteins. Microbes Infect. 2009;11:631–636. doi: 10.1016/j.micinf.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Charroux B, Royet J. Drosophila immune response: From systemic antimicrobial peptide production in fat body cells to local defense in the intestinal tract. Fly. 2010;4:40–47. doi: 10.4161/fly.4.1.10810. [DOI] [PubMed] [Google Scholar]
- Chen GY, Sakuma K, Kannagi R. Significance of NF-kappaB/GATA axis in tumor necrosis factor-alpha-induced expression of 6-sulfated cell recognition glycans in human T-lymphocytes. J Biol Chem. 2008;283:34563–34570. doi: 10.1074/jbc.M804271200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Zhao P, Liu C, Xu P, Gao Z, Xia Q, Xiang Z. Structures, regulatory regions, and inductive expression patterns of antimicrobial peptide genes in the silkworm Bombyx mori. Genomics. 2006;87:356–365. doi: 10.1016/j.ygeno.2005.11.018. [DOI] [PubMed] [Google Scholar]
- Chowdhury S, Taniai K, Hara S, Kadono-Okuda K, Kato Y, Yamamoto M, Xu J, Choi SK, Debnath NC, Choi HK, et al. cDNA cloning and gene expression of lebocin, a novel member of antibacterial peptides from the silkworm, Bombyx mori. Biochem Biophys Res Commun. 1995;214:271–278. doi: 10.1006/bbrc.1995.2284. [DOI] [PubMed] [Google Scholar]
- Dai YS, Cserjesi P, Markham BE, Molkentin JD. The transcription factors GATA4 and dHAND physically interact to synergistically activate cardiac gene expression through a p300-dependent mechanism. J Biol Chem. 2002;277:24390–24398. doi: 10.1074/jbc.M202490200. [DOI] [PubMed] [Google Scholar]
- De Gregorio E, Spellman PT, Tzou P, Rubin GM, Lemaitre B. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J. 2002;21:2568–2579. doi: 10.1093/emboj/21.11.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond G, Beckloff N, Weinberg A, Kisich KO. The roles of antimicrobial peptides in innate host defense. Curr Pharm Des. 2009;15:2377–2392. doi: 10.2174/138161209788682325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimarcq JL, Imler JL, Lanot R, Ezekowitz RA, Hoffmann JA, Janeway CA, Lagueux M. Treatment of l(2)mbn Drosophila tumorous blood cells with the steroid hormone ecdysone amplifies the inducibility of antimicrobial peptide gene expression. Insect Biochem Mol Biol. 1997;27:877–886. doi: 10.1016/s0965-1748(97)00072-6. [DOI] [PubMed] [Google Scholar]
- Dimarcq JL, Keppi E, Dunbar B, Lambert J, Reichhart JM, Hoffmann D, Rankine SM, Fothergill JE, Hoffmann JA. Insect immunity. Purification and characterization of a family of novel inducible antibacterial proteins from immunized larvae of the dipteran Phormia terranovae and complete amino-acid sequence of the predominant member, diptericin A. Eur J Biochem. 1988;171:17–22. doi: 10.1111/j.1432-1033.1988.tb13752.x. [DOI] [PubMed] [Google Scholar]
- Dunn PE, Drake D. Fate of bacteria injected into naive and immunized larvae of the tobacco hornworm, Manduca sexta. J Invertebr Pathol. 1983;41:77–85. [Google Scholar]
- Eisbacher M, Holmes ML, Newton A, Hogg PJ, Khachigian LM, Crossley M, Chong BH. Protein-protein interaction between Fli-1 and GATA-1 mediates synergistic expression of megakaryocyte-specific genes through cooperative DNA binding. Mol Cell Biol. 2003;23:3427–3441. doi: 10.1128/MCB.23.10.3427-3441.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engström Y, Kadalayil L, Sun SC, Samakovlis C, Hultmark D, Faye I. kappa B-like motifs regulate the induction of immune genes in Drosophila. J Mol Biol. 1993;232:327–333. doi: 10.1006/jmbi.1993.1392. [DOI] [PubMed] [Google Scholar]
- Fehlbaum P, Bulet P, Michaut L, Lagueux M, Broekaert WF, Hetru C, Hoffmann JA. Insect immunity. Septic injury of Drosophila induces the synthesis of a potent antifungal peptide with sequence homology to plant antifungal peptides. J Biol Chem. 1994;269:33159–33163. [PubMed] [Google Scholar]
- Ganesan S, Aggarwal K, Paquette N, Silverman N. NF-κB/Rel Proteins and the Humoral Immune Responses of Drosophila melanogaster. Curr Top Microbiol Immunol. 2011;349:25–60. doi: 10.1007/82_2010_107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon DF, Lewis SR, Haugen BR, James RA, McDermott MT, Wood WM, Ridgway EC. Pit-1 and GATA-2 interact and functionally cooperate to activate the thyrotropin beta-subunit promoter. J Biol Chem. 1997;272:24339–24347. doi: 10.1074/jbc.272.39.24339. [DOI] [PubMed] [Google Scholar]
- Gross CT, McGinnis W. DEAF-1, a novel protein that binds an essential region in a Deformed response element. EMBO J. 1996;15:1961–1970. [PMC free article] [PubMed] [Google Scholar]
- Gross I, Georgel P, Kappler C, Reichhart JM, Hoffmann JA. Drosophila immunity: a comparative analysis of the Rel proteins dorsal and Dif in the induction of the genes encoding diptericin and cecropin. Nucleic Acids Res. 1996;24:1238–1245. doi: 10.1093/nar/24.7.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara S, Yamakawa M. Moricin, a novel type of antibacterial peptide isolated from the silkworm, Bombyx mori. J Biol Chem. 1995;270:29923–29927. doi: 10.1074/jbc.270.50.29923. [DOI] [PubMed] [Google Scholar]
- Hara S, Yamakawa M. Production in Escherichia of moricin, a novel type antibacterial peptide from the silkworm, Bombyx mori. Biochem Biophys Res Commun. 1996;220:664–669. doi: 10.1006/bbrc.1996.0461. [DOI] [PubMed] [Google Scholar]
- Harshman LG, James AA. Differential gene expression in insects: transcriptional control. Annu Rev Entomol. 1998;43:671–700. doi: 10.1146/annurev.ento.43.1.671. [DOI] [PubMed] [Google Scholar]
- Hemmi H, Ishibashi J, Hara S, Yamakawa M. Solution structure of moricin, an antibacterial peptide, isolated from the silkworm Bombyx mori. FEBS Lett. 2002;518:33–38. doi: 10.1016/s0014-5793(02)02637-6. [DOI] [PubMed] [Google Scholar]
- Hetru C, Hoffmann JA. NF-kappaB in the immune response of Drosophila. Cold Spring Harb Perspect Biol. 2009;1:a000232. doi: 10.1101/cshperspect.a000232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imler JL, Bulet P. Antimicrobial peptides in Drosophila: structures, activities and gene regulation. Chem Immunol Allergy. 2005;86:1–21. doi: 10.1159/000086648. [DOI] [PubMed] [Google Scholar]
- Ip YT, Reach M, Engström Y, Kadalayil L, Cai H, González-Crespo S, Tatei K, Levine M. Dif, a dorsal-related gene that mediates an immune response in Drosophila. Cell. 1993;75:753–763. doi: 10.1016/0092-8674(93)90495-c. [DOI] [PubMed] [Google Scholar]
- Kadalayil L, Petersen UM, Engström Y. Adjacent GATA and kappa B-like motifs regulate the expression of a Drosophila immune gene. Nucleic Acids Res. 1997;25:1233–1239. doi: 10.1093/nar/25.6.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Goldman WE, Mellroth P, Steiner H, Fukase K, Kusumoto S, Harley W, Fox A, Golenbock D, Silverman N. Monomeric and polymeric gram-negative peptidoglycan but not purified LPS stimulate the Drosophila IMD pathway. Immunity. 2004;20:637–649. doi: 10.1016/s1074-7613(04)00104-9. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Silverman N. Bacterial recognition and signalling by the Drosophila IMD pathway. Cell Microbiol. 2005;7:461–469. doi: 10.1111/j.1462-5822.2005.00504.x. [DOI] [PubMed] [Google Scholar]
- Kanost MR, Jiang H, Yu XQ. Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol Rev. 2004;198:97–105. doi: 10.1111/j.0105-2896.2004.0121.x. [DOI] [PubMed] [Google Scholar]
- Kappler C, Meister M, Lagueux M, Gateff E, Hoffmann JA, Reichhart JM. Insect immunity. Two 17 bp repeats nesting a kappa B-related sequence confer inducibility to the diptericin gene and bind a polypeptide in bacteria-challenged Drosophila. EMBO J. 1993;12:1561–1568. doi: 10.1002/j.1460-2075.1993.tb05800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawana M, Lee ME, Quertermous EE, Quertermous T. Cooperative interaction of GATA-2 and AP1 regulates transcription of the endothelin-1 gene. Mol Cell Biol. 1995;15:4225–4231. doi: 10.1128/mcb.15.8.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerry S, TeKippe M, Gaddis NC, Aballay A. GATA transcription factor required for immunity to bacterial and fungal pathogens. PLoS One. 2006;1:e77. doi: 10.1371/journal.pone.0000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokoza V, Ahmed A, Woon Shin S, Okafor N, Zou Z, Raikhel AS. Blocking of Plasmodium transmission by cooperative action of Cecropin A and Defensin A in transgenic Aedes aegypti mosquitoes. Proc Natl Acad Sci USA. 2010;107:8111–8116. doi: 10.1073/pnas.1003056107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Kromer-Metzger E, Michaut L, Nicolas E, Meister M, Georgel P, Reichhart JM, Hoffmann JA. A recessive mutation, immune deficiency (imd), defines two distinct control pathways in the Drosophila host defense. Proc Natl Acad Sci USA. 1995;92:9465–9469. doi: 10.1073/pnas.92.21.9465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86:973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- Lemaitre B, Hoffmann J. The Host Defense of Drosophila melanogaster. Annu Rev Immunol. 2007;25:697–743. doi: 10.1146/annurev.immunol.25.022106.141615. [DOI] [PubMed] [Google Scholar]
- Levashina EA, Ohresser S, Lemaitre B, Imler JL. Two distinct pathways can control expression of the gene encoding the Drosophila antimicrobial peptide metchnikowin. J Mol Biol. 1998;278:515–527. doi: 10.1006/jmbi.1998.1705. [DOI] [PubMed] [Google Scholar]
- Li L, Zhu Q, He X, Sinha S, Halfon MS. Large-scale analysis of transcriptional cis-regulatory modules reveals both common features and distinct subclasses. Genome Biol. 2007;8:R101. doi: 10.1186/gb-2007-8-6-r101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry JA, Mackay JP. GATA-1: one protein, many partners. Int J Biochem Cell Biol. 2006;38:6–11. doi: 10.1016/j.biocel.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Marmaras VJ, Lampropoulou M. Regulators and signalling in insect haemocyte immunity. Cell Signal. 2009;21:186–195. doi: 10.1016/j.cellsig.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009;22:240–273. doi: 10.1128/CMR.00046-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oizumi Y, Hemmi H, Minami M, Asaoka A, Yamakawa M. Isolation, gene expression and solution structure of a novel moricin analogue, antibacterial peptide from a lepidopteran insect, Spodoptera litura. Biochim Biophys Acta. 2005;1752:83–92. doi: 10.1016/j.bbapap.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Patient RK, McGhee JD. The GATA family (vertebrates and invertebrates) Curr Opin Genet Dev. 2002;12:416–422. doi: 10.1016/s0959-437x(02)00319-2. [DOI] [PubMed] [Google Scholar]
- Petersen UM, Kadalayil L, Rehorn KP, Hoshizaki DK, Reuter R, Engström Y. Serpent regulates Drosophila immunity genes in the larval fat body through an essential GATA motif. EMBO J. 1999;18:4013–4022. doi: 10.1093/emboj/18.14.4013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao XJ, Yu XQ. Lipoteichoic acid and lipopolysaccharide can activate antimicrobial peptide expression in the tobacco hornworm Manduca sexta. Dev Comp Immunol. 2010;34:1119–1128. doi: 10.1016/j.dci.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnaparkhi GS, Duong HA, Courey AJ. Dorsal interacting protein 3 potentiates activation by Drosophila Rel homology domain proteins. Dev Comp Immunol. 2008;32:1290–1300. doi: 10.1016/j.dci.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed DE, Huang XM, Wohlschlegel JA, Levine MS, Senger K. DEAF-1 regulates immunity gene expression in Drosophila. Proc Natl Acad Sci USA. 2008;105:8351–8356. doi: 10.1073/pnas.0802921105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes JC, Muro-Pastor MI, Florencio FJ. The GATA family of transcription factors in Arabidopsis and rice. Plant Physiol. 2004;134:1718–1732. doi: 10.1104/pp.103.037788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronald PC, Beutler B. Plant and animal sensors of conserved microbial signatures. Science. 2010;330:1061–1064. doi: 10.1126/science.1189468. [DOI] [PubMed] [Google Scholar]
- Senger K, Armstrong GW, Rowell WJ, Kwan JM, Markstein M, Levine M. Immunity regulatory DNAs share common organizational features in Drosophila. Mol Cell. 2004;13:19–32. doi: 10.1016/s1097-2765(03)00500-8. [DOI] [PubMed] [Google Scholar]
- Senger K, Harris K, Levine M. GATA factors participate in tissue-specific immune responses in Drosophila larvae. Proc Natl Acad Sci USA. 2006;103:15957–15962. doi: 10.1073/pnas.0607608103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman N, Zhou R, Stöven S, Pandey N, Hultmark D, Maniatis T. A Drosophila IkappaB kinase complex required for Relish cleavage and antibacterial immunity. Genes Dev. 2000;14:2461–2471. doi: 10.1101/gad.817800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stöven S, Silverman N, Junell A, Hedengren-Olcott M, Erturk D, Engström Y, Maniatis T, Hultmark D. Caspase-mediated processing of the Drosophila NF-kappaB factor Relish. Proc Natl Acad Sci USA. 2003;100:5991–5996. doi: 10.1073/pnas.1035902100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji T, Hu X, Weber ANR, Ip YT. Toll and IMD pathways synergistically activate an innate immune response in Drosophila melanogaster. Mol Cell Biol. 2007;27:4578–4588. doi: 10.1128/MCB.01814-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanji T, Ip YT. Regulators of the Toll and Imd pathways in the Drosophila innate immune response. Trends Immunol. 2005;26:193–198. doi: 10.1016/j.it.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Tanji T, Yun EY, Ip YT. Heterodimers of NF-kappaB transcription factors DIF and Relish regulate antimicrobial peptide genes in Drosophila. Proc Natl Acad Sci USA. 2010;107:14715–14720. doi: 10.1073/pnas.1009473107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian C, Gao B, Rodriguez Mdel C, Lanz-Mendoza H, Ma B, Zhu S. Gene expression, antiparasitic activity, and functional evolution of the drosomycin family. Mol Immunol. 2008;45:3909–3916. doi: 10.1016/j.molimm.2008.06.025. [DOI] [PubMed] [Google Scholar]
- Tingvall TÖ, Roos E, Engström Y. The GATA factor Serpent is required for the onset of the humoral immune response in Drosophila embryos. Proc Natl Acad Sci USA. 2001;98:3884–3888. doi: 10.1073/pnas.061230198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardhanabhuti S, Wang J, Hannenhalli S. Position and distance specificity are important determinants of cis-regulatory motifs in addition to evolutionary conservation. Nucleic Acids Res. 2007;35:3203–3213. doi: 10.1093/nar/gkm201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Liu G, Kang D, Ekengren S, Steiner H, Hultmark D. A family of peptidoglycan recognition proteins in the fruit fly Drosophila melanogaster. Proc Natl Acad Sci USA. 2000;97:13772–13777. doi: 10.1073/pnas.97.25.13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Borge-Renberg K, Mellroth P, Steiner H, Hultmark D. Functional diversity of the Drosophila PGRP-LC gene cluster in the response to lipopolysaccharide and peptidoglycan. J Biol Chem. 2003;278:26319–26322. doi: 10.1074/jbc.C300184200. [DOI] [PubMed] [Google Scholar]
- Wicker C, Reichhart JM, Hoffmann D, Hultmark D, Samakovlis C, Hoffmann JA. Insect immunity. Characterization of a Drosophila cDNA encoding a novel member of the diptericin family of immune peptides. J Biol Chem. 1990;265:22493–22498. [PubMed] [Google Scholar]
- Zhang Y, Rath N, Hannenhalli S, Wang Z, Cappola T, Kimura S, Atochina-Vasserman E, Lu MM, Beers MF, Morrisey EE. GATA and Nkx factors synergistically regulate tissue-specific gene expression and development in vivo. Development. 2007;134:189–198. doi: 10.1242/dev.02720. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Johnson TJ, Myers AA, Kanost MR. Identification by subtractive suppression hybridization of bacteria-induced genes expressed in Manduca sexta fat body. Insect Biochem Mol Biol. 2003;33:541–559. doi: 10.1016/s0965-1748(03)00028-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.